Pericytes, Mesenchymal Stem Cells and the Wound Healing Process
Abstract
:1. Pericyte Morphology
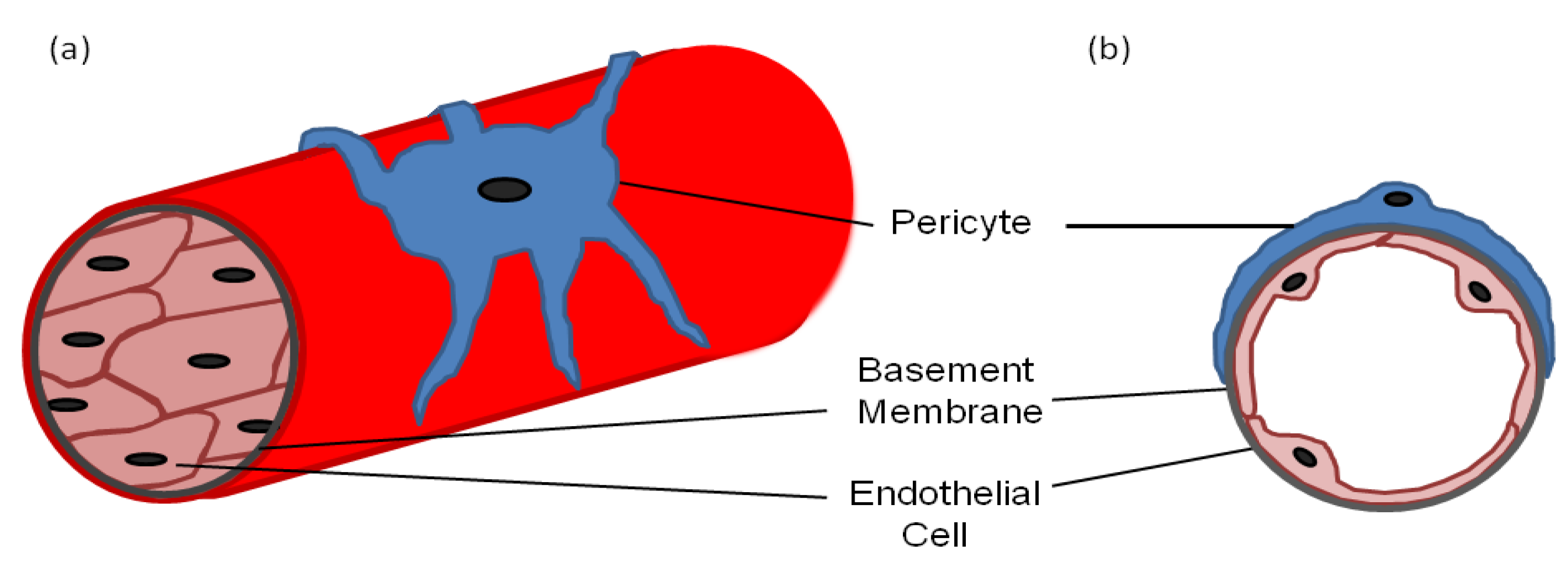
2. Markers for Isolating/Identifying Pericytes
3. Pericytes and the Formation of Blood Vessels
4. Pericytes and the Regulation and Maintenance of Blood Vessels
5. Mesenchymal Stem Cell Properties of Pericytes
6. Mesenchymal Stem Cells and Wound Healing
7. Pericytes and Wound Healing
8. Discussion
Conflicts of Interest
References
- Hirschi, K.K.; D’Amore, P.A. Pericytes in the microvasculature. Cardiovasc. Res. 1996, 32, 687–698. [Google Scholar]
- Da Silva Meirelles, L.; Caplan, A.I.; Nardi, N.B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 2008, 9, 2287–2299. [Google Scholar] [CrossRef]
- Hungerford, J.E.; Little, C.D. Developmental biology of the vascular smooth muscle cell: Building a multilayered vessel wall. J. Vasc. Res. 1999, 36, 2–27. [Google Scholar] [CrossRef]
- Etchevers, H.C.; Vincent, C.; le Douarin, N.M.; Couly, G.F. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 2001, 128, 1059–1068. [Google Scholar]
- DeRuiter, M.C.; Poelmann, R.E.; VanMunsteren, J.C.; Mironov, V.; Markwald, R.R.; Gittenberger-de Groot, A.C. Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ. Res. 1997, 80, 444–451. [Google Scholar] [CrossRef]
- Rajantie, I.; Ilmonen, M.; Alminaite, A.; Ozerdem, U.; Alitalo, K.; Salven, P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood 2004, 104, 2084–2086. [Google Scholar] [CrossRef]
- Mandarino, L.J.; Sundarraj, N.; Finlayson, J.; Hassell, H.R. Regulation of fibronectin and laminin synthesis by retinal capillary endothelial cells and pericytes in vitro. Exp. Eye Res. 1993, 57, 609–621. [Google Scholar] [CrossRef]
- Sims, D.E. Recent advances in pericyte biology-implications for health and disease. Can. J. Cardiol. 1991, 7, 431–443. [Google Scholar]
- Larson, D.M.; Carson, M.P.; Haudenschild, C.C. Junctional transfer of small molecules in cultured bovine brain microvascular endothelial cells and pericytes. Microvasc. Res. 1987, 34, 184–199. [Google Scholar] [CrossRef]
- Rucker, H.K.; Wynder, H.J.; Thomas, W.E. Cellular mechanisms of CNS pericytes. Brain Res. Bull. 2000, 51, 363–369. [Google Scholar] [CrossRef]
- Bergers, G.; Song, S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005, 7, 452–464. [Google Scholar] [CrossRef]
- Gerhardt, H.; Betsholtz, C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003, 314, 15–23. [Google Scholar] [CrossRef]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef]
- Zimmermann, K.W. Der Feinere Bau der Blutkapillaren. Z. Anat. Entwicklungsgesch. 1923, 68, 29–109. [Google Scholar] [CrossRef]
- Nehls, V.; Drenckhahn, D. Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J. Cell Biol. 1991, 113, 147–154. [Google Scholar] [CrossRef]
- Shepro, D.; Morel, N.M. Pericyte physiology. FASEB J. 1993, 7, 1031–1038. [Google Scholar]
