ELISPOT Assays in 384-Well Format: Up to 30 Data Points with One Million Cells
Abstract
:1. Introduction
2. Experimental Section
2.1. Peripheral Blood Mononuclear Cells
2.2. Human IFN-γ ELISPOT Assays
2.3. Fluorescence Detection of PBMC
3. Results and Discussion
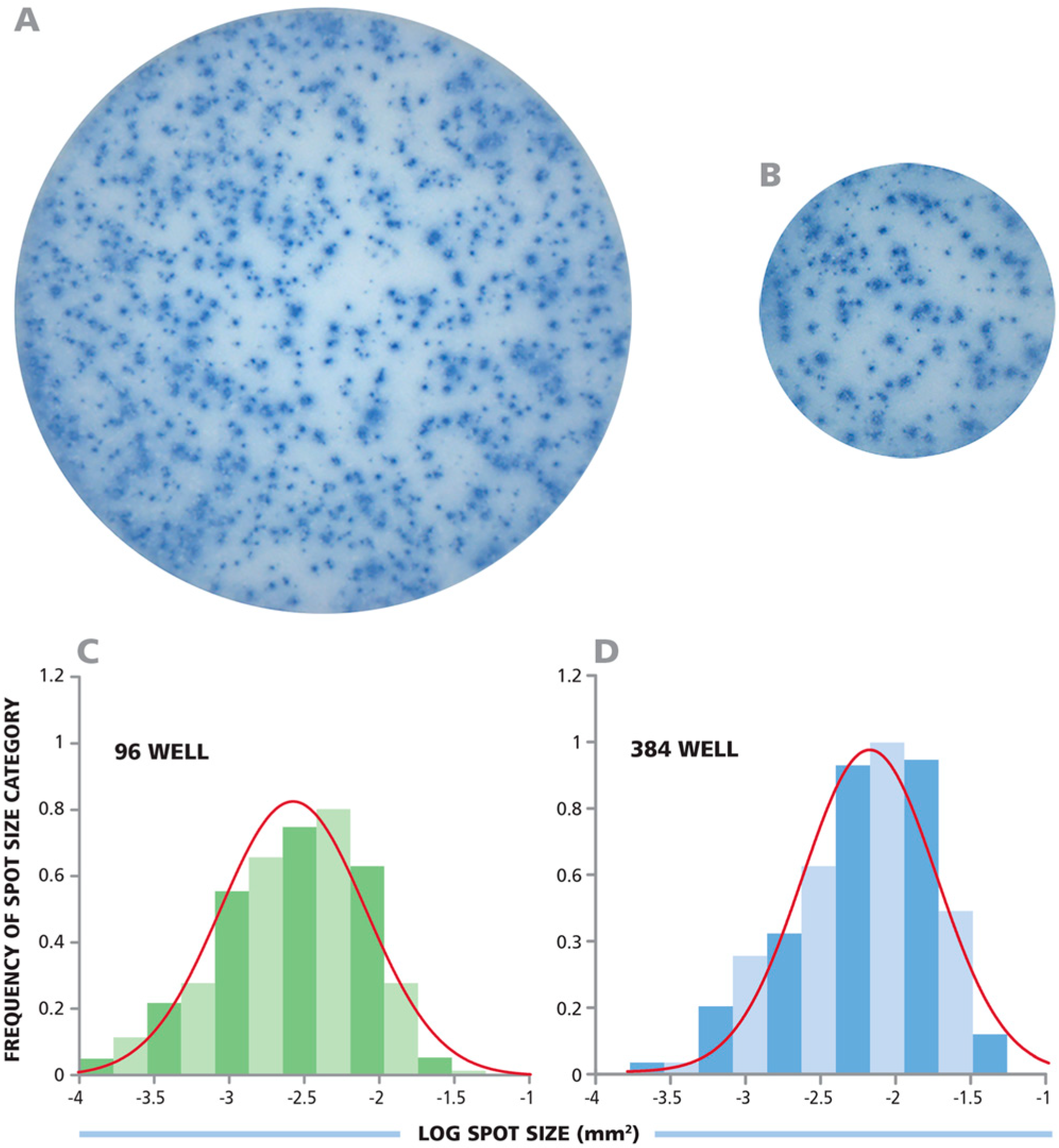
3.1. CD8+ Cell-Derived IFN-γ ELISPOTs Show Identical Size Distributions in 96-Well and 384-Well Assays
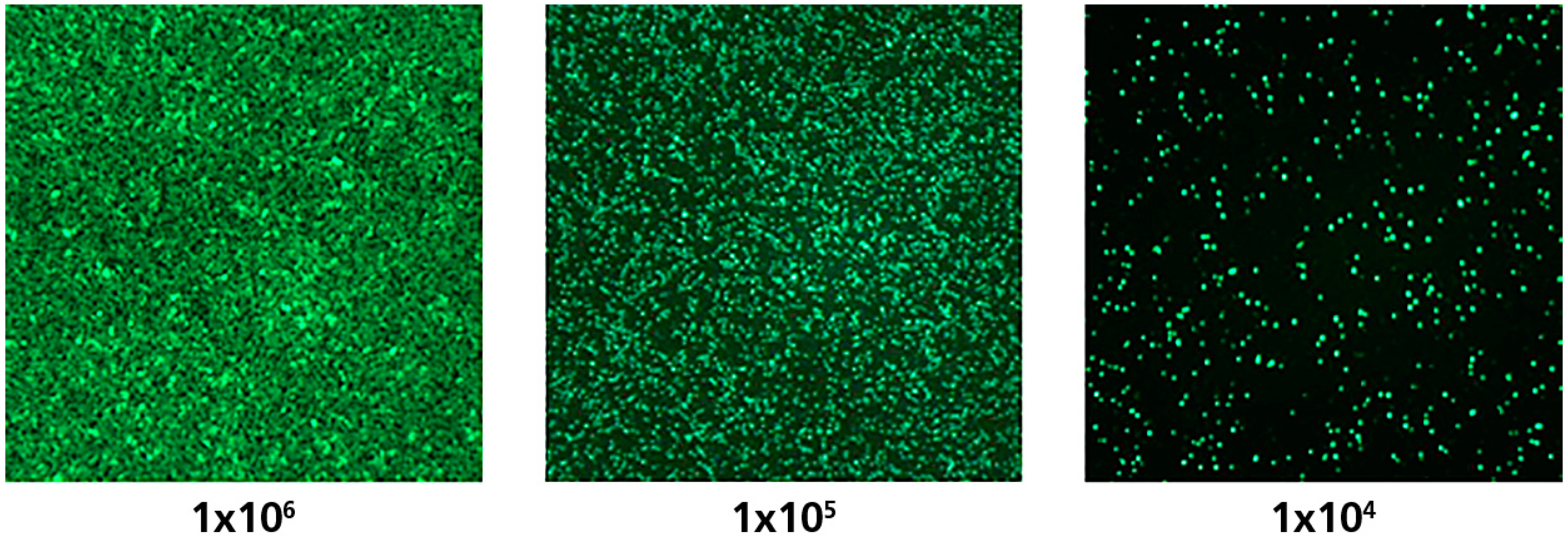
3.2. In Both the 384-and 96-Well Plate, a Linear Relationship between the Number of PBMC Plated and Spot Forming Units (SFU) Is Seen

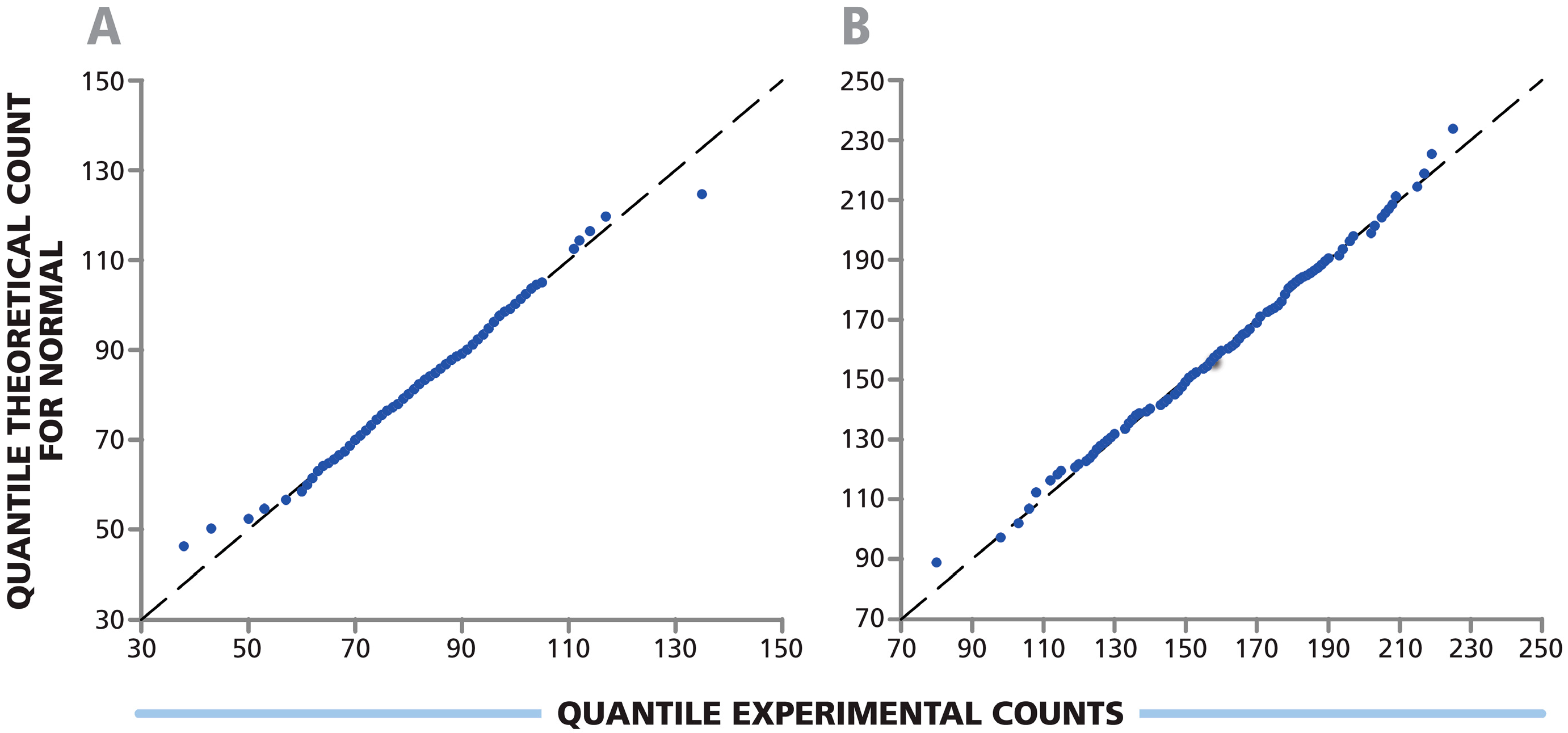
3.3. Spot Counts in Replicate Wells of a 384-Well Plate Follow Normal Distribution
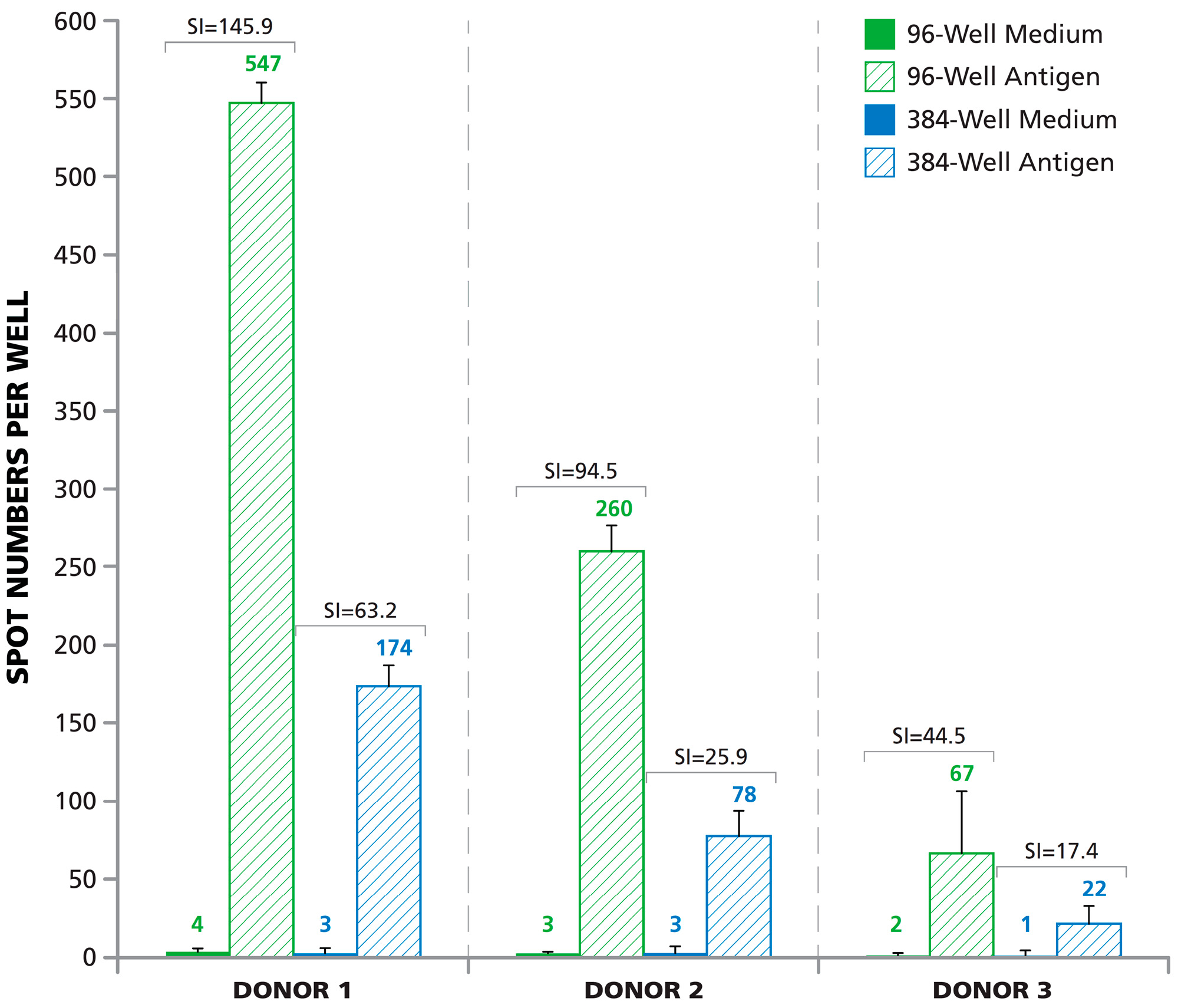
3.4. SFU in 384-Well Plates Are One-Third Those in 96-Well Plates
| TEST | 96-WELL | 384-WELL | 96W/384W |
|---|---|---|---|
