Comparative Multi-Donor Study of IFNγ Secretion and Expression by Human PBMCs Using ELISPOT Side-by-Side with ELISA and Flow Cytometry Assays
Abstract
:1. Introduction
2. Experimental Section
2.1. Cells
2.2. ELISPOT Assay
2.3. Flow Cytometry Assay
2.4. ELISA
3. Results and Discussion
3.1. ELISPOT
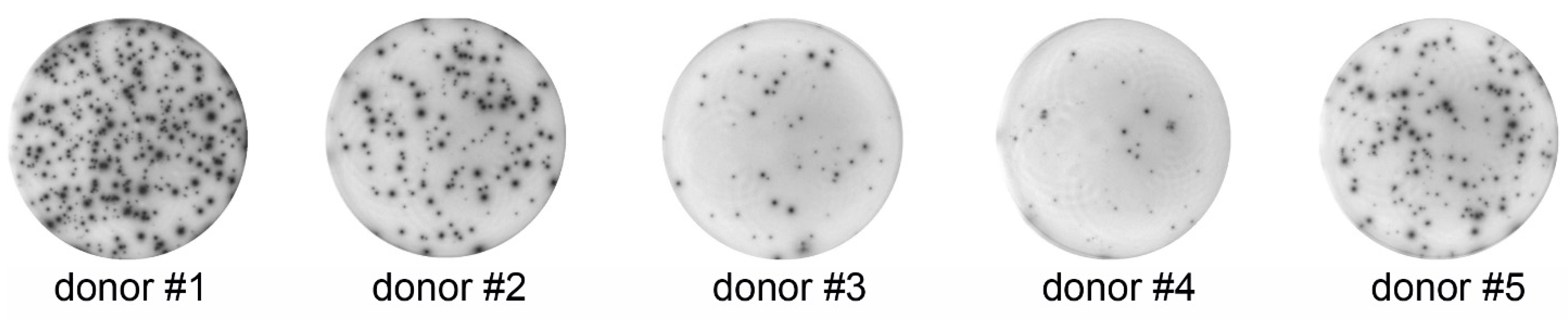
| Donor | Spots Per Well ± SD | Spot-Forming Cells (%) ± SD |
|---|---|---|
| 1 | 326 ± 11.15 | 6.52 ± 0.23 |
| 2 | 155 ± 17.76 | 3.10 ± 0.36 |
| 3 | 47 ± 5.74 | 0.94 ± 0.12 |
| 4 | 23 ± 0.71 | 0.46 ± 0.02 |
| 5 | 143 ± 12.40 | 2.90 ± 0.23 |
3.2. ELISPOT vs. ELISA
| Donor | ELISA (pg/mL) ± SD |
|---|---|
| 1 | 212 ± 4.38 |
| 2 | 1449 ± 0.00 |
| 3 | 48 ± 2.46 |
| 4 | 268 ± 8.19 |
| 5 | 266 ± 1.803 |
3.3. ELISPOT vs. Flow Cytometry
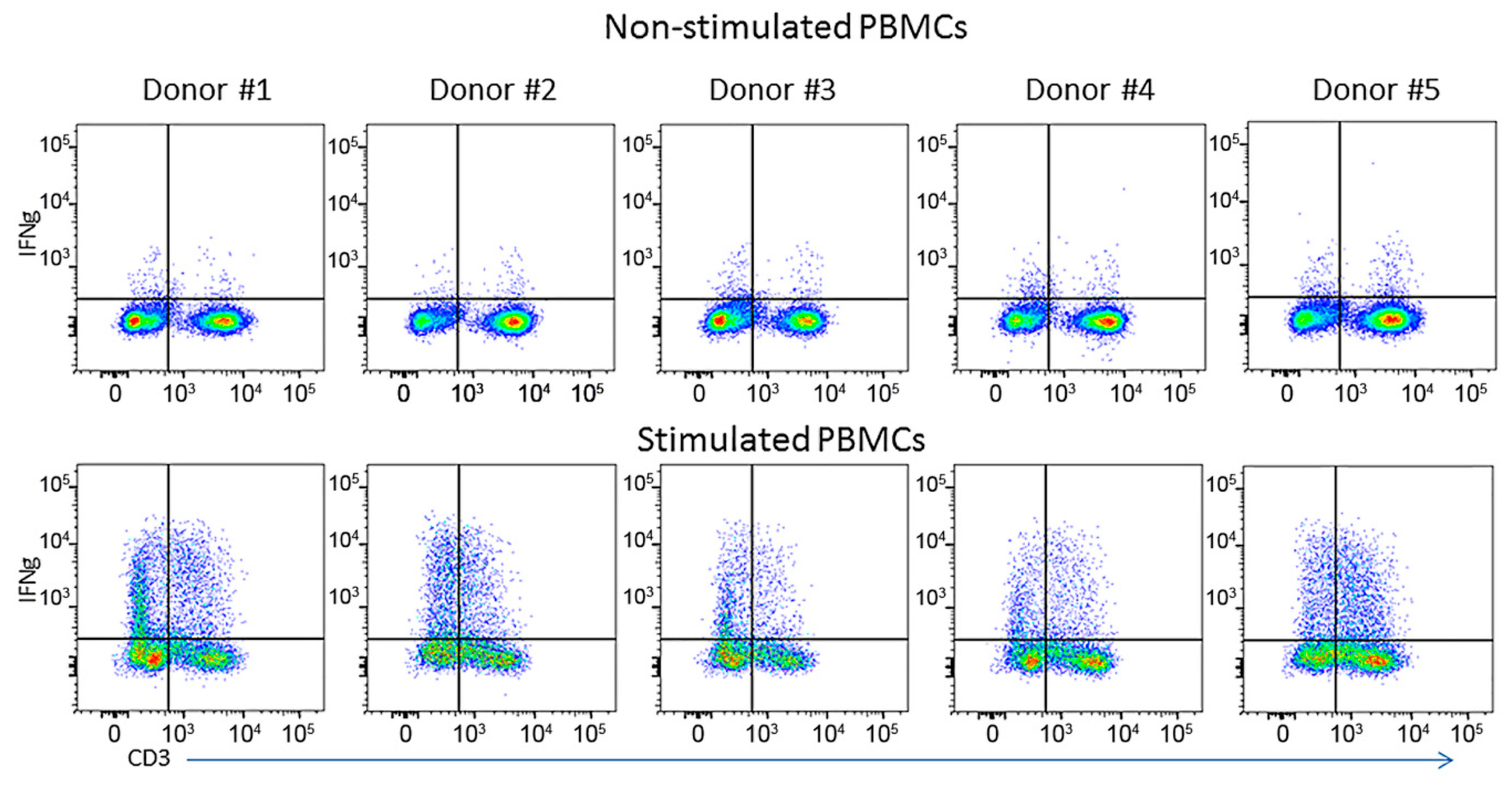
| Donor | Non-Stimulated Cells, % | Stimulated Cells, % | ||||
|---|---|---|---|---|---|---|
| CD3 Negative Cells | T-cells | All Cells | CD3 Negegative Cells | T-Cells | All Cells | |
| 1 | 0.61 | 0.65 | 1.26 | 21.0 | 12.40 | 33.40 |
| 2 | 0.33 | 0.50 | 0.83 | 18.50 | 13.80 | 32.30 |
| 3 | 1.18 | 0.75 | 1.93 | 16.50 | 6.54 | 23.04 |
| 4 | 1.02 | 0.77 | 1.79 | 8.29 | 7.83 | 16.12 |
| 5 | 0.59 | 0.74 | 1.33 | 7.84 | 15.50 | 23.34 |
| Cells | Rankings of Donors |
|---|---|
| Non-stimulated CD3 neg. cells | 3 > 4 > 1 > 5 > 2 |
| Non-stimulated T-cells | 4 > 3 > 5 > 1 > 2 |
| All non-stimulated cells | 3 > 4 > 5 > 1 > 2 |
| Stimulated CD3 neg. cells | 1 > 2 > 3 > 4 > 5 |
| Stimulated T-cells | 5 > 2 > 1 > 4 > 3 |
| All stimulated cells | 1 > 2 > 5 > 3 > 4 |
| Donor | Stimulated Cells | Fraction of IFNγ- Secreting Cells (%) | |
|---|---|---|---|
| Flow Cytometry (“All Cells”, %) | ELISPOT (SFCs ± SD, %) | ||
| 1 | 33.4 | 6.52 ± 0.23 | 19.52 |
| 2 | 32.3 | 3.1 ± 0.36 | 9.6 |
| 3 | 23.04 | 0.94 ± 0.12 | 4.08 |
| 4 | 16.12 | 0.46 ± 0.02 | 2.85 |
| 5 | 23.34 | 2.9 ± 0.23 | 4.28 |
3.4. ELISA vs. Flow Cytometry
3.5. Discussion
| Donor | Normalized Values | ||
|---|---|---|---|
| ELISPOT | Flow Cytometry(All stimulated cells) | ELISA | |
| 1 | 14.17 | 2.07 | 4.42 |
| 2 | 6.74 | 2.00 | 30.19 |
| 3 | 2.04 | 1.43 | 1.00 |
| 4 | 1.0 | 1.00 | 5.58 |
| 5 | 6.30 | 1.45 | 5.54 |
