Choline Metabolites Reverse Differentially the Habituation Deficit and Elevated Memory of Tau Null Drosophila
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drosophila Culture and Strains
2.2. Drug Treatment
2.3. Choline, Acetylcholine, and Phosphatidylcholine Analysis
2.4. Associative Learning and Memory
2.5. Habituation to Electric Foot shock
2.6. Statistical Analysis of Behavioral Experiments
3. Results
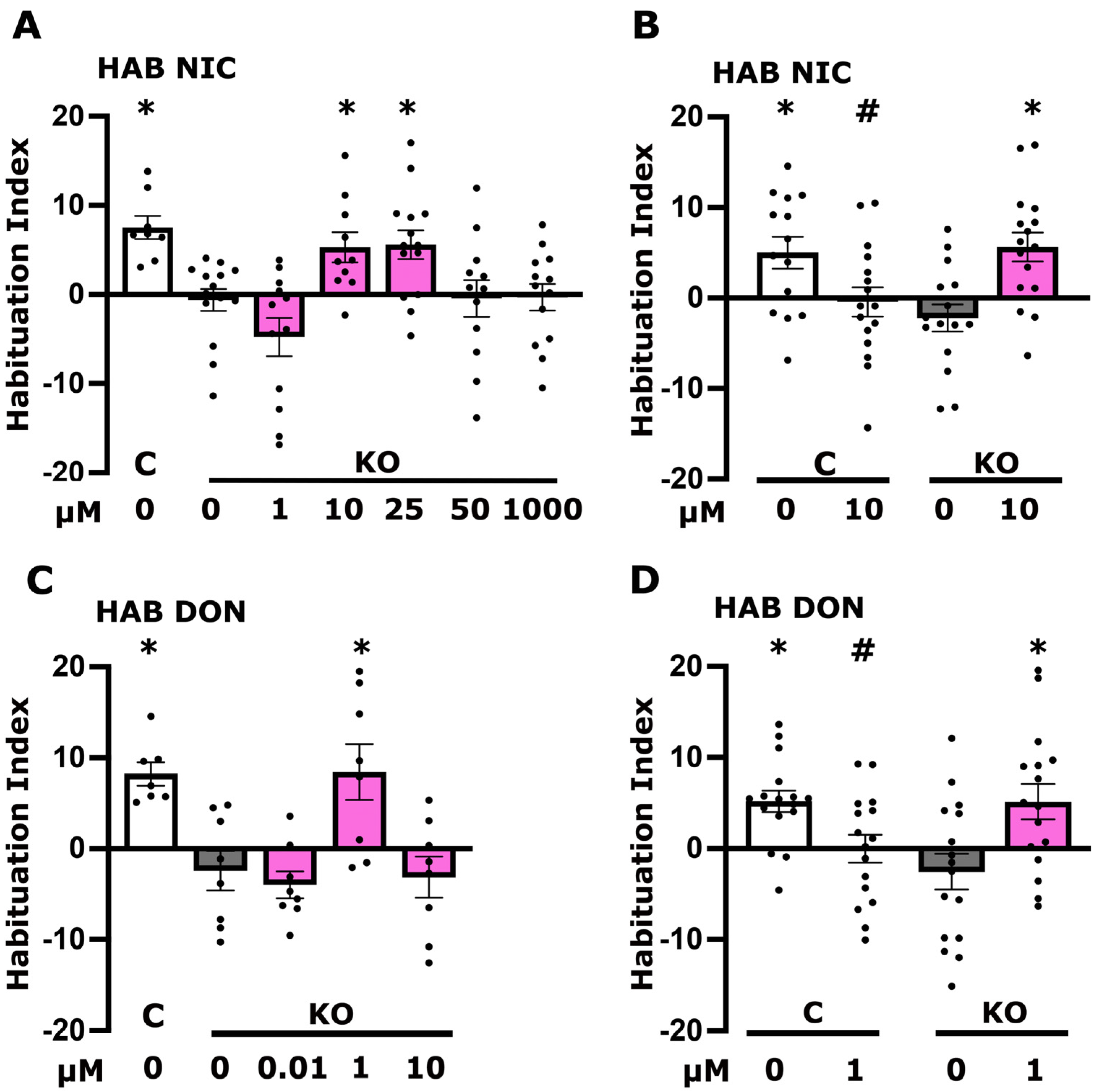
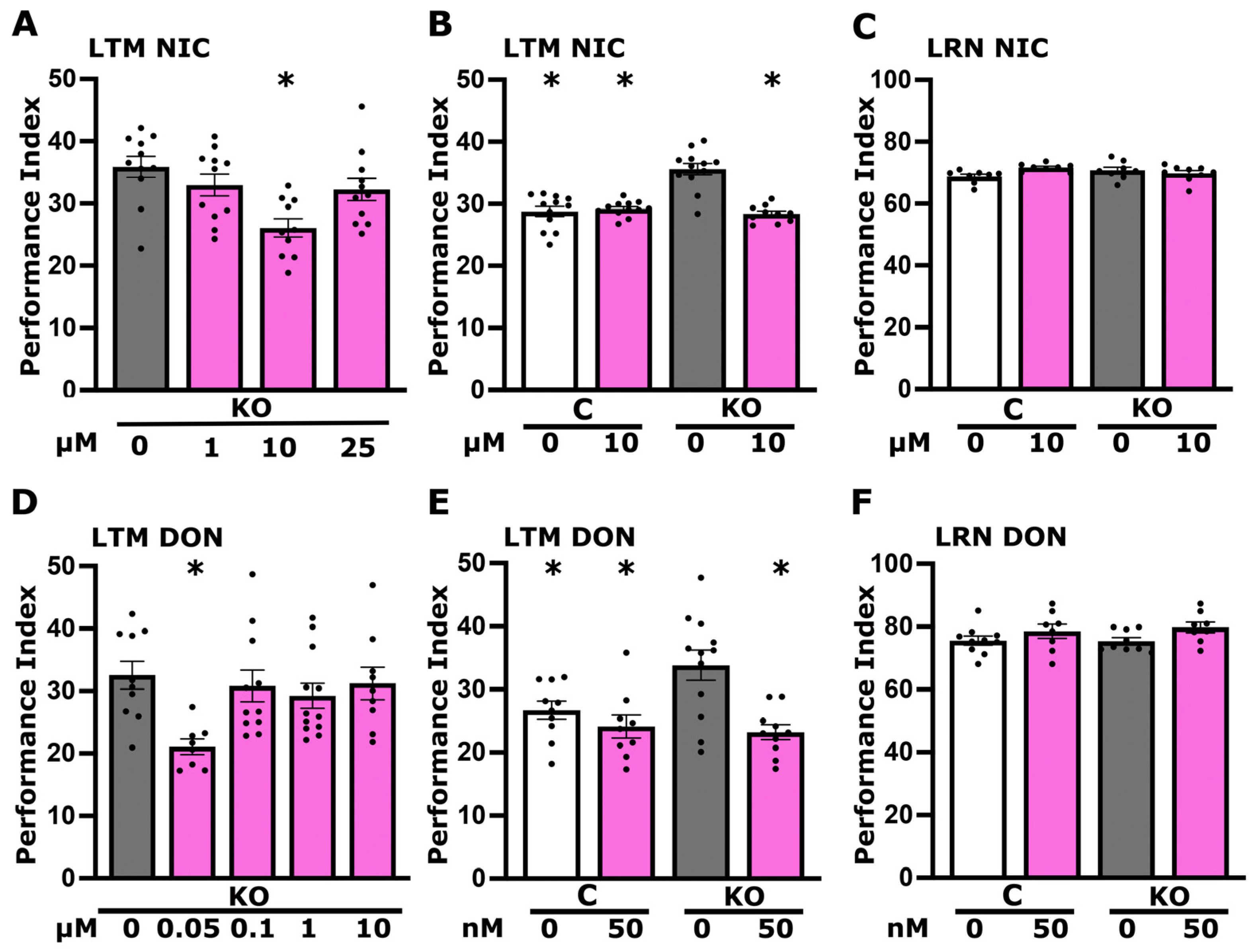
3.1. Increased Dietary Choline Reverses the Defective Habituation and Elevated Long-Term Memory of dTauKO Flies
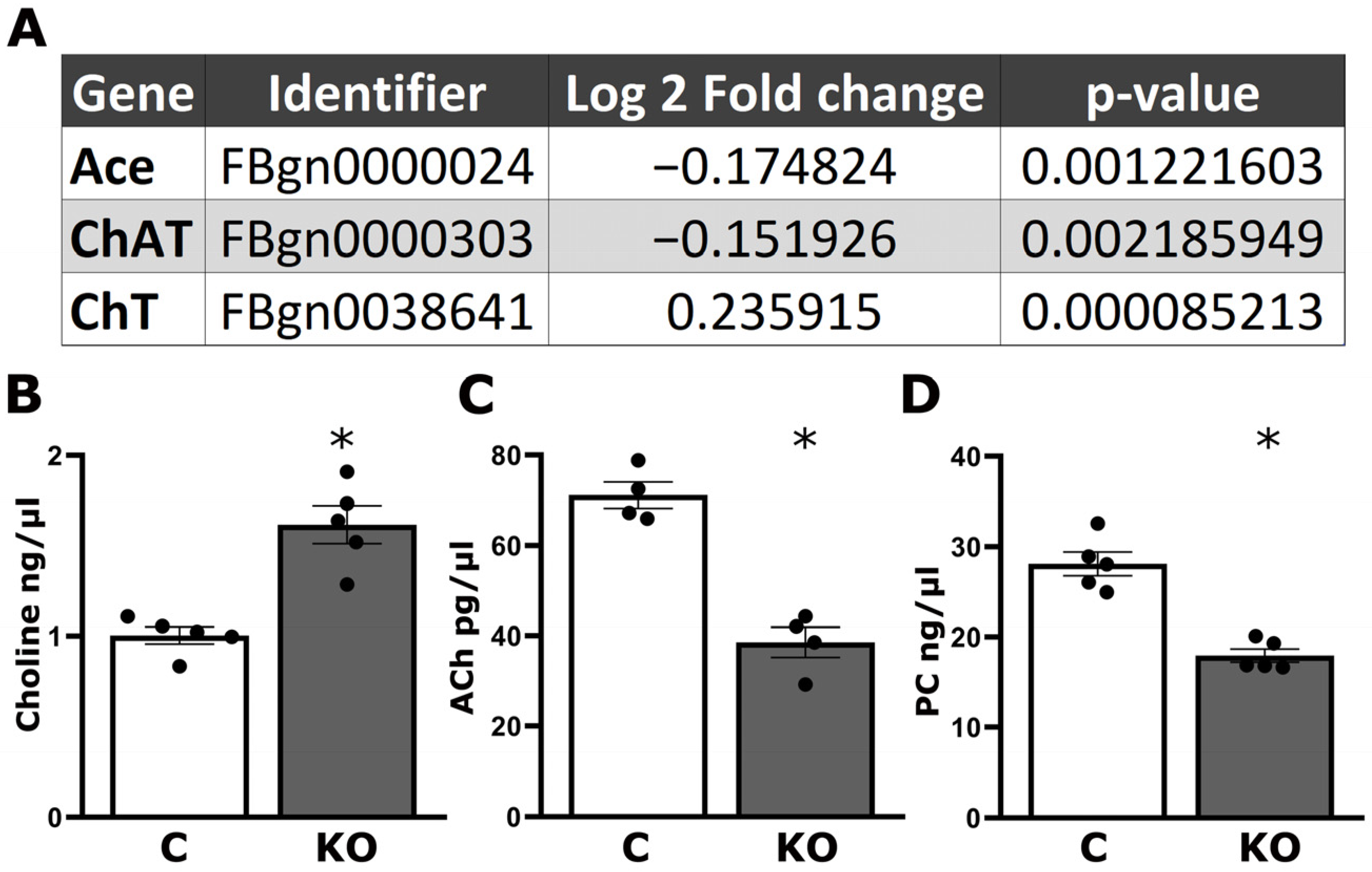
3.2. Altered Cholinergic Neurotransmission Underlies Defective Habituation of dTau Null Mutants
3.3. Betaine but Not CDP-Choline Plays a Role in Foot Shock Habituation Disrupted in dTau Null Mutants
3.4. Altered Cholinergic Signaling Underlies the Elevated Long-Term Memory of dTau KO Flies
3.5. CDP-Choline Levels but Not Betaine Play a Role in LTM Regulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cleveland, D.W.; Hwo, S.Y.; Kirschner, M.W. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J. Mol. Biol. 1977, 116, 207–225. [Google Scholar] [CrossRef] [PubMed]
- Creekmore, B.C.; Watanabe, R.; Lee, E.B. Neurodegenerative Disease Tauopathies. Annu. Rev. Pathol. 2024, 19, 345–370. [Google Scholar] [CrossRef]
- Montalto, G.; Ricciarelli, R. Tau, tau kinases, and tauopathies: An updated overview. Biofactors 2023, 49, 502–511. [Google Scholar] [CrossRef]
- Giong, H.K.; Subramanian, M.; Yu, K.; Lee, J.S. Non-Rodent Genetic Animal Models for Studying Tauopathy: Review of Drosophila, Zebrafish, and C. elegans Models. Int. J. Mol. Sci. 2021, 22, 8465. [Google Scholar] [CrossRef]
- Sanchez-Varo, R.; Mejias-Ortega, M.; Fernandez-Valenzuela, J.J.; Nunez-Diaz, C.; Caceres-Palomo, L.; Vegas-Gomez, L.; Sanchez-Mejias, E.; Trujillo-Estrada, L.; Garcia-Leon, J.A.; Moreno-Gonzalez, I.; et al. Transgenic Mouse Models of Alzheimer’s Disease: An Integrative Analysis. Int. J. Mol. Sci. 2022, 23, 5404. [Google Scholar] [CrossRef] [PubMed]
- Trojanowski, J.Q.; Lee, V.M. Pathological tau: A loss of normal function or a gain in toxicity? Nat. Neurosci. 2005, 8, 1136–1137. [Google Scholar] [CrossRef]
