Actin Cytoskeleton Remodeling Accompanied by Redistribution of Adhesion Proteins Drives Migration of Cells in Different EMT States
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines, Constructs, and Transfections
2.2. Antibodies and Reagents
2.3. Immunofluorescence
2.4. Live-Cell Imaging, Tracking, and Kymograph Analysis
2.5. Co-Immunoprecipitation and Western Blotting
3. Results
3.1. EGF-Induced EMT of IAR-20 Epithelial Cells
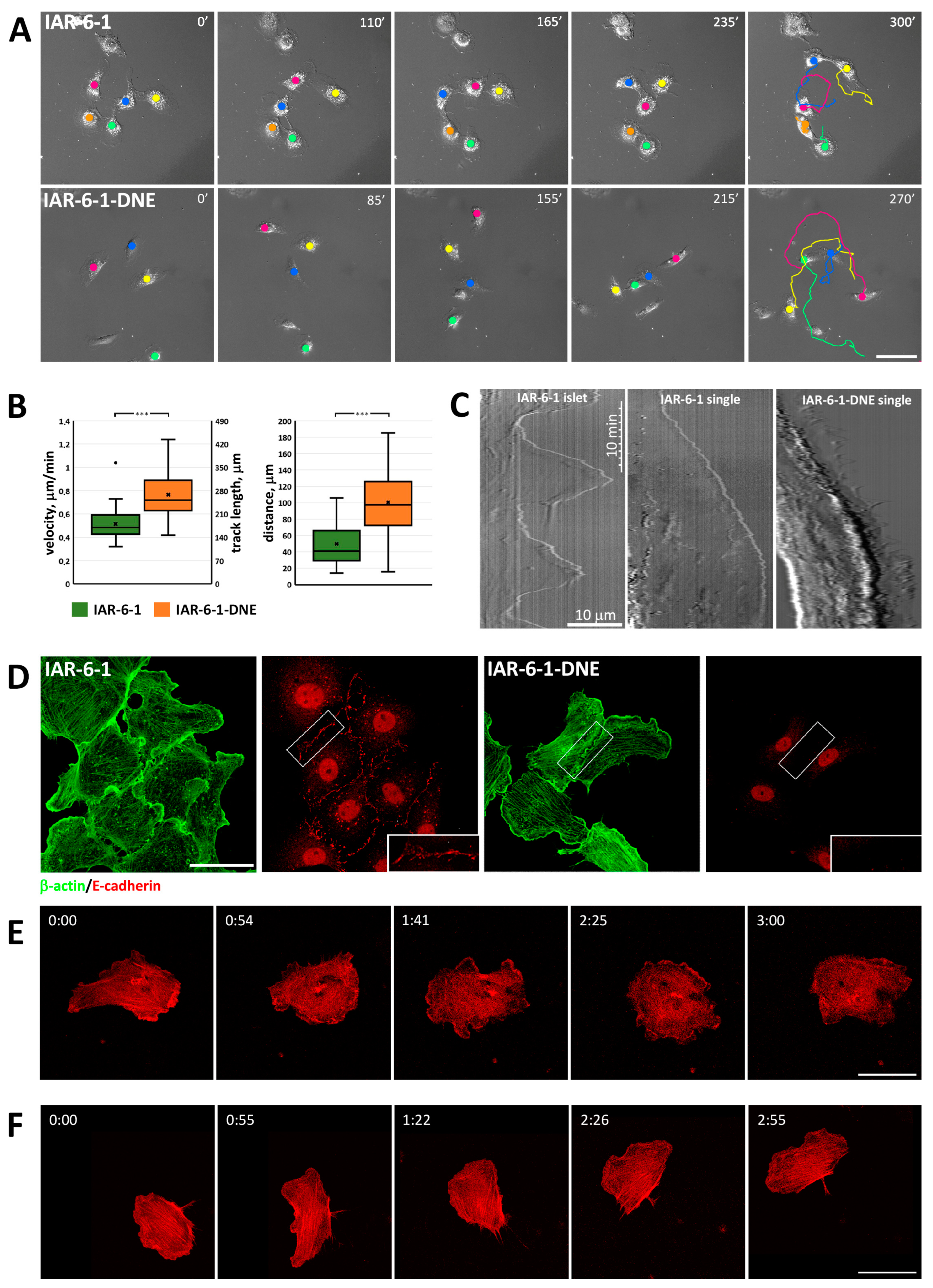
3.2. Migratory Activity of IAR-6-1 Cells Exhibiting Hybrid Epithelial–Mesenchymal Phenotype and Their Descendant IAR-6-1-DNE Cells
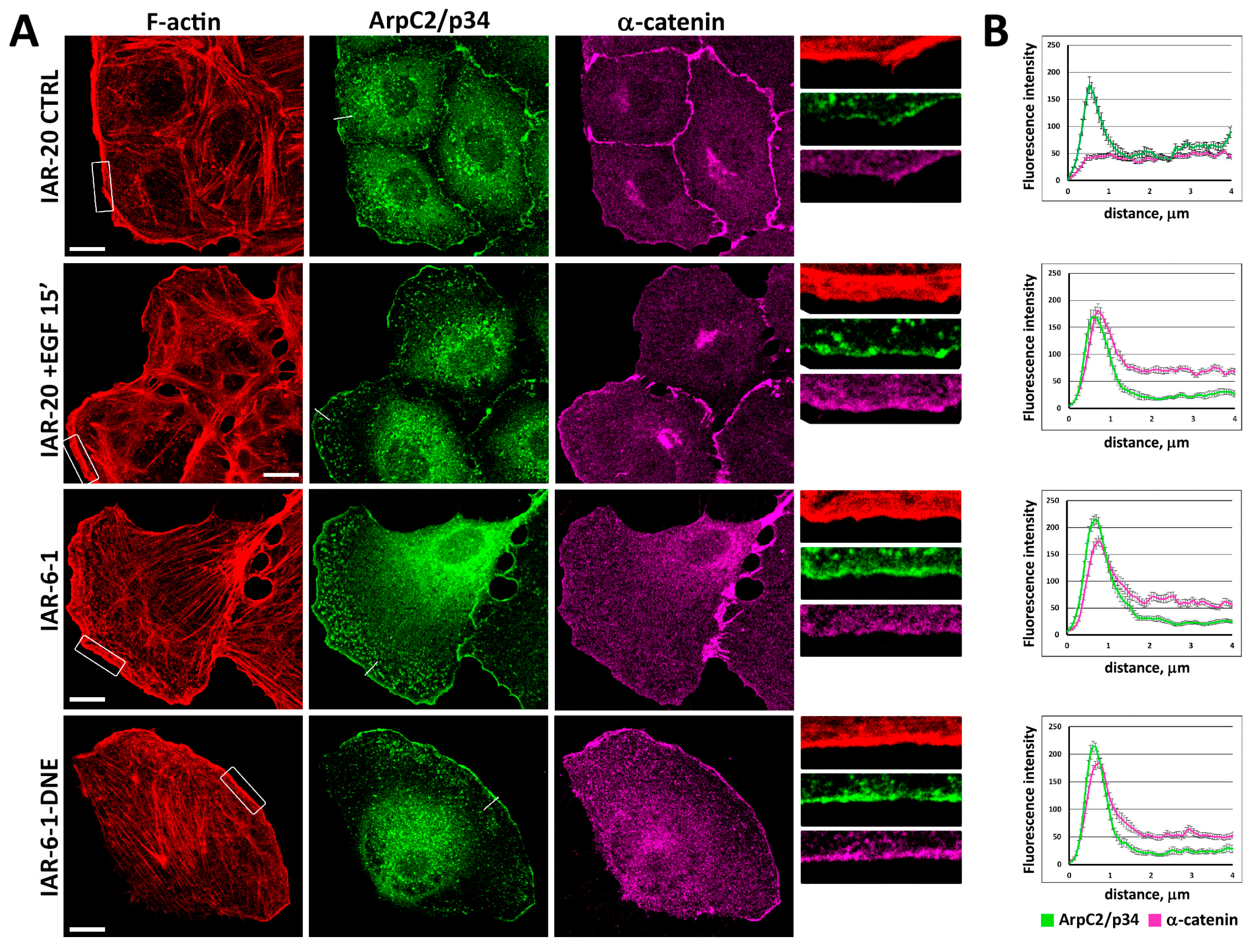
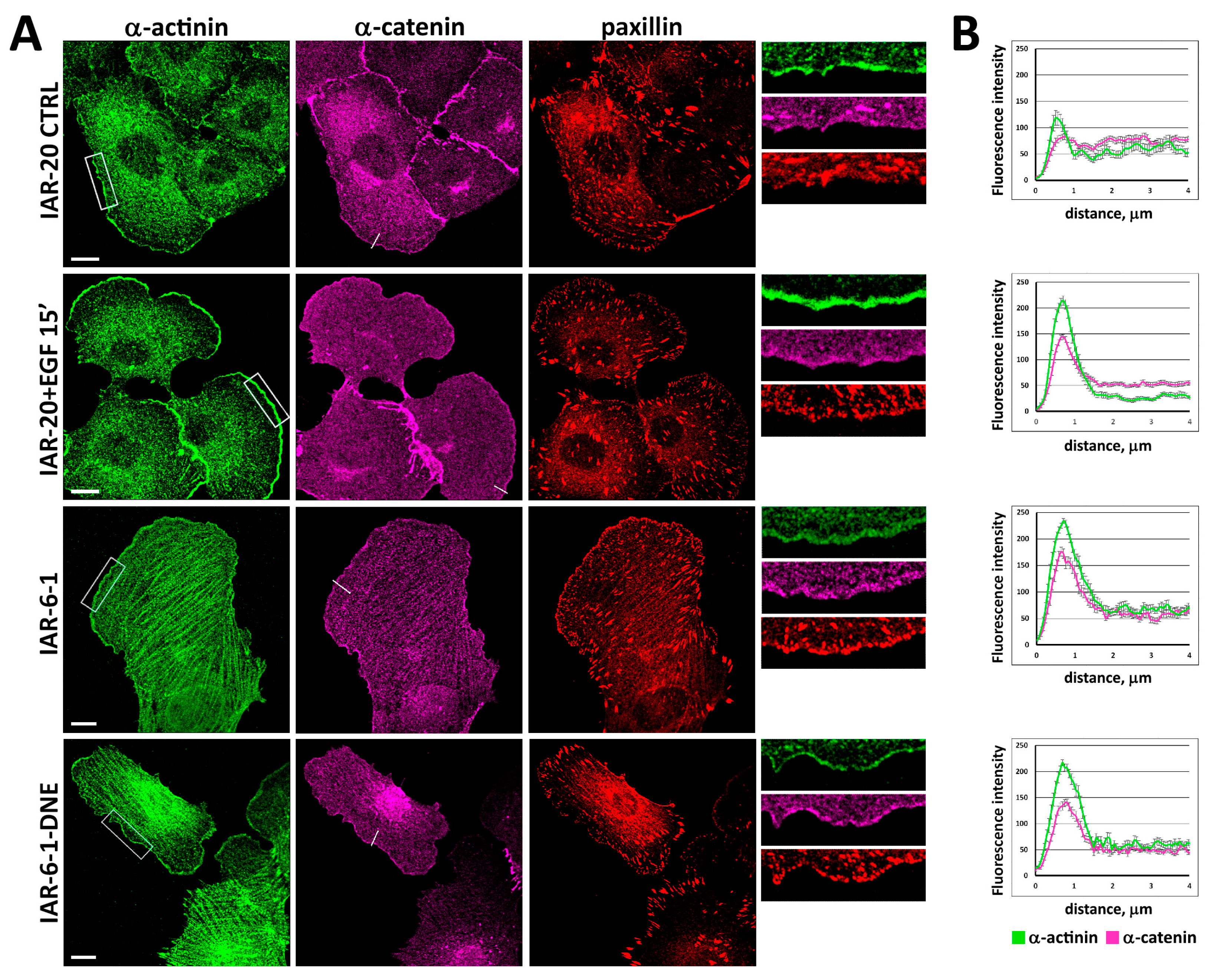
3.3. Redistribution of α-Catenin in Cells in Different EMT States
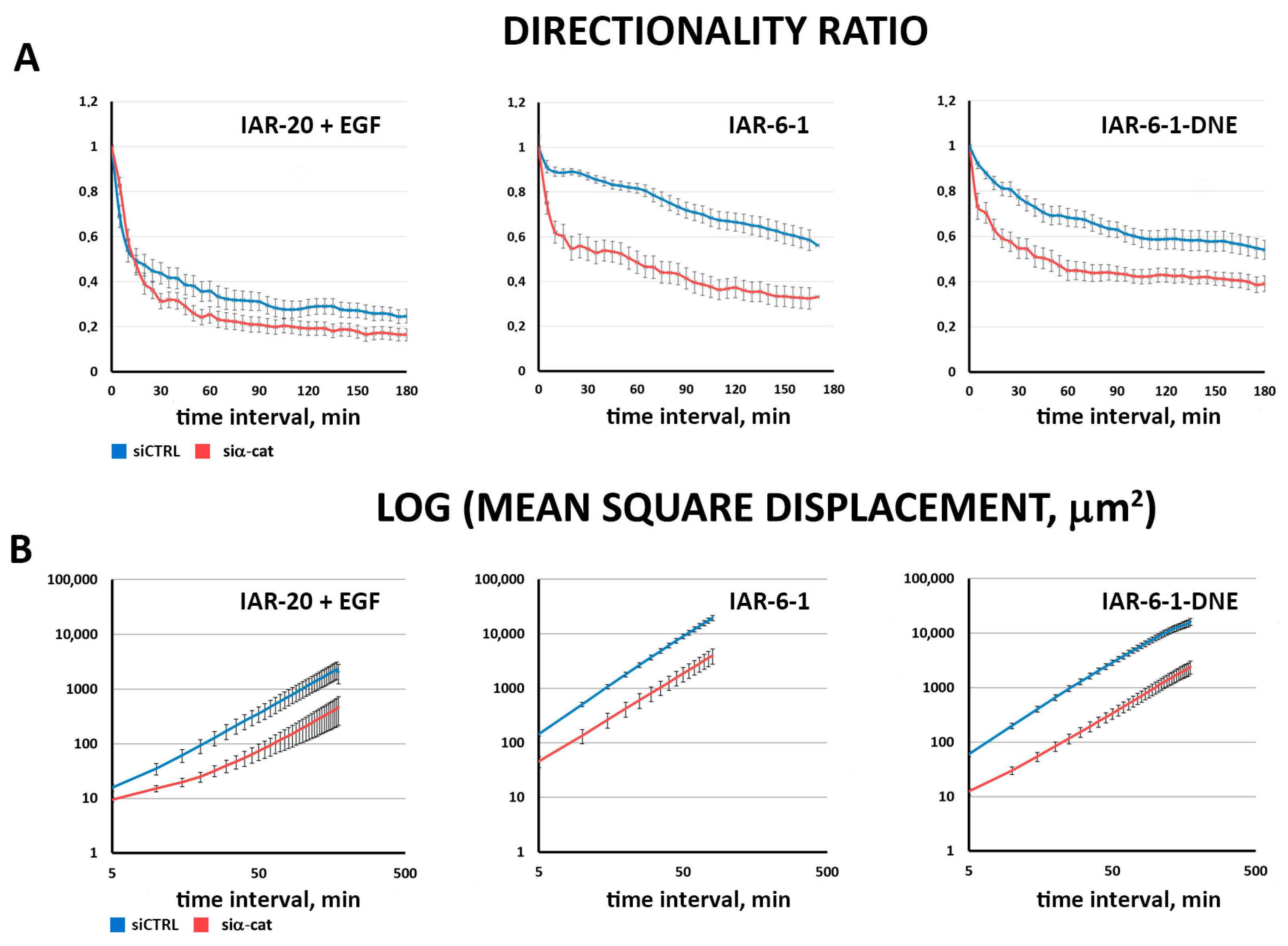
3.4. α-Catenin Is Involved in Cell Migration
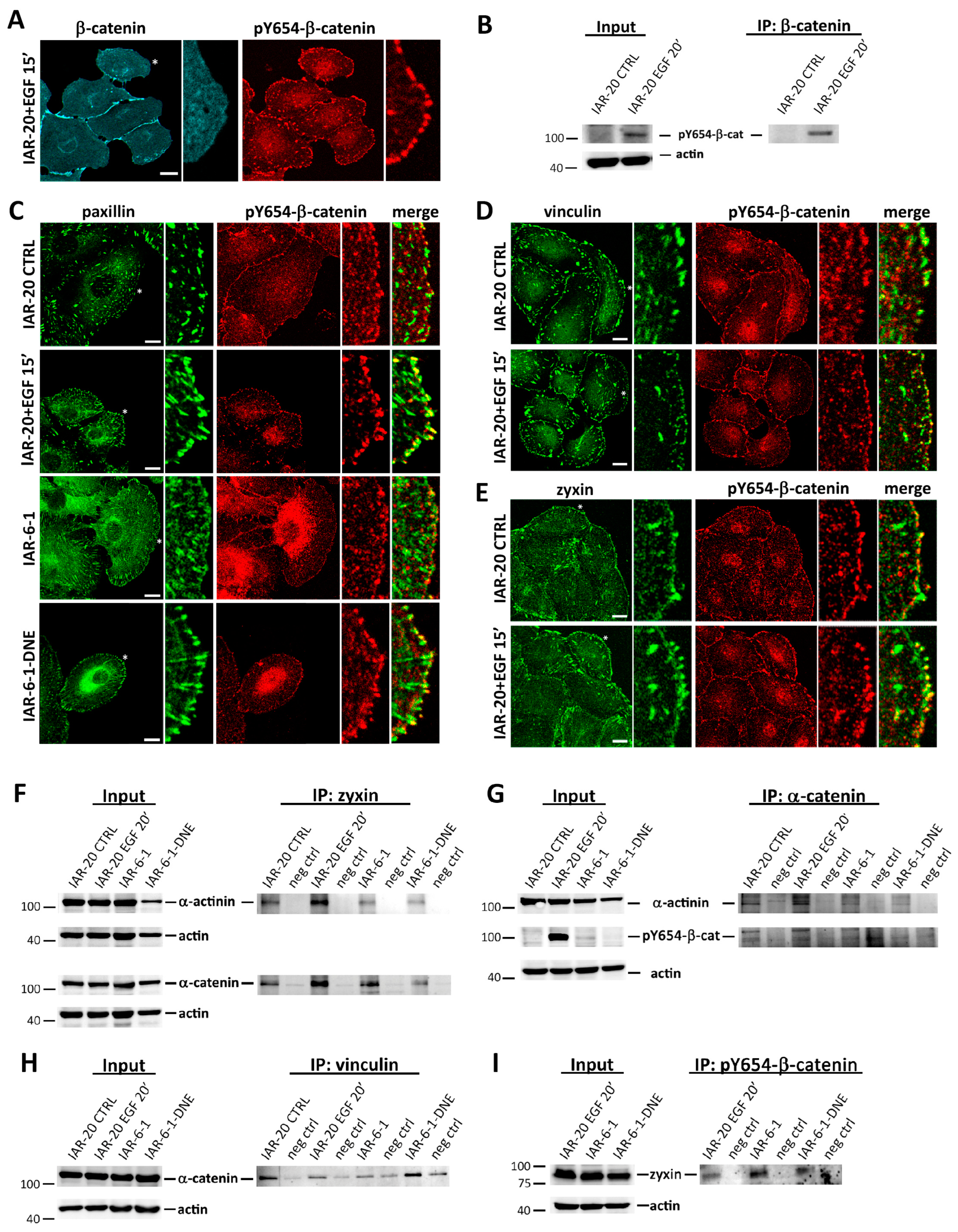
3.5. pY654-β-Catenin Enrichment at Integrin Adhesion Complexes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nieto, M.A.; Huang, R.Y.Y.J.; Jackson, R.A.A.; Thiery, J.P.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial–Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Derynck, R.; Weinberg, R.A. EMT and Cancer: More Than Meets the Eye. Dev. Cell 2019, 49, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic EMT: A Multi-tool for Tumor Progression. EMBO J. 2021, 40, e108647. [Google Scholar] [CrossRef] [PubMed]
- Pastushenko, I.; Blanpain, C. EMT Transition States during Tumor Progression and Metastasis. Trends Cell Biol. 2019, 29, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. Dynamic Contacts: Rearranging Adherens Junctions to Drive Epithelial Remodelling. Nat. Rev. Mol. Cell Biol. 2014, 15, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Braga, V. Spatial Integration of E-Cadherin Adhesion, Signalling and the Epithelial Cytoskeleton. Curr. Opin. Cell Biol. 2016, 42, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Mège, R.M.; Ishiyama, N. Integration of Cadherin Adhesion and Cytoskeleton at Adherens Junctions. Cold Spring Harb. Perspect. Biol. 2017, 9, a028738. [Google Scholar] [CrossRef] [PubMed]