| Rankings of Donors | 1 > 2 > 5 > 3 > 4 | 1 > 2 > 5 > 3 > 4 | 2 > 4 ≥ 5 > 1 > 3 |
4. Conclusions
Acknowledgments
Author contributions
Conflicts of Interest
References
- Akira, S. Pathogen recognition by innate immunity and its signaling. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Cao, X. The immune potential and immunopathology of cytokine-producing b cell subsets: A comprehensive review. J. Autoimmun. 2014, 55, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Colonna, M. Innate lymphoid cells in homeostasis, infection, chronic inflammation and tumors of the gastrointestinal tract. Curr. Opin. Gastroenterol. 2013, 29, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J., III; Ahmed, C.M.; Wilson, T.D.; Johnson, H.M. Regulation of interferon gamma signaling by suppressors of cytokine signaling and regulatory t cells. Front. Immunol. 2013, 4. [Google Scholar] [CrossRef]
- Wheelock, E.F. Interferon-like virus-inhibitor induced in human leukocytes by phytohemagglutinin. Science 1965, 149, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, E.F.; Sibley, W.A. Circulating virus, interferon and antibody after vaccination with the 17-d strain of yellow-fever virus. N. Engl. J. Med. 1965, 273, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Czerkinsky, C.C.; Nilsson, L.A.; Nygren, H.; Ouchterlony, O.; Tarkowski, A. A solid-phase enzyme-linked immunospot (elispot) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods 1983, 65, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhny, A.E. Chemistry and biology of the elispot assay. Methods Mol. Biol. 2005, 302, 15–31. [Google Scholar] [PubMed]
- Kalyuzhny, A.E. Elispot assay on membrane microplates. Methods Mol. Biol. 2009, 536, 355–365. [Google Scholar] [PubMed]
- Sedgwick, J.D. Elispot assay: A personal retrospective. Methods Mol. Biol. 2005, 302, 3–14. [Google Scholar] [PubMed]
- Sedgwick, J.D.; Holt, P.G. A solid-phase immunoenzymatic technique for the enumeration of specific antibody-secreting cells. J. Immunol. Methods 1983, 57, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Viera, K.; Petr, C.; Marie, N.; Eva, T. Simultaneous analysis of cytokines and co-stimulatory molecules concentrations by elisa technique and of probabilities of measurable concentrations of interleukins il-2, il-4, il-5, il-6, cxcl8 (il-8), il-10, il-13 occurring in plasma of healthy blood donors. Mediat. Inflamm. 2006, 2006. [Google Scholar] [CrossRef]