| 1 | 670.0 | 177.5 | 3.8 |
| 2 | 577.5 | 177.3 | 3.3 |
| 3 | 547.3 | 173.8 | 3.1 |
| 4 | 499.3 | 164.0 | 3.0 |
| 5 | 423.0 | 118.5 | 3.6 |
| 6 | 344.5 | 102.8 | 3.4 |
| 7 | 260.0 | 77.8 | 3.3 |
| 8 | 140.3 | 52.3 | 2.7 |
| 9 | 126.0 | 35.5 | 3.5 |
| 10 | 66.8 | 21.8 | 3.1 |
| 11 | 26.0 | 7.5 | 3.5 |
| MEAN 3.3 ± 0.3 |
3.5. Matching Dose Response Curves Are Seen in 96- and 384-Well Format
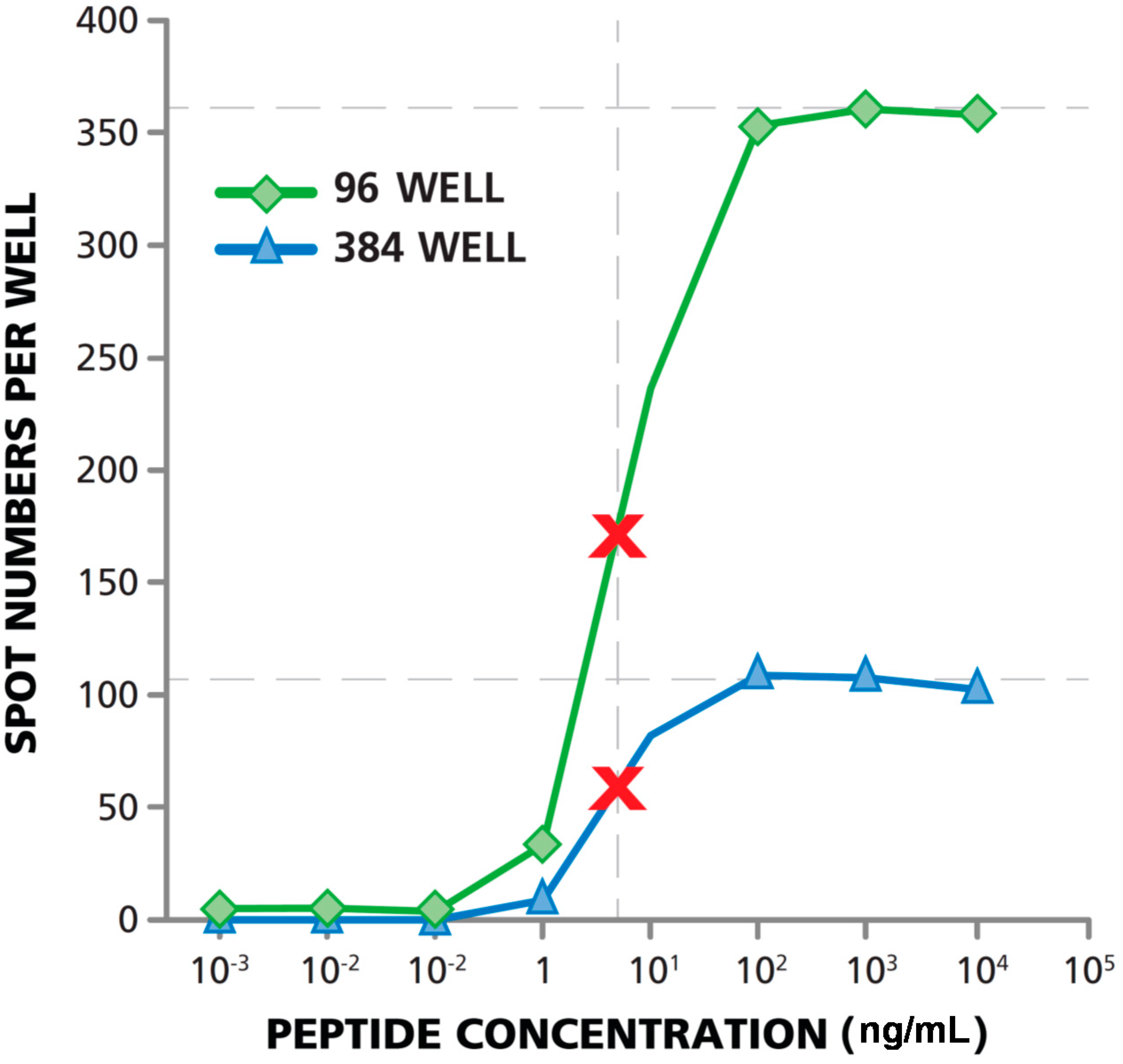
3.6. Lower Signal-to-Noise Performance of 384-Well Assays
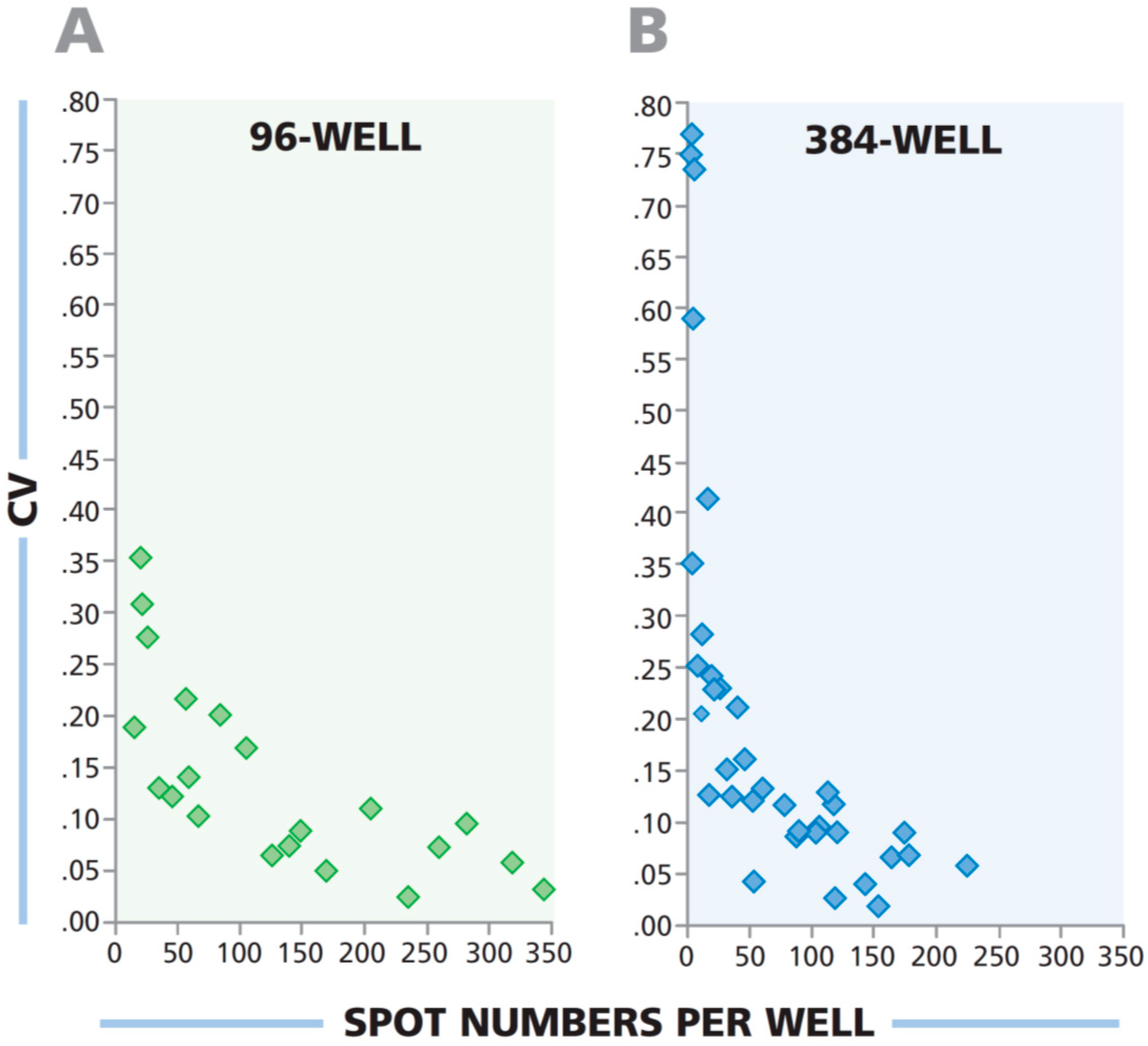
3.7. Low Spot Counts From 384-Well Plate Have a Higher Coefficient of Variation Than Corresponding Wells From 96-Well Plate
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lehmann, P.V.; Sundararaman, S. When results of T-cell immune monitoring match/do not match clinical outcomes of tumor vaccine trials: What more could and