- Bouleau, S.; Tricoire, H. Drosophila models of Alzheimer’s disease: Advances, limits, and perspectives. J. Alzheimer’s Dis. 2015, 45, 1015–1038. [Google Scholar] [CrossRef]
- Heidary, G.; Fortini, M.E. Identification and characterization of the Drosophila tau homolog. Mech. Dev. 2001, 108, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Burnouf, S.; Gronke, S.; Augustin, H.; Dols, J.; Gorsky, M.K.; Werner, J.; Kerr, F.; Alic, N.; Martinez, P.; Partridge, L. Deletion of endogenous Tau proteins is not detrimental in Drosophila. Sci. Rep. 2016, 6, 23102. [Google Scholar] [CrossRef]
- Bolkan, B.J.; Kretzschmar, D. Loss of Tau results in defects in photoreceptor development and progressive neuronal degeneration in Drosophila. Dev. Neurobiol. 2014, 74, 1210–1225. [Google Scholar] [CrossRef]
- Papanikolopoulou, K.; Roussou, I.G.; Gouzi, J.Y.; Samiotaki, M.; Panayotou, G.; Turin, L.; Skoulakis, E.M.C. Drosophila Tau Negatively Regulates Translation and Olfactory Long-Term Memory, But Facilitates Footshock Habituation and Cytoskeletal Homeostasis. J. Neurosci. 2019, 39, 8315–8329. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.L. Learning and memory using Drosophila melanogaster: A focus on advances made in the fifth decade of research. Genetics 2023, 224, iyad085. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, S.F.; Froudarakis, E.I.; Kanellopoulos, A.; Skoulakis, E.M. Protection from premature habituation requires functional mushroom bodies in Drosophila. Learn. Mem. 2007, 14, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Foka, K.; Georganta, E.M.; Semelidou, O.; Skoulakis, E.M.C. Loss of the Schizophrenia-linked Furin protein from Drosophila mushroom body neurons results in antipsychotic-reversible habituation deficits. J. Neurosci. 2022, 42, 7496–7511. [Google Scholar] [CrossRef] [PubMed]
- Roussou, I.G.; Papanikolopoulou, K.; Savakis, C.; Skoulakis, E.M.C. Drosophila Bruton’s Tyrosine Kinase Regulates Habituation Latency and Facilitation in Distinct Mushroom Body Neurons. J. Neurosci. 2019, 39, 8730–8743. [Google Scholar] [CrossRef] [PubMed]
- Semelidou, O.; Acevedo, S.F.; Skoulakis, E.M. Temporally specific engagement of distinct neuronal circuits regulating olfactory habituation in Drosophila. Elife 2018, 7, e39569. [Google Scholar] [CrossRef] [PubMed]
