Estrogen Modulates Glycerol Permeability in Sertoli Cells through Downregulation of Aquaporin-9
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Cell Culture and Experimental Groups
2.3. Reverse Transcriptase Polymerase Chain Reaction (RT-PCR) and Quantitative RT-PCR (qPCR)
2.4. Preparation of Cellular Suspension
2.5. Stopped-Flow Light Scattering
2.6. Statistical Analysis
3. Results
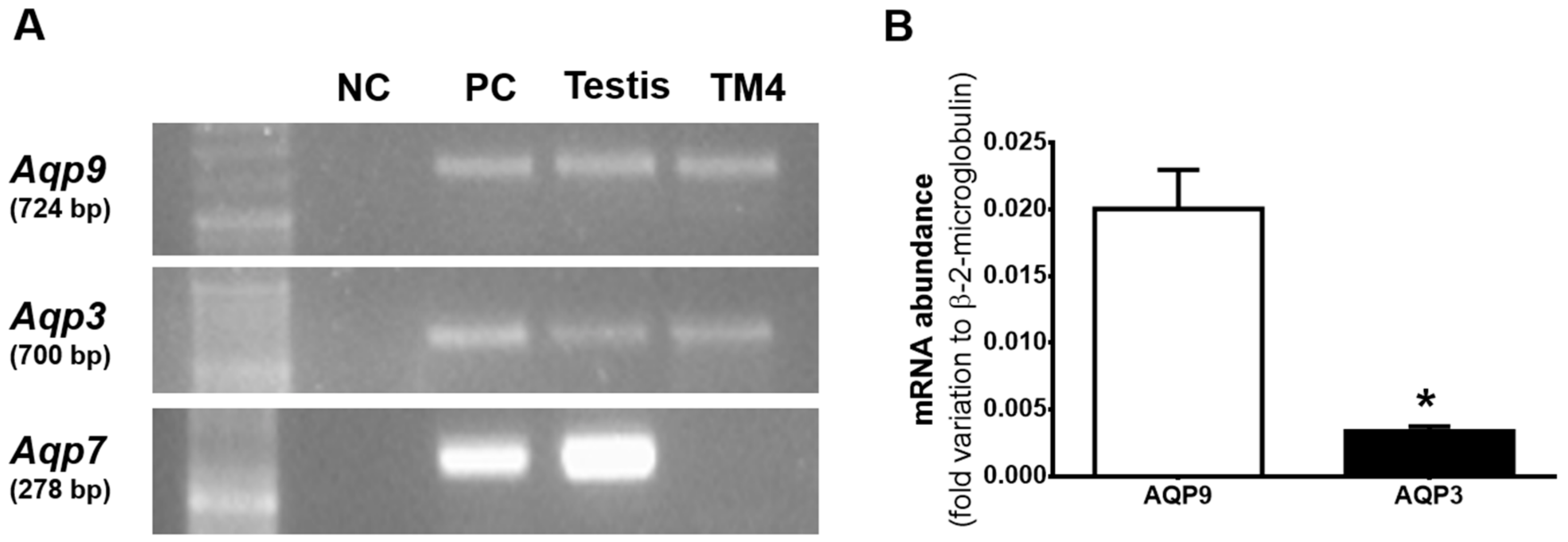
3.1. Aqp3 and Aqp9 are Expressed in mSCs but not AQP7
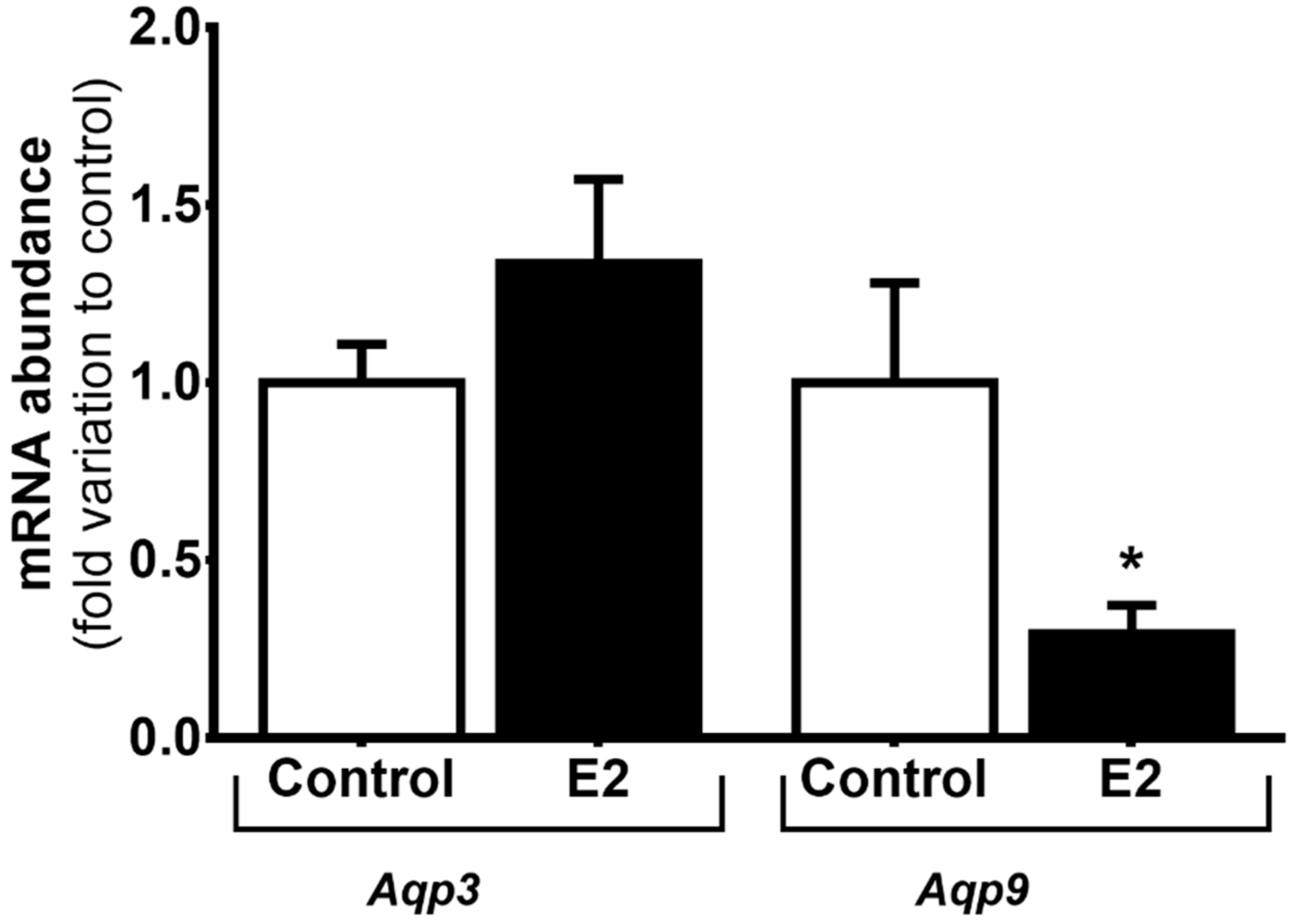
3.2. High Levels of E2 Downregulate Aqp9 mRNA Expression in mSCs
3.3. Permeability of mSCs to Glycerol is Decreased after Exposure to High Levels of E2
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clarke, M.; Pearl, C.A. Alterations in the estrogen environment of the testis contribute to declining sperm production in aging rats. Syst. Biol. Reprod. Med. 2014, 60, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Hampl, R.; Kubatova, J.; Sobotka, V.; Heracek, J. Steroids in semen, their role in spermatogenesis, and the possible impact of endocrine disruptors. Horm. Mol. Biol. Clin. Investig. 2013, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, R.; Alves, M.; Silva, J.; Barros, A.; Ferraz, L.; Sousa, M.; Sá, R.; Oliveira, P. Expression of estrogen receptors alpha (ER-α), beta (ER-β), and G protein-coupled receptor 30 (GPR30) in testicular tissue of men with Klinefelter syndrome. Horm. Metab. Res. 2016, 48, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Leavy, M.; Trottmann, M.; Liedl, B.; Reese, S.; Stief, C.; Freitag, B.; Baugh, J.; Spagnoli, G.; Kolle, S. Effects of Elevated beta-Estradiol Levels on the Functional Morphology of the Testis—New Insights. Sci. Rep. 2017, 7, 39931. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, J.P.; Kowalik, A.; Gallardi, R.L.; Egeler, O.; Clubb, B.H. Glycerol Disrupts Tight Junction—Associated Actin Microfilaments, Occludin, and Microtubules in Sertoli Cells. J. Androl. 2000, 21, 625–635. [Google Scholar] [PubMed]
- Crisóstomo, L.; Alves, M.G.; Calamita, G.; Sousa, M.; Oliveira, P.F. Glycerol and testicular activity: The good, the bad and the ugly. Mol. Hum. Reprod. 2017, 23, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, J.P.; Barr, K.J.; Buckingham, K.D. Sustained azoospermia in squirrel monkey, Saimiri sciureus, resulting from a single intratesticular glycerol injection. Contraception 1989, 39, 447–457. [Google Scholar] [CrossRef]
- Crisostomo, L.; Alves, M.G.; Gorga, A.; Sousa, M.; Riera, M.F.; Galardo, M.N.; Meroni, S.B.; Oliveira, P.F. Molecular Mechanisms and Signaling Pathways Involved in the Nutritional Support of Spermatogenesis by Sertoli Cells. Methods Mol. Biol. 2018, 1748, 129–155. [Google Scholar] [PubMed]