- Berx, G.; van Roy, F. Involvement of Members of the Cadherin Superfamily in Cancer. Cold Spring Harb. Perspect. Biol. 2009, 1, a003129. [Google Scholar] [CrossRef]
- Zhitnyak, I.Y.; Rubtsova, S.N.; Litovka, N.I.; Gloushankova, N.A. Early Events in Actin Cytoskeleton Dynamics and E-Cadherin-Mediated Cell-Cell Adhesion during Epithelial-Mesenchymal Transition. Cells 2020, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Rimm, D.L.; Koslov, E.R.; Kebriaei, P.; Cianci, C.D.; Morrow, J.S. Alpha 1(E)-Catenin Is an Actin-Binding and -Bundling Protein Mediating the Attachment of F-Actin to the Membrane Adhesion Complex. Proc. Natl. Acad. Sci. USA 1995, 92, 8813–8817. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ringwald, M.; Kemler, R. Uvomorulin-Catenin Complex Formation Is Regulated by a Specific Domain in the Cytoplasmic Region of the Cell Adhesion Molecule. Proc. Natl. Acad. Sci. USA 1990, 87, 4246–4250. [Google Scholar] [CrossRef] [PubMed]
- Huber, O.; Krohn, M.; Kemler, R. A Specific Domain in Alpha-Catenin Mediates Binding to Beta-Catenin or Plakoglobin. J. Cell Sci. 1997, 110, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Pokutta, S.; Choi, H.-J.; Ahlsen, G.; Hansen, S.D.; Weis, W.I. Structural and Thermodynamic Characterization of Cadherin·β-Catenin·α-Catenin Complex Formation. J. Biol. Chem. 2014, 289, 13589–13601. [Google Scholar] [CrossRef] [PubMed]
- Nieset, J.E.; Redfield, A.R.; Jin, F.; Knudsen, K.A.; Johnson, K.R.; Wheelock, M.J. Characterization of the Interactions of α-Catenin with α-Actinin and β-Catenin/Plakoglobin. J. Cell Sci. 1997, 110, 1013–1022. [Google Scholar] [CrossRef]