- Ramaswami, M. Network plasticity in adaptive filtering and behavioral habituation. Neuron 2014, 82, 1216–1229. [Google Scholar] [CrossRef] [PubMed]
- Avery, S.N.; McHugo, M.; Armstrong, K.; Blackford, J.U.; Woodward, N.D.; Heckers, S. Disrupted Habituation in the Early Stage of Psychosis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- McDiarmid, T.A.; Bernardos, A.C.; Rankin, C.H. Habituation is altered in neuropsychiatric disorders-A comprehensive review with recommendations for experimental design and analysis. Neurosci. Biobehav. Rev. 2017, 80, 286–305. [Google Scholar] [CrossRef]
- Shih, M.M.; Davis, F.P.; Henry, G.L.; Dubnau, J. Nuclear Transcriptomes of the Seven Neuronal Cell Types That Constitute the Drosophila Mushroom Bodies. G3 2019, 9, 81–94. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Maioli, F.; Martelli, M.; Servadei, L.; Brunetti, N.; Porcellini, E.; Licastro, F. Homocysteine and folate as risk factors for dementia and Alzheimer disease. Am. J. Clin. Nutr. 2005, 82, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.; Liu, Z.; Cervantes-Sandoval, I.; Davis, R.L. Drosophila SLC22A Transporter Is a Memory Suppressor Gene that Influences Cholinergic Neurotransmission to the Mushroom Bodies. Neuron 2016, 90, 581–595. [Google Scholar] [CrossRef] [PubMed]
- Vourkou, E.; Paspaliaris, V.; Bourouliti, A.; Zerva, M.C.; Prifti, E.; Papanikolopoulou, K.; Skoulakis, E.M.C. Differential Effects of Human Tau Isoforms to Neuronal Dysfunction and Toxicity in the Drosophila CNS. Int. J. Mol. Sci. 2022, 23, 12985. [Google Scholar] [CrossRef]
- Rosenthal, J.S.; Yuan, Q. Constructing and Tuning Excitatory Cholinergic Synapses: The Multifaceted Functions of Nicotinic Acetylcholine Receptors in Drosophila Neural Development and Physiology. Front. Cell. Neurosci. 2021, 15, 720560. [Google Scholar] [CrossRef] [PubMed]
- Schildein, S.; Huston, J.P.; Schwarting, R.K. Open field habituation learning is improved by nicotine and attenuated by mecamylamine administered posttrial into the nucleus accumbens. Neurobiol. Learn. Mem. 2002, 77, 277–290. [Google Scholar] [CrossRef]