should we measure? In The Tumor Immunoenvironment; Shurin, M.R., Umansky, V., Malyguine, A., Eds.; Springer Dordrecht: New York, NY, USA, 2013; Chapter 32; pp. 725–740. [Google Scholar]
- Karulin, A.Y.; Hesse, M.D.; Tary-Lehmann, M.; Lehmann, P.V. Single-cytokine-producing cd4 memory cells predominate in type 1 and type 2 immunity. J. Immunol. 2000, 164, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Kuerten, S.; Nowacki, T.M.; Kleen, T.O.; Asaad, R.J.; Lehmann, P.V.; Tary-Lehmann, M. Dissociated production of perforin, granzyme b, and ifn-gamma by hiv-specific cd8(+) cells in hiv infection. AIDS Res. Hum. Retrov. 2008, 24, 62–71. [Google Scholar] [CrossRef]
- Nowacki, T.M.; Kuerten, S.; Zhang, W.; Shive, C.L.; Kreher, C.R.; Boehm, B.O.; Lehmann, P.V.; Tary-Lehmann, M. Granzyme b production distinguishes recently activated cd8(+) memory cells from resting memory cells. Cell. Immunol. 2007, 247, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Targoni, O.S.; Lehmann, P.V. Endogenous myelin basic protein inactivates the high avidity t cell repertoire. J. Exp. Med. 1998, 187, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Herzog, B.A.; Ott, P.A.; Dittrich, M.T.; Quast, S.; Karulin, A.Y.; Kalbacher, H.; Karges, W.; Tary-Lehmann, M.; Lehmann, P.V.; Boehm, B.O.; et al. Increased in vivo frequency of ia-2 peptide-reactive ifngamma+/il-4- t cells in type 1 diabetic subjects. J. Autoimmun. 2004, 23, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Herzog, B.A.; Quast, S.; Hofstetter, H.H.; Boehm, B.O.; Tary-Lehmann, M.; Durinovic-Bello, I.; Berner, B.R.; Lehmann, P.V. Islet-cell antigen-reactive t cells show different expansion rates and th1/th2 differentiation in type 1 diabetic patients and healthy controls. Clin. Immunol. 