- Yonemura, S.; Wada, Y.; Watanabe, T.; Nagafuchi, A.; Shibata, M. Alpha-Catenin as a Tension Transducer That Induces Adherens Junction Development. Nat. Cell Biol. 2010, 12, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-J.; Pokutta, S.; Cadwell, G.W.; Bobkov, A.A.; Bankston, L.A.; Liddington, R.C.; Weis, W.I. AE-Catenin Is an Autoinhibited Molecule That Coactivates Vinculin. Proc. Natl. Acad. Sci. USA 2012, 109, 8576–8581. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, N.; Tanaka, N.; Abe, K.; Yang, Y.J.; Abbas, Y.M.; Umitsu, M.; Nagar, B.; Bueler, S.A.; Rubinstein, J.L.; Takeichi, M.; et al. An Autoinhibited Structure of α-Catenin and Its Implications for Vinculin Recruitment to Adherens Junctions. J. Biol. Chem. 2013, 288, 15913–15925. [Google Scholar] [CrossRef] [PubMed]
- Pokutta, S.; Drees, F.; Takai, Y.; Nelson, W.J.; Weis, W.I. Biochemical and Structural Definition of the L-Afadin- and Actin-Binding Sites of Alpha-Catenin. J. Biol. Chem. 2002, 277, 18868–18874. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Tan, J.; Anderson, K.L.; Hanein, D.; Volkmann, N.; Weis, W.I.; Nelson, W.J.; Dunn, A.R. Cell Adhesion. The Minimal Cadherin-Catenin Complex Binds to Actin Filaments under Force. Science 2014, 346, 1254211. [Google Scholar] [CrossRef] [PubMed]
- Drees, F.; Pokutta, S.; Yamada, S.; Nelson, W.J.; Weis, W.I. Alpha-Catenin Is a Molecular Switch That Binds E-Cadherin-Beta-Catenin and Regulates Actin-Filament Assembly. Cell 2005, 123, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Vasioukhin, V.; Bauer, C.; Degenstein, L.; Wise, B.; Fuchs, E. Hyperproliferation and Defects in Epithelial Polarity upon Conditional Ablation of Alpha-Catenin in Skin. Cell 2001, 104, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.M.; Kwiatkowski, A.V.; Yang, C.; Korobova, F.; Pokutta, S.; Svitkina, T.; Weis, W.I.; Nelson, W.J. AlphaE-Catenin Regulates Actin Dynamics Independently of Cadherin-Mediated Cell-Cell Adhesion. J. Cell Biol. 2010, 189, 339–352. [Google Scholar] [CrossRef]