- Govind, A.P.; Vezina, P.; Green, W.N. Nicotine-induced upregulation of nicotinic receptors: Underlying mechanisms and relevance to nicotine addiction. Biochem. Pharmacol. 2009, 78, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.; Shaw, A.; Lambert, M.; Perry, H.H.; Lowenstein, E.; Valenzuela, D.; Velazquez-Ulloa, N.A. Developmental nicotine exposure affects larval brain size and the adult dopaminergic system of Drosophila melanogaster. BMC Dev. Biol. 2018, 18, 13. [Google Scholar] [CrossRef]
- Best, J.D.; Berghmans, S.; Hunt, J.J.; Clarke, S.C.; Fleming, A.; Goldsmith, P.; Roach, A.G. Non-associative learning in larval zebrafish. Neuropsychopharmacology 2008, 33, 1206–1215. [Google Scholar] [CrossRef]
- Ayikobua, E.T.; Semuyaba, I.; Eze, D.E.; Kalange, M.; Nansunga, M.; Okpanachi, A.O.; Safiriyu, A.A. Combined Donepezil and Ethanolic Extract of Propolis Improved Memory Better Than Donepezil and Propolis Monotherapy in Wild Type Drosophila melanogaster. Evid.-Based Complement. Altern. Med. 2018, 2018, 3717328. [Google Scholar] [CrossRef]
- Siddique, Y.H.; Naz, F.; Rahul; Varshney, H.; Idrisi, M.; Shahid, M. Effect of donepezil hydrochloride on the transgenic Drosophila expressing human Abeta-42. Int. J. Neurosci. 2023, 21, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Adibhatla, R.M.; Hatcher, J.F. Cytidine 5′-diphosphocholine (CDP-choline) in stroke and other CNS disorders. Neurochem. Res. 2005, 30, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Secades, J.J.; Frontera, G. CDP-choline: Pharmacological and clinical review. Methods Find. Exp. Clin. Pharmacol. 1995, 17 (Suppl. B), 1–54. [Google Scholar] [PubMed]
- Uslu, G.; Savci, V.; Buyukuysal, L.R.; Goktalay, G. CDP-choline attenuates scopolamine induced disruption of prepulse inhibition in rats: Involvement of central nicotinic mechanism. Neurosci. Lett. 2014, 569, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Ueland, P.M. Choline and betaine in health and disease. J. Inherit. Metab. Dis. 2011, 34, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R. The metabolic burden of methyl donor deficiency with focus on the betaine homocysteine methyltransferase pathway. Nutrients 2013, 5, 3481–3495. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Balan, S.; Toyoshima, M.; Maekawa, M.; Ohba, H.; Watanabe, A.; Iwayama, Y.; Fujita, Y.; Tan, Y.; Hisano, Y.; et al. Investigation of betaine as a novel psychotherapeutic for schizophrenia. EBioMedicine 2019, 45, 432–446. [Google Scholar] [CrossRef]