- Voller, A. The enzyme-linked immunosorbent assay (elisa) (theory, technique and applications). La Ricerca in clinica e in laboratorio 1978, 8, 289–298. [Google Scholar] [PubMed]
- Ljungstrom, I.; Engvall, E.; Ruitenberg, E.J. Proceedings: Elisa, enzyme linked immunosorbent assay—a new technique for sero-diagnosis of trichinosis. Parasitology 1974, 69, xxiv. [Google Scholar] [PubMed]
- Keustermans, G.C.; Hoeks, S.B.; Meerding, J.M.; Prakken, B.J.; de Jager, W. Cytokine assays: An assessment of the preparation and treatment of blood and tissue samples. Methods 2013, 61, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Freer, G.; Rindi, L. Intracellular cytokine detection by fluorescence-activated flow cytometry: Basic principles and recent advances. Methods 2013, 61, 30–38. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, S.C. Vaccine applications of flow cytometry. Methods 2012, 57, 383–391. [Google Scholar]
- Nomura, L.; Maino, V.C.; Maecker, H.T. Standardization and optimization of multiparameter intracellular cytokine staining. Cytom. Part A 2008, 73, 984–991. [Google Scholar] [CrossRef]
- Bailey, T.; Stark, S.; Grant, A.; Hartnett, C.; Tsang, M.; Kalyuzhny, A. A multidonor elispot study of il-1 beta, il-2, il-4, il-6, il-13, ifn-gamma and tnf-alpha release by cryopreserved human peripheral blood mononuclear cells. J. Immunol. Methods 2002, 270, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Palzer, S.; Hartnett, C.; Bailey, T.; Tsang, M.; Kalyuzhny, A.E. A cell-detachment solution can reduce background staining in the elispot assay. Methods Mol. Biol. 2005, 302, 87–94. [Google Scholar] [PubMed]
- Kalyuzhny, A.; Stark, S. A simple method to reduce the background and improve well-to-well reproducibility of staining in elispot assays. J. Immunol. Methods 2001, 257, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Hagen, J.; Houchins, J.P.; Kalyuzhny, A.E. Combining elispot and elisa to measure amounts of cytokines secreted by a single cell. Methods Mol. Biol. 2012, 792, 115–122. [Google Scholar] [PubMed]