- Wood, M.N.; Ishiyama, N.; Singaram, I.; Chung, C.M.; Flozak, A.S.; Yemelyanov, A.; Ikura, M.; Cho, W.; Gottardi, C.J. α-Catenin Homodimers Are Recruited to Phosphoinositide-Activated Membranes to Promote Adhesion. J. Cell Biol. 2017, 216, 3767–3783. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, V.; Platek, A.; Hiver, S.; Enomoto, H.; Takeichi, M. Catenins Steer Cell Migration via Stabilization of Front-Rear Polarity. Dev. Cell 2017, 43, 463–479.e5. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Melamed, S.; Damouny-Khoury, H.; Amer, M.; Feld, L.; Nadjar-Boger, E.; Sheetz, M.P.; Wolfenson, H. α-Catenin Links Integrin Adhesions to F-Actin to Regulate ECM Mechanosensing and Rigidity Dependence. J. Cell Biol. 2022, 221, e202102121. [Google Scholar] [CrossRef]
- Kanchanawong, P.; Calderwood, D.A. Organization, Dynamics and Mechanoregulation of Integrin-Mediated Cell–ECM Adhesions. Nat. Rev. Mol. Cell Biol. 2023, 24, 142–161. [Google Scholar] [CrossRef] [PubMed]
- Roca-Cusachs, P.; del Rio, A.; Puklin-Faucher, E.; Gauthier, N.C.; Biais, N.; Sheetz, M.P. Integrin-Dependent Force Transmission to the Extracellular Matrix by α-Actinin Triggers Adhesion Maturation. Proc. Natl. Acad. Sci. USA 2013, 110, E1361–E1370. [Google Scholar] [CrossRef] [PubMed]
- Crawford, A.W.; Michelsen, J.W.; Beckerle, M.C. An Interaction between Zyxin and Alpha-Actinin. J. Cell Biol. 1992, 116, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.M.; Rademacher, J.; Bagshaw, R.D.; Wortmann, C.; Barth, C.; van Unen, J.; Alp, K.M.; Giudice, G.; Eccles, R.L.; Heinrich, L.E.; et al. Systems Analysis of RhoGEF and RhoGAP Regulatory Proteins Reveals Spatially Organized RAC1 Signalling from Integrin Adhesions. Nat. Cell Biol. 2020, 22, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Montesano, R.; Saint Vincent, L.; Drevon, C.; Tomatis, L. Production of Epithelial and Mesenchymal Tumours with Rat Liver Cells Transformed in Vitro. Int. J. cancer 1975, 16, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.W.; Schell, M.J. Neuronal IP 3 3-Kinase Is an F-Actin–Bundling Protein: Role in Dendritic Targeting and Regulation of Spine Morphology. Mol. Biol. Cell 2009, 20, 5166–5180. [Google Scholar] [CrossRef]