- Yoshihara, S.; Jiang, X.; Morikawa, M.; Ogawa, T.; Ichinose, S.; Yabe, H.; Kakita, A.; Toyoshima, M.; Kunii, Y.; Yoshikawa, T.; et al. Betaine ameliorates schizophrenic traits by functionally compensating for KIF3-based CRMP2 transport. Cell Rep. 2021, 35, 108971. [Google Scholar] [CrossRef] [PubMed]
- Noyes, N.C.; Davis, R.L. Genetics and Molecular Biology of Memory Suppression. Neuroscientist 2022, 1–13. [Google Scholar] [CrossRef]
- Roselli, C.; Ramaswami, M.; Boto, T.; Cervantes-Sandoval, I. The Making of Long-Lasting Memories: A Fruit Fly Perspective. Front. Behav. Neurosci. 2021, 15, 662129. [Google Scholar] [CrossRef]
- Crocker, A.; Guan, X.J.; Murphy, C.T.; Murthy, M. Cell-Type-Specific Transcriptome Analysis in the Drosophila Mushroom Body Reveals Memory-Related Changes in Gene Expression. Cell Rep. 2016, 15, 1580–1596. [Google Scholar] [CrossRef] [PubMed]
- Barnstedt, O.; Owald, D.; Felsenberg, J.; Brain, R.; Moszynski, J.P.; Talbot, C.B.; Perrat, P.N.; Waddell, S. Memory-Relevant Mushroom Body Output Synapses Are Cholinergic. Neuron 2016, 89, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Molina-Fernandez, C.; Ugalde, M.B.; Tognarelli, E.I.; Angel, C.; Campusano, J.M. Muscarinic ACh Receptors Contribute to Aversive Olfactory Learning in Drosophila. Neural Plast. 2015, 2015, 658918. [Google Scholar] [CrossRef] [PubMed]
- Chambers, R.P.; Call, G.B.; Meyer, D.; Smith, J.; Techau, J.A.; Pearman, K.; Buhlman, L.M. Nicotine increases lifespan and rescues olfactory and motor deficits in a Drosophila model of Parkinson’s disease. Behav. Brain Res. 2013, 253, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, M.; Wallis, T.P.; Saber, S.H.; Meunier, F.A. Phospholipases in neuronal function: A role in learning and memory? J. Neurochem. 2020, 153, 300–333. [Google Scholar] [CrossRef] [PubMed]