- Sims, D.E. Diversity within pericytes. Clin. Exp. Pharmacol. Physiol. 2000, 27, 842–846. [Google Scholar] [CrossRef]
- Risau, W.; Engelhardt, B.; Wekerle, H. Immune function of the blood-brain barrier: Incomplete presentation of protein (auto-)antigens by rat brain microvascular endothelium in vitro. J. Cell Biol. 1990, 110, 1757–1766. [Google Scholar] [CrossRef]
- Herman, I.M.; D’Amore, P.A. Microvascular pericytes contain muscle and non muscle actins. J. Cell Biol. 1985, 101, 43–52. [Google Scholar] [CrossRef]
- Wakui, S. Epidermal growth factor receptor at endothelial cell and pericytes interdigitation in human granulation tissue. Microvasc. Res. 1992, 44, 255–262. [Google Scholar] [CrossRef]
- Jackson, J.A.; Carlson, E.C. Inhibition of bovine retinal microvascular pericyte proliferation in vitro by adenosine. Am. J. Physiol. 1992, 263, H634–H640. [Google Scholar]
- Dodge, A.B.; D’Amore, P.A. Cell-Cell interactions in diabetic angiopathy. Diabetes Care. 1992, 15, 1168–1180. [Google Scholar]
- Noden, D.M. Embryonic origins and assembly of blood vessels. Am. Rev. Respir. Dis. 1989, 140, 1097–1103. [Google Scholar] [CrossRef]
- Risau, W.; Sariola, H.; Zerwes, H.G.; Sasse, J.; Ekblom, P.; Kemler, R.; Doetschman, T. Vasculogenesis and angiogenesis in embryonic-stem-cell-derived embryoid bodies. Development 1988, 102, 471–478. [Google Scholar]
- Cleaver, O.; Tonissen, K.F.; Saha, M.S.; Krieg, P.A. Neovascularization of the Xenopus embryo. Dev. Dyn. 1997, 210, 66–77. [Google Scholar] [CrossRef]
- Ambler, C.A.; Nowicki, J.L.; Burke, A.C.; Bautch, V.L. Assembly of trunk and limb blood vessels involves extensive migration and vasculogenesis of somite-derived angioblasts. Dev. Biol. 2001, 234, 352–364. [Google Scholar] [CrossRef]
- Nakamura, H. Electron microscopic study of the prenatal development of the thoracic aorta in the rat. Am. J. Anat. 1988, 181, 406–418. [Google Scholar] [CrossRef]
- Antonelli-Orlidge, A.; Saunders, K.B.; Smith, S.R.; D’Amore, P.A. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc. Natl. Acad. Sci. USA 1989, 86, 4544–4548. [Google Scholar] [CrossRef]
- Orlidge, A.; D’Amore, P.A. Inhibition of capillary endothelial cell growth by pericytes and smooth muscle cells. J. Cell Biol. 1987, 105, 1455–1462. [Google Scholar] [CrossRef]
- Hirschi, K.K.; Rohovsky, S.A.; D’Amore, P.A. PDGF, TGF-beta, and heterotypic cell-cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J. Cell Biol. 1998, 141, 805–814. [Google Scholar] [CrossRef]
- Crocker, D.J.; Murad, T.M.; Geer, J.C. Role of the pericyte in wound healing. An ultrastructural study. Exp. Mol. Pathol. 1970, 13, 51–65. [Google Scholar] [CrossRef]
- Oh, S.P.; Seki, T.; Goss, K.A.; Imamura, T.; Yi, Y.; Donahoe, P.K.; Li, L.; Miyazono, K.; ten Dijke, P.; Kim, S.; et al. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc. Natl. Acad. Sci. USA 2000, 97, 2626–2631. [Google Scholar] [CrossRef]