- Gorelik, R.; Gautreau, A. Quantitative and Unbiased Analysis of Directional Persistence in Cell Migration. Nat. Protoc. 2014, 9, 1931–1943. [Google Scholar] [CrossRef] [PubMed]
- Ayollo, D.V.; Zhitnyak, I.Y.; Vasiliev, J.M.; Gloushankova, N.A. Rearrangements of the Actin Cytoskeleton and E-Cadherin–Based Adherens Junctions Caused by Neoplasic Transformation Change Cell–Cell Interactions. PLoS ONE 2009, 4, e8027. [Google Scholar] [CrossRef] [PubMed]
- Rubtsova, S.N.; Zhitnyak, I.Y.; Gloushankova, N.A. A Novel Role of E-Cadherin-Based Adherens Junctions in Neoplastic Cell Dissemination. PLoS ONE 2015, 10, e0133578. [Google Scholar] [CrossRef] [PubMed]
- Klingelhöfer, J.; Laur, O.Y.; Troyanovsky, R.B.; Troyanovsky, S.M. Dynamic Interplay between Adhesive and Lateral E-Cadherin Dimers. Mol. Cell. Biol. 2002, 22, 7449–7458. [Google Scholar] [CrossRef] [PubMed]
- Troyanovsky, S.M. Adherens Junction: The Ensemble of Specialized Cadherin Clusters. Trends Cell Biol. 2023, 33, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Hazan, R.B.; Kang, L.; Roe, S.; Borgen, P.I.; Rimm, D.L. Vinculin Is Associated with the E-Cadherin Adhesion Complex. J. Biol. Chem. 1997, 272, 32448–32453. [Google Scholar] [CrossRef] [PubMed]
- Roura, S.; Miravet, S.; Piedra, J.; García de Herreros, A.; Duñach, M. Regulation of E-Cadherin/Catenin Association by Tyrosine Phosphorylation. J. Biol. Chem. 1999, 274, 36734–36740. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, J.; Kerstens, A.; Danuser, G.; Schwartz, M.A.; Waterman-Storer, C.M. Integrin-Dependent Actomyosin Contraction Regulates Epithelial Cell Scattering. J. Cell Biol. 2005, 171, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, P.J.; Ebner, R.; Lopez, A.R.; Derynck, R. TGF-Beta Induced Transdifferentiation of Mammary Epithelial Cells to Mesenchymal Cells: Involvement of Type I Receptors. J. Cell Biol. 1994, 127, 2021–2036. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.; Srivastava, J.; Madson, N.; Wittmann, T.; Barber, D.L. Dynamic Actin Remodeling during Epithelial–Mesenchymal Transition Depends on Increased Moesin Expression. Mol. Biol. Cell 2011, 22, 4750–4764. [Google Scholar] [CrossRef] [PubMed]