- Bernardino, R.L.; Marinelli, R.A.; Maggio, A.; Gena, P.; Cataldo, I.; Alves, M.G.; Svelto, M.; Oliveira, P.F.; Calamita, G. Hepatocyte and Sertoli Cell Aquaporins, Recent Advances and Research Trends. Int. J. Mol. Sci. 2016, 17, 1096. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.D.; Lobo, C.H.; Carvalho, A.A.; Moura, A.A.; Rodrigues, A.P. Structure, function, and localization of aquaporins: Their possible implications on gamete cryopreservation. Genet. Mol. Res. 2013, 12, 6718–6732. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M.; Morato, R.; Rodriguez-Gil, J.E.; Bonet, S.; Prieto-Martinez, N. Aquaporins in the male reproductive tract and sperm: Functional implications and cryobiology. Reprod. Domest. Anim. 2017, 52, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Soler, N.M.; Fisher, J.S.; Sharpe, R.; Hill, E.; Van Hoek, A.; Brown, D.; Breton, S. Aquaporin 9 expression in the developing rat epididymis is modulated by steroid hormones. Reproduction 2010, 139, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Wellejus, A.; Jensen, H.E.; Loft, S.; Jonassen, T.E. Expression of aquaporin 9 in rat liver and efferent ducts of the male reproductive system after neonatal diethylstilbestrol exposure. J. Histochem. Cytochem. 2008, 56, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Morinaga, T.; Nakakoshi, M.; Hirao, A.; Imai, M.; Ishibashi, K. Mouse aquaporin 10 gene (AQP10) is a pseudogene. Biochem. Biophys. Res. Commun. 2002, 294, 630–634. [Google Scholar] [CrossRef]
- Hess, R.A. Oestrogen in fluid transport in efferent ducts of the male reproductive tract. Rev. Reprod. 2000, 5, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Jarow, J.P.; Chen, H.; Rosner, W.; Trentacoste, S.; Zirkin, B.R. Assessment of the androgen environment within the human testis: Minimally invasive method to obtain intratesticular fluid. J. Androl. 2001, 22, 640–645. [Google Scholar] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Maggio, A.; Bernardino, R.L.; Gena, P.; Alves, M.G.; Oliveira, P.F.; Calamita, G. A Stopped-Flow Light Scattering Methodology for Assessing the Osmotic Water Permeability of Whole Sertoli Cells. Methods Mol. Biol. 2018, 1748, 279–286. [Google Scholar] [PubMed]
- Campos, E.; Moura, T.F.; Oliva, A.; Leandro, P.; Soveral, G. Lack of Aquaporin 3 in bovine erythrocyte membranes correlates with low glycerol permeation. Biochem. Biophys. Res. Commun. 2011, 408, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Gena, P.; Méndez-Giménez, L.; Rosito, A.; Valentí, V.; Rotellar, F.; Sola, I.; Moncada, R.; Silva, C.; Svelto, M. Reduced hepatic aquaporin-9 and glycerol permeability are related to insulin resistance in non-alcoholic fatty liver disease. Int. J. Obes. 2014, 38, 1213. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Gena, P.; Ferri, D.; Rosito, A.; Rojek, A.; Nielsen, S.; Marinelli, R.A.; Frühbeck, G.; Svelto, M. Biophysical assessment of aquaporin-9 as principal facilitative pathway in mouse liver import of glucogenetic glycerol. Biol. Cell 2012, 104, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Ma, T.; Verkman, A. Erythrocyte water permeability and renal function in double knockout mice lacking aquaporin-1 and aquaporin-3. J. Biol. Chem. 2001, 276, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Hess, R.A.; Bunick, D.; Lee, K.-H.; Bahr, J.; Taylor, J.A.; Korach, K.S.; Lubahn, D.B. A role for oestrogens in the male reproductive system. Nature 1997, 390, 509. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.S.; Nanjappa, M.K.; Ko, C.; Prins, G.S.; Hess, R.A. Estrogens in male physiology. Physiol. Rev. 2017, 97, 995–1043. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.S.; Turner, K.J.; Fraser, H.M.; Saunders, P.T.; Brown, D.; Sharpe, R.M. Immunoexpression of aquaporin-1 in the efferent ducts of the rat and marmoset monkey during development, its modulation by estrogens, and its possible role in fluid resorption. Endocrinology 1998, 139, 3935–3945. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Soler, N.; Isnard-Bagnis, C.; Herak-Kramberger, C.; Sabolic, I.; Van Hoek, A.; Brown, D.; Breton, S. Expression of aquaporin 9 in the adult rat epididymal epithelium is modulated by androgens. Biol. Reprod. 2002, 66, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, R.L.; Costa, A.R.; Martins, A.D.; Silva, J.; Barros, A.; Sousa, M.; Sá, R.; Alves, M.G.; Oliveira, P.F. Estradiol modulates Na+-dependent HCO3- transporters altering intracellular pH and ion transport in human Sertoli cells: A role on male fertility? Biol. Cell 2016, 108, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Bernardino, R.L.; Martins, A.D.; Jesus, T.T.; Sá, R.; Sousa, M.; Alves, M.G.; Oliveira, P.F. Estrogenic regulation of bicarbonate transporters from SLC4 family in rat Sertoli cells. Mol. Cell. Biochem. 2015, 408, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Lebeck, J.; Gena, P.; O’Neill, H.; Skowronski, M.T.; Lund, S.; Calamita, G.; Praetorius, J. Estrogen prevents increased hepatic aquaporin-9 expression and glycerol uptake during starvation. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 302, G365–G374. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.A.; Carnes, K.; França, L.R.; Hermo, L.; Hess, R.A. Aquaporin-1 and -9 are differentially regulated by oestrogen in the efferent ductule epithelium and initial segment of the epididymis. Biol. Cell 2005, 97, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Krzeczunowicz, D.; Ruz, R. Cell specificity of aquaporins 0, 3, and 10 expressed in the testis, efferent ducts, and epididymis of adult rats. J. Androl. 2004, 25, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K. New members of mammalian aquaporins: AQP10–AQP12, in Aquaporins. In Handbook of Experimental Pharmacology; Barrett, J.E., Ed.; Springer: Berlin, Germany, 2009; Volume 190, pp. 251–262. [Google Scholar]