- Oshima, M.; Oshima, H.; Taketo, M.M. TGF-beta receptor type II deficiency results in defects of yolk sac hematopoiesis and vasculogenesis. Dev. Biol. 1996, 179, 297–302. [Google Scholar] [CrossRef]
- Li, D.Y.; Sorensen, L.K.; Brooke, B.S.; Urness, L.D.; Davis, E.C.; Taylor, D.G.; Boak, B.B.; Wendel, D.P. Defective angiogenesis in mice lacking endoglin. Science 1999, 284, 1534–1537. [Google Scholar] [CrossRef]
- Goumans, M.J.; Valdimarsdottir, G.; Itoh, S.; Rosendahl, A.; Sideras, P.; ten Dijke, P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002, 21, 1743–1753. [Google Scholar] [CrossRef]
- Carvalho, R.L.; Jonker, L.; Goumans, M.J.; Larsson, J.; Bouwman, P.; Karlsson, S.; Dijke, P.T.; Arthur, H.M.; Mummery, C.L. Defective paracrine signalling by TGF-beta in yolk sac vasculature of endoglin mutant mice: A paradigm for hereditary haemorrhagic telangiectasia. Development 2004, 131, 6237–6247. [Google Scholar] [CrossRef]
- Desmoulière, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef]
- Verbeek, M.M.; Otte-Höller, I.; Wesseling, P.; Ruiter, D.J.; de Waal, R.M. Induction of alpha-smooth muscle actin expression in cultured human brain pericytes by transforming growth factor-beta 1. Am. J. Pathol. 1994, 144, 372–382. [Google Scholar]
- Egginton, S.; Hudlicka, O.; Brown, M.D.; Graciotti, L.; Granata, A.L. In vivo pericyte-endothelial cell interaction during angiogenesis in adult cardiac and skeletal muscle. Microvasc Res. 1996, 2, 213–228. [Google Scholar]
- Martin, A.R.; Bailie, J.R.; Robson, T.; McKeown, S.R.; Al-Assar, O.; McFarland, A.; Hirst, D.G. Retinal pericytes control expression of nitric oxide synthase and endothelin-1 in microvascular endothelial cells. Microvasc.Res. 2000, 59, 131–139. [Google Scholar] [CrossRef]
- Holmgren, L.; Glaser, A.; Pfeifer-Ohlsson, S.; Ohlsson, R. Angiogenesis during human extraembryonic development involves the spatiotemporal control of PDGF ligand and receptor gene expression. Development 1991, 113, 749–754. [Google Scholar]
- Mousseau, Y.; Mollard, S.; Richard, L.; Nizou, A.; Faucher-Durand, K.; Cook-Moreau, J.; Qiu, H.; Baaj, Y.; Funalot, B.; Fourcade, L.; et al. Fingolimod inhibits PDGF-B-induced migration of vascular smooth muscle cell by down-regulating the S1PR1/S1PR3 pathway. Biochimie 2012, 94, 2523–2531. [Google Scholar] [CrossRef]
- Yi, N.; Chen, S.Y.; Ma, A.; Chen, P.S.; Yao, B.; Liang, T.M.; Liu, C. Tunicamycin inhibits PDGF-BB-Induced proliferation and migration of vascular smooth muscle cells through induction of HO-1. Anat Rec. 2012, 295, 1462–1472. [Google Scholar] [CrossRef]
- D’Amore, P.A.; Smith, S.R. Growth factor effects on cells of the vascular wall: A survey. Growth Factors 1993, 8, 61–75. [Google Scholar] [CrossRef]