- Aiello, N.M.; Maddipati, R.; Norgard, R.J.; Balli, D.; Li, J.; Yuan, S.; Yamazoe, T.; Black, T.; Sahmoud, A.; Furth, E.E.; et al. EMT Subtype Influences Epithelial Plasticity and Mode of Cell Migration. Dev. Cell 2018, 45, 681–695.e4. [Google Scholar] [CrossRef] [PubMed]
- Hoschuetzky, H.; Aberle, H.; Kemler, R. Beta-Catenin Mediates the Interaction of the Cadherin-Catenin Complex with Epidermal Growth Factor Receptor. J. Cell Biol. 1994, 127, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Shibamoto, S.; Hayakawa, M.; Takeuchi, K.; Hori, T.; Oku, N.; Miyazawa, K.; Kitanfura, N.; Takeichi, M.; Ito, F. Tyrosine Phosphorylation of β-Catenin and Plakoglobin Enhanced by Hepatocyte Growth Factor and Epidermal Growth Factor in Human Carcinoma Cells. Cell Adhes. Commun. 1994, 1, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.H.; Weis, W.I. The Structure of the Beta-Catenin/E-Cadherin Complex and the Molecular Basis of Diverse Ligand Recognition by Beta-Catenin. Cell 2001, 105, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Valenta, T.; Hausmann, G.; Basler, K. The Many Faces and Functions of β-Catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef] [PubMed]
- Coluccia, A.M.L.; Vacca, A.; Duñach, M.; Mologni, L.; Redaelli, S.; Bustos, V.H.; Benati, D.; Pinna, L.A.; Gambacorti-Passerini, C. Bcr-Abl Stabilizes β-Catenin in Chronic Myeloid Leukemia through Its Tyrosine Phosphorylation. EMBO J. 2007, 26, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- van Veelen, W.; Le, N.H.; Helvensteijn, W.; Blonden, L.; Theeuwes, M.; Bakker, E.R.M.; Franken, P.F.; van Gurp, L.; Meijlink, F.; van der Valk, M.A.; et al. β-Catenin Tyrosine 654 Phosphorylation Increases Wnt Signalling and Intestinal Tumorigenesis. Gut 2011, 60, 1204–1212. [Google Scholar] [CrossRef]