- Minegishi, T.; Kastian, R.F.; Inagaki, N. Mechanical regulation of synapse formation and plasticity. Semin. Cell Dev. Biol. 2023, 140, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Schverer, M.; O’Mahony, S.M.; O’Riordan, K.J.; Donoso, F.; Roy, B.L.; Stanton, C.; Dinan, T.G.; Schellekens, H.; Cryan, J.F. Dietary phospholipids: Role in cognitive processes across the lifespan. Neurosci. Biobehav. Rev. 2020, 111, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Rema, V.; Bali, K.K.; Ramachandra, R.; Chugh, M.; Darokhan, Z.; Chaudhary, R. Cytidine-5-diphosphocholine supplement in early life induces stable increase in dendritic complexity of neurons in the somatosensory cortex of adult rats. Neuroscience 2008, 155, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Teather, L.A.; Wurtman, R.J. Dietary CDP-choline supplementation prevents memory impairment caused by impoverished environmental conditions in rats. Learn. Mem. 2005, 12, 39–43. [Google Scholar] [CrossRef]
- Parkhitko, A.A.; Wang, L.; Filine, E.; Jouandin, P.; Leshchiner, D.; Binari, R.; Asara, J.M.; Rabinowitz, J.D.; Perrimon, N. A genetic model of methionine restriction extends Drosophila health- and lifespan. Proc. Natl. Acad. Sci. USA 2021, 118, e2110387118. [Google Scholar] [CrossRef]
- Ostrakhovitch, E.A.; Tabibzadeh, S. Homocysteine and age-associated disorders. Ageing Res. Rev. 2019, 49, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Bussière, M.; Vance, J.E.; Campenot, R.B.; Vance, D.E. Compartmentalization of choline and acetylcholine metabolism in cultured sympathetic neurons. J. Biochem. 2001, 130, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Fonnum, F.; Malthe-Sorenssen, D. Molecular properties of choline acetyltransferase and their importance for the compartmentation of acetylcholine synthesis. Prog. Brain Res. 1972, 36, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, D.A.; Carlson, L.; Park, J.L.; Ross, C.D. Enzymes of acetylcholine metabolism in the rat inferior colliculus. Brain Res. 2021, 1766, 147518. [Google Scholar] [CrossRef]
- McGuire, S.E.; Le, P.T.; Davis, R.L. The role of Drosophila mushroom body signaling in olfactory memory. Science 2001, 293, 1330–1333. [Google Scholar] [CrossRef]
- Hamid, R.; Sant, H.S.; Kulkarni, M.N. Choline Transporter regulates olfactory habituation via a neuronal triad of excitatory, inhibitory and mushroom body neurons. PLoS Genet. 2021, 17, e1009938. [Google Scholar] [CrossRef]