- Tilton, R.G.; Kilo, C.; Williamson, J.R. Pericyte-Endothelial relationships in cardiac and skeletal muscle capillaries. Microvasc.Res. 1979, 18, 325–335. [Google Scholar] [CrossRef]
- Das, A.; Frank, R.N.; Weber, M.L.; Kennedy, A.; Reidy, C.A.; Mancini, M.A. ATP causes retinal pericytes to contract in vitro. Exp. Eye Res. 1988, 46, 349–362. [Google Scholar] [CrossRef]
- Kelley, C.; D’Amore, P.; Hechtman, H.B.; Shepro, D. Microvascular pericyte contractility in vitro: Comparison with other cells of the vascular wall. J. Cell Biol. 1987, 104, 483–490. [Google Scholar] [CrossRef]
- Singhal, P.C.; Scharschmidt, L.A.; Gibbons, N.; Hays, R.M. Contraction and relaxation of cultured mesangial cells on a silicone rubber surface. Kidney Int. 1986, 30, 862–873. [Google Scholar] [CrossRef]
- Joyce, N.C.; DeCamilli, P.; Boyles, J. Pericytes, like vascular smooth muscle cells, are immunocytochemically positive for cyclic GMP-dependent protein kinase. Microvasc.Res. 1984, 28, 206–219. [Google Scholar] [CrossRef]
- Wang, S.; Voisin, M.B.; Larbi, K.Y.; Dangerfield, J.; Scheiermann, C.; Tran, M.; Maxwell, P.H.; Sorokin, L.; Nourshargh, S. Venular basement membranes contain specific matrix protein low expression regions that act as exit points for emigrating neutrophils. J. Exp. Med. 2006, 203, 1519–1532. [Google Scholar] [CrossRef] [Green Version]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Yancopoulos, G.D.; Davis, S.; Gale, N.W.; Rudge, J.S.; Wiegand, S.J.; Holash, J. Vascular-specific growth factors and blood vessel formation. Nature 2000, 407, 242–248. [Google Scholar] [CrossRef]
- Song, S.; Ewald, A.J.; Stallcup, W.; Werb, Z.; Bergers, G. PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival. Nat. Cell Biol. 2005, 7, 870–879. [Google Scholar] [CrossRef]
- Dulmovits, B.M.; Herman, I.M. Microvascular remodeling and wound healing: A role for pericytes. Int. J. Biochem. Cell Biol. 2012, 44, 1800–1812. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Paquet-Fifield, S.; Schlüter, H.; Li, A.; Aitken, T.; Gangatirkar, P.; Blashki, D.; Koelmeyer, R.; Pouliot, N.; Palatsides, M.; Ellis, S.; et al. A role for pericytes as microenvironmental regulators of human skin tissue regeneration. J. Clin. Invest. 2009, 119, 2795–2806. [Google Scholar]
- Farrington-Rock, C.; Crofts, N.J.; Doherty, M.J.; Ashton, B.A.; Griffin-Jones, C.; Canfield, A.E. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation 2004, 110, 2226–2232. [Google Scholar] [CrossRef]
- Richardson, R.L.; Hausman, G.J.; Campion, D.R. Response of pericytes to thermal lesion in the inguinal fat pad of 10-day-old rats. Acta Anat (Basel) 1982, 114, 41–57. [Google Scholar] [CrossRef]
- Caplan, A.I. All MSCs are pericytes? Cell Stem Cell 2008, 3, 229–230. [Google Scholar] [CrossRef]