- Taurin, S.; Sandbo, N.; Qin, Y.; Browning, D.; Dulin, N.O. Phosphorylation of β-Catenin by Cyclic AMP-Dependent Protein Kinase. J. Biol. Chem. 2006, 281, 9971–9976. [Google Scholar] [CrossRef] [PubMed]
- Hinck, L.; Näthke, I.S.; Papkoff, J.; Nelson, W.J. Dynamics of Cadherin/Catenin Complex Formation: Novel Protein Interactions and Pathways of Complex Assembly. J. Cell Biol. 1994, 125, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Sieg, D.J.; Hauck, C.R.; Ilic, D.; Klingbeil, C.K.; Schaefer, E.; Damsky, C.H.; Schlaepfer, D.D. FAK Integrates Growth-Factor and Integrin Signals to Promote Cell Migration. Nat. Cell Biol. 2000, 2, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Umesh, V.; Rape, A.D.; Ulrich, T.A.; Kumar, S. Microenvironmental Stiffness Enhances Glioma Cell Proliferation by Stimulating Epidermal Growth Factor Receptor Signaling. PLoS ONE 2014, 9, e101771. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Oh, D.; Dubey, A.K.; Yao, M.; Yang, B.; Groves, J.T.; Sheetz, M. EGFR Family and Src Family Kinase Interactions: Mechanics Matters? Curr. Opin. Cell Biol. 2018, 51, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Plopper, G.E.; McNamee, H.P.; Dike, L.E.; Bojanowski, K.; Ingber, D.E. Convergence of Integrin and Growth Factor Receptor Signaling Pathways within the Focal Adhesion Complex. Mol. Biol. Cell 1995, 6, 1349–1365. [Google Scholar] [CrossRef] [PubMed]
- Moro, L. Integrins Induce Activation of EGF Receptor: Role in MAP Kinase Induction and Adhesion-Dependent Cell Survival. EMBO J. 1998, 17, 6622–6632. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.; Jacquemet, G.; Byron, A.; Jones, M.C.; Warwood, S.; Selley, J.N.; Knight, D.; Humphries, J.D.; Humphries, M.J. Defining the Phospho-Adhesome through the Phosphoproteomic Analysis of Integrin Signalling. Nat. Commun. 2015, 6, 6265. [Google Scholar] [CrossRef] [PubMed]
- Seetharaman, S.; Etienne-Manneville, S. Cytoskeletal Crosstalk in Cell Migration. Trends Cell Biol. 2020, 30, 720–735. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Svitkina, T.M. Adenomatous Polyposis Coli (APC) in Cell Migration. Eur. J. Cell Biol. 2022, 101, 151228. [Google Scholar] [CrossRef] [PubMed]