- Madeira, A.; Fernández-Veledo, S.; Camps, M.; Zorzano, A.; Moura, T.F.; Ceperuelo-Mallafré, V.; Vendrell, J.; Soveral, G. Human aquaporin-11 is a water and glycerol channel and localizes in the vicinity of lipid droplets in human adipocytes. Obesity 2014, 22, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Schellenberg, M.; Liu, L.Y.; Dayanandan, B.; Zhang, T.; Mandato, C.A.; Smith, C.E. Membrane domain specificity in the spatial distribution of aquaporins 5, 7, 9, and 11 in efferent ducts and epididymis of rats. J. Histochem. Cytochem. 2008, 56, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.-H.; Cooper, T. Aquaporin AQP11 in the testis: Molecular identity and association with the processing of residual cytoplasm of elongated spermatids. Reproduction 2010, 139, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Eng, F.; Wiebe, J.P.; Alima, L.H. Long-term alterations in the permeability of the blood-testis barrier following a single intratesticular injection of dilute aqueous glycerol. J. Androl. 1994, 15, 311–317. [Google Scholar] [PubMed]
- Chiadak, J.D.; Gena, P.; Gregoire, F.; Bolaky, N.; Delforge, V.; Perret, J.; Calamita, G.; Delporte, C. Lipopolysaccharide Modifies Glycerol Permeability and Metabolism in 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2017, 18, 2566. [Google Scholar] [CrossRef] [PubMed]
- Tsukaguchi, H.; Weremowicz, S.; Morton, C.C.; Hediger, M.A. Functional and molecular characterization of the human neutral solute channel aquaporin-9. Am. J. Physiol. 1999, 277, F685–F696. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.; Escusa, K.; Luna, S.; Tapia, L.; Dofitas, B.; Morales, M. Revisiting oestrogen antagonists (clomiphene or tamoxifen) as medical empiric therapy for idiopathic male infertility: A meta-analysis. Andrology 2013, 1, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Jungwirth, A.; Giwercman, A.; Tournaye, H.; Diemer, T.; Kopa, Z.; Dohle, G.; Krausz, C. European Association of Urology Guidelines on Male Infertility: The 2012 Update. Eur. Urol. 2012, 62, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, S.S.; Tannir, J.; Brannigan, R.E. The metabolic syndrome and male infertility. J. Androl. 2008, 29, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Kirschner, M.A.; Berkowitz, R.; Ertel, N.H. Increased estrogen production in obese men. J. Clin. Endocrinol. Metab. 1979, 48, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Calamita, G.; Perret, J.; Delporte, C. Aquaglyceroporins: Drug targets for metabolic diseases? Front. Physiol. 2018, 9, 851. [Google Scholar] [CrossRef] [PubMed]