- Kristensson, K.; Olsson, Y. Accumulation of protein tracers in pericytes of the central nervous system following systemic injection in immature mice. Acta Neurol. Scand. 1973, 49, 189–194. [Google Scholar] [CrossRef]
- Thomas, W.E. Brain macrophages: On the role of pericytes and perivascular cells. Brain Res. Brain Res. Rev. 1999, 1, 42–57. [Google Scholar] [CrossRef]
- Diaz-Flores, L.; Gutierrez, R.; Lopez-Alonso, A.; Gonzalez, R.; Varela, H. Pericytes as a supplementary source of osteoblasts in periosteal osteogenesis. Clin. Orthop. Relat. Res. 1992, 275, 280–286. [Google Scholar]
- Kirton, J.P.; Crofts, N.J.; George, S.J.; Brennan, K.; Canfield, A.E. Wnt/beta-catenin signaling stimulates chondrogenic and inhibits adipogenic differentiation of pericytes: Potential relevance to vascular disease? Circ. Res. 2007, 101, 581–589. [Google Scholar] [CrossRef]
- Hirschi, K.K.; Rohovsky, S.A.; Beck, L.H.; Smith, S.R.; D’Amore, P.A. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ. Res. 1999, 84, 298–305. [Google Scholar] [CrossRef]
- Nicosia, R.F.; Villaschi, S. Rat aortic smooth muscle cells become pericytes during angiogenesis in vitro. Lab. Invest. 1995, 73, 658–666. [Google Scholar]
- Mansilla, E.; Marín, G.H.; Drago, H.; Sturla, F.; Salas, E.; Gardiner, C.; Bossi, S.; Lamonega, R.; Guzmán, A.; Nuñez, A.; et al. Bloodstream cells phenotypically identical to human mesenchymal bone marrow stem cells circulate in large amounts under the influence of acute large skin damage: New evidence for their use in regenerative medicine. Transplant. Proc. 2006, 38, 967–969. [Google Scholar] [CrossRef]
- Nakagawa, H.; Akita, S.; Fukui, M.; Fujii, T.; Akino, K. Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br. J. Dermatol. 2005, 153, 29–36. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007, 13, 1299–2312. [Google Scholar] [CrossRef]
- Badillo, A.T.; Redden, R.A.; Zhang, L.; Doolin, E.J.; Liechty, K.W. Treatment of diabetic wounds with fetal murine mesenchymal stromal cells enhances wound closure. Cell Tissue Res. 2007, 329, 301–311. [Google Scholar] [CrossRef]
- Chen, L.; Tredget, E.E.; Wu, P.Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008, 3, e1886. [Google Scholar] [CrossRef]
- Altman, A.M.; Matthias, N.; Yan, Y.; Song, Y.H.; Bai, X.; Chiu, E.S.; Slakey, D.P.; Alt, E.U. Dermal matrix as a carrier for in vivo delivery of human adipose-derived stem cells. Biomaterials 2008, 29, 1431–1442. [Google Scholar] [CrossRef]
- Zebardast, N.; Lickorish, D.; Davies, J.E. Human umbilical cord perivascular cells (HUCPVC): A mesenchymal cell source for dermal wound healing. Organogenesis 2010, 6, 197–203. [Google Scholar] [CrossRef]
- Shumakov, V.I.; Onishchenko, N.A.; Rasulov, M.F.; Krasheninnikov, M.E.; Zaidenov, V.A. Mesenchymal bone marrow stem cells more effectively stimulate regeneration of deep burn wounds than embryonic fibroblasts. Bull. Exp. Biol.Med. 2003, 136, 192–195. [Google Scholar] [CrossRef]