- Chai, G.S.; Jiang, X.; Ni, Z.F.; Ma, Z.W.; Xie, A.J.; Cheng, X.S.; Wang, Q.; Wang, J.Z.; Liu, G.P. Betaine attenuates Alzheimer-like pathological changes and memory deficits induced by homocysteine. J. Neurochem. 2013, 124, 388–396. [Google Scholar] [CrossRef]
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and tauopathies. Brain Res. Bull. 2016, 126 Pt 3, 238–292. [Google Scholar]
- Mesulam, M.M. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J. Comp. Neurol. 2013, 521, 4124–4144. [Google Scholar] [CrossRef]
- Giacobini, E.; Becker, R.E. One hundred years after the discovery of Alzheimer’s disease. A turning point for therapy? J. Alzheimer’s Dis. 2007, 12, 37–52. [Google Scholar] [CrossRef]
- Ikonomovic, M.D.; Abrahamson, E.E.; Isanski, B.A.; Wuu, J.; Mufson, E.J.; DeKosky, S.T. Superior frontal cortex cholinergic axon density in mild cognitive impairment and early Alzheimer disease. Arch. Neurol. 2007, 64, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharmacal Res. 2013, 36, 375–399. [Google Scholar] [CrossRef] [PubMed]
- Heilman, K.M.; Nadeau, S.E. Emotional and Neuropsychiatric Disorders Associated with Alzheimer’s Disease. Neurotherapeutics 2022, 19, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Creese, B.; Aarsland, D.; Kales, H.C.; Lyketsos, C.G.; Sweet, R.A.; Ballard, C. Psychosis in Alzheimer disease—Mechanisms, genetics and therapeutic opportunities. Nat. Rev. Neurol. 2022, 18, 131–144. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerva, M.-C.; Triantafylloudis, C.; Paspaliaris, V.; Skoulakis, E.M.C.; Papanikolopoulou, K. Choline Metabolites Reverse Differentially the Habituation Deficit and Elevated Memory of Tau Null Drosophila. Cells 2024, 13, 746. https://doi.org/10.3390/cells13090746
Zerva M-C, Triantafylloudis C, Paspaliaris V, Skoulakis EMC, Papanikolopoulou K. Choline Metabolites Reverse Differentially the Habituation Deficit and Elevated Memory of Tau Null Drosophila. Cells. 2024; 13(9):746. https://doi.org/10.3390/cells13090746
Chicago/Turabian StyleZerva, Maria-Christina, Christos Triantafylloudis, Vassilis Paspaliaris, Efthimios M. C. Skoulakis, and Katerina Papanikolopoulou. 2024. "Choline Metabolites Reverse Differentially the Habituation Deficit and Elevated Memory of Tau Null Drosophila" Cells 13, no. 9: 746. https://doi.org/10.3390/cells13090746