2005, 115, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Li Pira, G.; Ivaldi, F.; Starc, N.; Landi, F.; Locatelli, F.; Rutella, S.; Tripodi, G.; Manca, F. Miniaturized and high-throughput assays for analysis of t-cell immunity specific for opportunistic pathogens and hiv. Clin. Vaccine Immunol. 2014, 21, 488–495. [Google Scholar] [CrossRef] [PubMed]
- PBMC Searchable Database. Available online: www.epbmc.immunospot.com (accessed on 13 January 2015).
- Ramachandran, H.; Laux, J.; Moldovan, I.; Caspell, R.; Lehmann, P.V.; Subbramanian, R.A. Optimal thawing of cryopreserved peripheral blood mononuclear cells for use in high-throughput human immune monitoring studies. Cells 2012, 1, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lehmann, P.V. Objective, user-independent elispot data analysis based on scientifically validated principles. Methods Mol. Biol. 2012, 792, 155–171. [Google Scholar] [PubMed]
- Karulin, A.Y.; Karacsony, K; Zhang, W; Targoni, O.S.; Moldovan, I.; Dittrich, M.; Sundararaman, S.; Lehmann, P.V. Elispots produced by cd8 and cd4 cells follow log normal distribution. Cells 2015, 4. In Press. [Google Scholar]
- Zhang, W.; Caspell, R.; Karulin, A.Y.; Ahmad, M.; Haicheur, N.; Abdelsalam, A.; Johannesen, K.; Vignard, V.; Dudzik, P.; Georgakopoulou, K.; et al. Elispot assays provide reproducible results among different laboratories for t-cell immune monitoring--even in hands of elispot-inexperienced investigators. J. Immunotoxicol. 2009, 6, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Karulin, A.Y.; Caspell, R.; Dittrich, M.; Sundararaman, S.; Lehmann, P.V. Normal distribution of CD8+ T-cell derived elispot counts within replicates justifies the reliance on parametric statistics for identifying positive responses. Cells 2015, 4. In Press. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanson, J.; Sundararaman, S.; Caspell, R.; Karacsony, E.; Karulin, A.Y.; Lehmann, P.V. ELISPOT Assays in 384-Well Format: Up to 30 Data Points with One Million Cells. Cells 2015, 4, 71-83. https://doi.org/10.3390/cells4010071
Hanson J, Sundararaman S, Caspell R, Karacsony E, Karulin AY, Lehmann PV. ELISPOT Assays in 384-Well Format: Up to 30 Data Points with One Million Cells. Cells. 2015; 4(1):71-83. https://doi.org/10.3390/cells4010071
Chicago/Turabian StyleHanson, Jodi, Srividya Sundararaman, Richard Caspell, Edith Karacsony, Alexey Y. Karulin, and Paul V. Lehmann. 2015. "ELISPOT Assays in 384-Well Format: Up to 30 Data Points with One Million Cells" Cells 4, no. 1: 71-83. https://doi.org/10.3390/cells4010071