- Sasaki, M.; Abe, R.; Fujita, Y.; Ando, S.; Inokuma, D.; Shimizu, H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 2008, 180, 2581–2587. [Google Scholar]
- Huang, S.; Lu, G.; Wu, Y.; Jirigala, E.; Xu, Y.; Ma, K.; Fu, X. Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J. Dermatol.Sci. 2012, 66, 29–36. [Google Scholar] [CrossRef]
- Miller, F.N.; Sims, D.E.; Schuschke, D.A.; Abney, D.L. Differentiation of light-dye effects in the microcirculation. Microvasc. Res. 1992, 44, 166–184. [Google Scholar] [CrossRef]
- Sims, D.E.; Miller, F.N.; Horne, M.M.; Edwards, M.J. Interleukin-2 alters the positions of capillary and venule pericytes in rat cremaster muscle. J. Submicrosc. Cytol. Pathol. 1994, 26, 507–513. [Google Scholar]
- Canfield, A.E.; Allen, T.D.; Grant, M.E.; Schor, S.L.; Schor, A.M. Modulation of extracellular matrix biosynthesis by bovine retinal pericytes in vitro: Effects of the substratum and cell density. J. Cell Sci. 1990, 96, 159–169. [Google Scholar]
- Schor, A.M.; Canfield, A.E.; Sloan, P.; Schor, S.L. Differentiation of pericytes in culture is accompanied by changes in the extracellular matrix. In Vitro Cell Dev.Biol. 1991, 27A, 651–659. [Google Scholar]
- Rajkumar, V.S.; Shiwen, X.; Bostrom, M.; Leoni, P.; Muddle, J.; Ivarsson, M.; Gerdin, B.; Denton, C.P.; Bou-Gharios, G.; Black, C.M.; et al. Platelet-Derived growth factor-beta receptor activation is essential for fibroblast and pericyte recruitment during cutaneous wound healing. Am. J. Pathol. 2006, 169, 2254–2265. [Google Scholar] [CrossRef]
- Sundberg, C.; Ljungström, M.; Lindmark, G.; Gerdin, B.; Rubin, K. Microvascular pericytes express platelet-derived growth factor-beta receptors in human healing wounds and colorectal adenocarcinoma. Am. J. Pathol. 1993, 143, 1377–1388. [Google Scholar]
- Popescu, F.C.; Busuioc, C.J.; Mogoşanu, G.D.; Pop, O.T.; Pârvănescu, H.; Lascăr, I.; Nicolae, C.I.; Mogoantă, L. Pericytes and myofibroblasts reaction in experimental thermal third degree skin burns. Rom. J. Morphol. Embryol. 2011, 52 (3 Suppl), 1011–1017. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mills, S.J.; Cowin, A.J.; Kaur, P. Pericytes, Mesenchymal Stem Cells and the Wound Healing Process. Cells 2013, 2, 621-634. https://doi.org/10.3390/cells2030621
Mills SJ, Cowin AJ, Kaur P. Pericytes, Mesenchymal Stem Cells and the Wound Healing Process. Cells. 2013; 2(3):621-634. https://doi.org/10.3390/cells2030621
Chicago/Turabian StyleMills, Stuart J., Allison J. Cowin, and Pritinder Kaur. 2013. "Pericytes, Mesenchymal Stem Cells and the Wound Healing Process" Cells 2, no. 3: 621-634. https://doi.org/10.3390/cells2030621
APA StyleMills, S. J., Cowin, A. J., & Kaur, P. (2013). Pericytes, Mesenchymal Stem Cells and the Wound Healing Process. Cells, 2(3), 621-634. https://doi.org/10.3390/cells2030621