- Madeira, A.; Moura, T.F.; Soveral, G. Aquaglyceroporins: Implications in adipose biology and obesity. Cell. Mol. Life Sci. 2015, 72, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.D.; Moreira, A.C.; Sá, R.; Monteiro, M.P.; Sousa, M.; Carvalho, R.A.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Leptin modulates human Sertoli cells acetate production and glycolytic profile: A novel mechanism of obesity-induced male infertility? Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Hofny, E.R.; Ali, M.E.; Abdel-Hafez, H.Z.; Kamal, E.E.-D.; Mohamed, E.E.; El-Azeem, H.G.A.; Mostafa, T. Semen parameters and hormonal profile in obese fertile and infertile males. Fertil. Steril. 2010, 94, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Meneses, M.J.; Silva, B.M.; Sousa, M.; Alves, M.G.; Oliveira, P.F. New insights on hormones and factors that modulate Sertoli cell metabolism. Histol. Histopathol. 2016, 31, 499–513. [Google Scholar] [PubMed]
- Rojek, A.M.; Skowronski, M.T.; Füchtbauer, E.M.; Füchtbauer, A.C.; Fenton, R.A.; Agre, P.; Frøkiær, J.; Nielsen, S. Defective glycerol metabolism in aquaporin 9 (AQP9) knockout mice. Proc. Natl. Acad. Sci. USA 2007, 104, 3609–3614. [Google Scholar] [CrossRef] [PubMed]
- Nicchia, G.P.; Frigeri, A.; Nico, B.; Ribatti, D.; Svelto, M. Tissue distribution and membrane localization of aquaporin-9 water channel: Evidence for sex-linked differences in liver. J. Histochem. Cytochem. 2001, 49, 1547–1556. [Google Scholar] [CrossRef] [PubMed]


| GENE | SEQUENCE 5′-3′ | ANNEALING T° | C |
|---|---|---|---|
| AQP3 (NM_016689.2) | FWD: GGACCCTCATCCTTGTGATGTT RVS: TCGTAGTACAGCCCAAAAACAA | 63 °C | 40 |
| AQP7 (NM_007473.4) | FWD: CTACAGAAGAATATGGTGCGAGA RVS: CAGGAACTGACCCAGCACAT | 63 °C | 40 |
| AQP9 (NM_022026.3) | FWD: CTGAGAAGGACCGAGCCAAG RVS: ATGATGACGCTGAGTTCGTGT | 60 °C | 40 |
| β-2-MICROGLOBULIN (NM_009735.3) | FWD: GCTTCAGTCGTCAGCATGGC RVS: GGATTTCAATGTGAGGCGGGT | 58 °C | 30 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardino, R.L.; Carrageta, D.F.; Silva, A.M.; Calamita, G.; Alves, M.G.; Soveral, G.; Oliveira, P.F. Estrogen Modulates Glycerol Permeability in Sertoli Cells through Downregulation of Aquaporin-9. Cells 2018, 7, 153. https://doi.org/10.3390/cells7100153
Bernardino RL, Carrageta DF, Silva AM, Calamita G, Alves MG, Soveral G, Oliveira PF. Estrogen Modulates Glycerol Permeability in Sertoli Cells through Downregulation of Aquaporin-9. Cells. 2018; 7(10):153. https://doi.org/10.3390/cells7100153
Chicago/Turabian StyleBernardino, Raquel L., David F. Carrageta, Ana M. Silva, Giuseppe Calamita, Marco G. Alves, Graça Soveral, and Pedro F. Oliveira. 2018. "Estrogen Modulates Glycerol Permeability in Sertoli Cells through Downregulation of Aquaporin-9" Cells 7, no. 10: 153. https://doi.org/10.3390/cells7100153
APA StyleBernardino, R. L., Carrageta, D. F., Silva, A. M., Calamita, G., Alves, M. G., Soveral, G., & Oliveira, P. F. (2018). Estrogen Modulates Glycerol Permeability in Sertoli Cells through Downregulation of Aquaporin-9. Cells, 7(10), 153. https://doi.org/10.3390/cells7100153