- Jason, J.; Archibald, L.K.; Nwanyanwu, O.C.; Byrd, M.G.; Kazembe, P.N.; Dobbie, H.; Jarvis, W.R. Comparison of serum and cell-specific cytokines in humans. Clin. Diagn. Lab. Immunol. 2001, 8, 1097–1103. [Google Scholar] [PubMed]
- Kabilan, L.; Andersson, G.; Lolli, F.; Ekre, H.P.; Olsson, T.; Troye-Blomberg, M. Detection of intracellular expression and secretion of interferon-gamma at the single-cell level after activation of human t cells with tetanus toxoid in vitro. Eur. J. Immunol. 1990, 20, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Tanguay, S.; Killion, J.J. Direct comparison of elispot and elisa-based assays for detection of individual cytokine-secreting cells. Lymphokine Cytokine Res. 1994, 13, 259–263. [Google Scholar] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagen, J.; Zimmerman, R.; Goetz, C.; Bonnevier, J.; Houchins, J.P.; Reagan, K.; Kalyuzhny, A.E. Comparative Multi-Donor Study of IFNγ Secretion and Expression by Human PBMCs Using ELISPOT Side-by-Side with ELISA and Flow Cytometry Assays. Cells 2015, 4, 84-95. https://doi.org/10.3390/cells4010084
Hagen J, Zimmerman R, Goetz C, Bonnevier J, Houchins JP, Reagan K, Kalyuzhny AE. Comparative Multi-Donor Study of IFNγ Secretion and Expression by Human PBMCs Using ELISPOT Side-by-Side with ELISA and Flow Cytometry Assays. Cells. 2015; 4(1):84-95. https://doi.org/10.3390/cells4010084
Chicago/Turabian StyleHagen, Jodi, Ryan Zimmerman, Christine Goetz, Jody Bonnevier, Jeffrey P. Houchins, Kevin Reagan, and Alexander E. Kalyuzhny. 2015. "Comparative Multi-Donor Study of IFNγ Secretion and Expression by Human PBMCs Using ELISPOT Side-by-Side with ELISA and Flow Cytometry Assays" Cells 4, no. 1: 84-95. https://doi.org/10.3390/cells4010084
APA StyleHagen, J., Zimmerman, R., Goetz, C., Bonnevier, J., Houchins, J. P., Reagan, K., & Kalyuzhny, A. E. (2015). Comparative Multi-Donor Study of IFNγ Secretion and Expression by Human PBMCs Using ELISPOT Side-by-Side with ELISA and Flow Cytometry Assays. Cells, 4(1), 84-95. https://doi.org/10.3390/cells4010084