- Juanes, M.A.; Bouguenina, H.; Eskin, J.A.; Jaiswal, R.; Badache, A.; Goode, B.L. Adenomatous Polyposis Coli Nucleates Actin Assembly to Drive Cell Migration and Microtubule-Induced Focal Adhesion Turnover. J. Cell Biol. 2017, 216, 2859–2875. [Google Scholar] [CrossRef] [PubMed]
- Juanes, M.A.; Isnardon, D.; Badache, A.; Brasselet, S.; Mavrakis, M.; Goode, B.L. The Role of APC-Mediated Actin Assembly in Microtubule Capture and Focal Adhesion Turnover. J. Cell Biol. 2019, 218, 3415–3435. [Google Scholar] [CrossRef]
- Faux, M.C.; Coates, J.L.; Kershaw, N.J.; Layton, M.J.; Burgess, A.W. Independent Interactions of Phosphorylated β-Catenin with E-Cadherin at Cell-Cell Contacts and APC at Cell Protrusions. PLoS ONE 2010, 5, e14127. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Leung, L.; Brocardo, M.; Henderson, J.; Flegg, C.; Henderson, B.R. Membrane Localization of Adenomatous Polyposis Coli Protein at Cellular Protrusions. J. Biol. Chem. 2006, 281, 17140–17149. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilnitskaya, A.S.; Litovka, N.I.; Rubtsova, S.N.; Zhitnyak, I.Y.; Gloushankova, N.A. Actin Cytoskeleton Remodeling Accompanied by Redistribution of Adhesion Proteins Drives Migration of Cells in Different EMT States. Cells 2024, 13, 780. https://doi.org/10.3390/cells13090780
Ilnitskaya AS, Litovka NI, Rubtsova SN, Zhitnyak IY, Gloushankova NA. Actin Cytoskeleton Remodeling Accompanied by Redistribution of Adhesion Proteins Drives Migration of Cells in Different EMT States. Cells. 2024; 13(9):780. https://doi.org/10.3390/cells13090780
Chicago/Turabian StyleIlnitskaya, Alla S., Nikita I. Litovka, Svetlana N. Rubtsova, Irina Y. Zhitnyak, and Natalya A. Gloushankova. 2024. "Actin Cytoskeleton Remodeling Accompanied by Redistribution of Adhesion Proteins Drives Migration of Cells in Different EMT States" Cells 13, no. 9: 780. https://doi.org/10.3390/cells13090780








