Action of the Metalloproteinases in Gonadal Remodeling during Sex Reversal in the Sequential Hermaphroditism of the Teleostei Fish Synbranchus marmoratus (Synbranchiformes: Synbranchidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Preparation for Light Microscopy
Immunohistochemistry for Metalloproteinases (MMPs), 3β-Hidroxysteroid Dehydrogenase(3β-HSD) Enzyme and Proliferating Cell Nuclear Antigen (PCNA)
2.3. Sex Steroid Levels
2.4. Statistical Analysis
3. Results
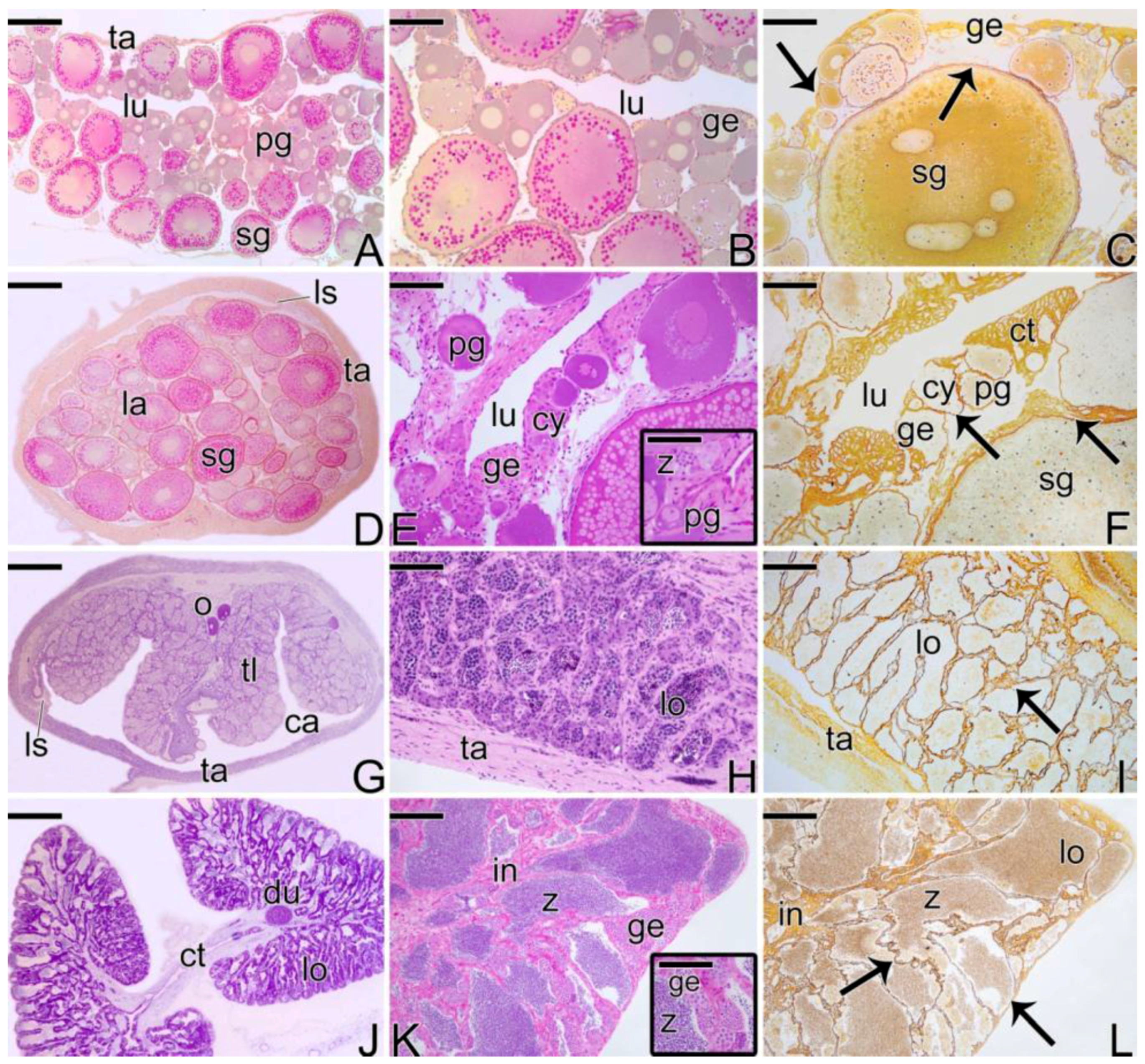
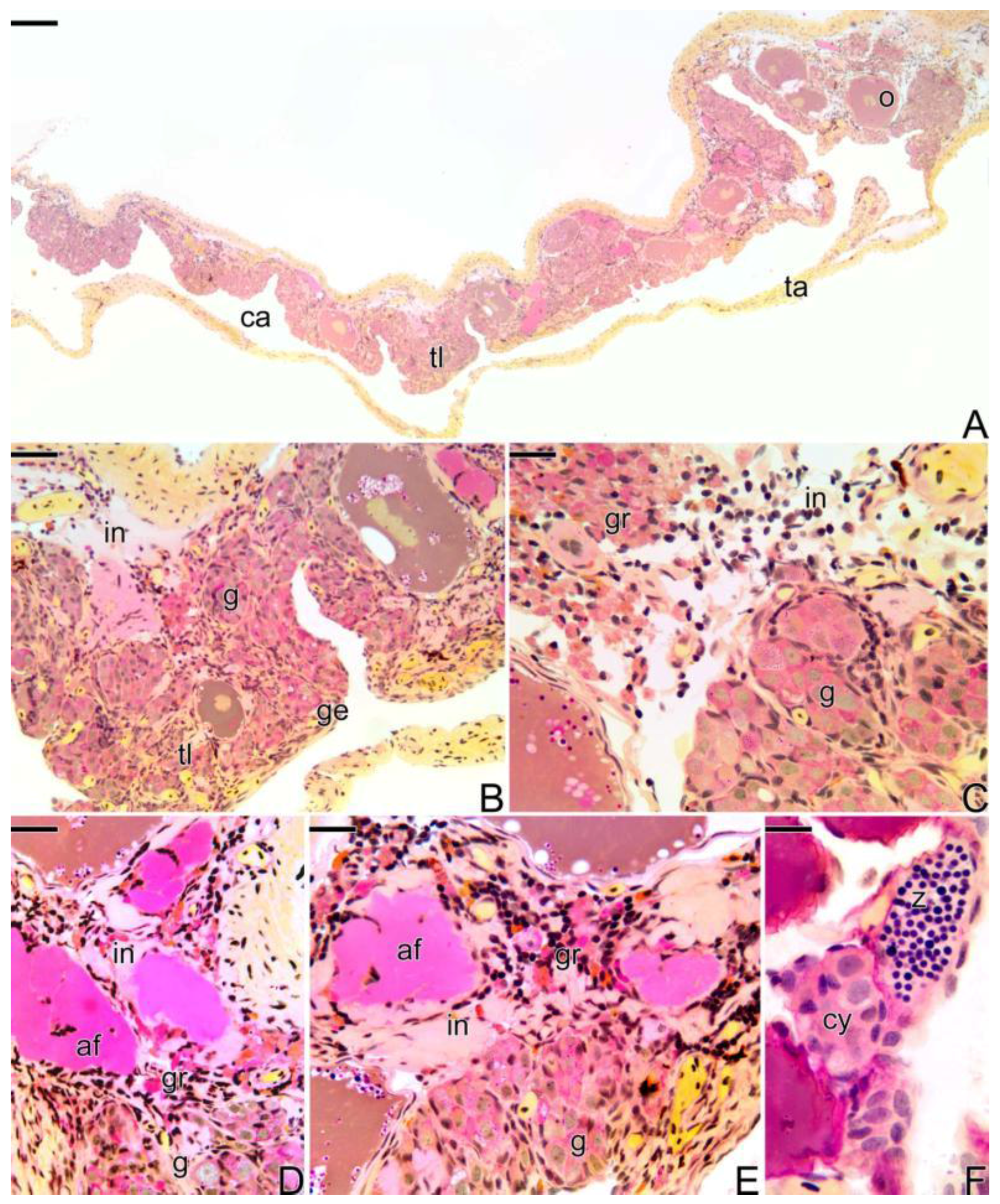
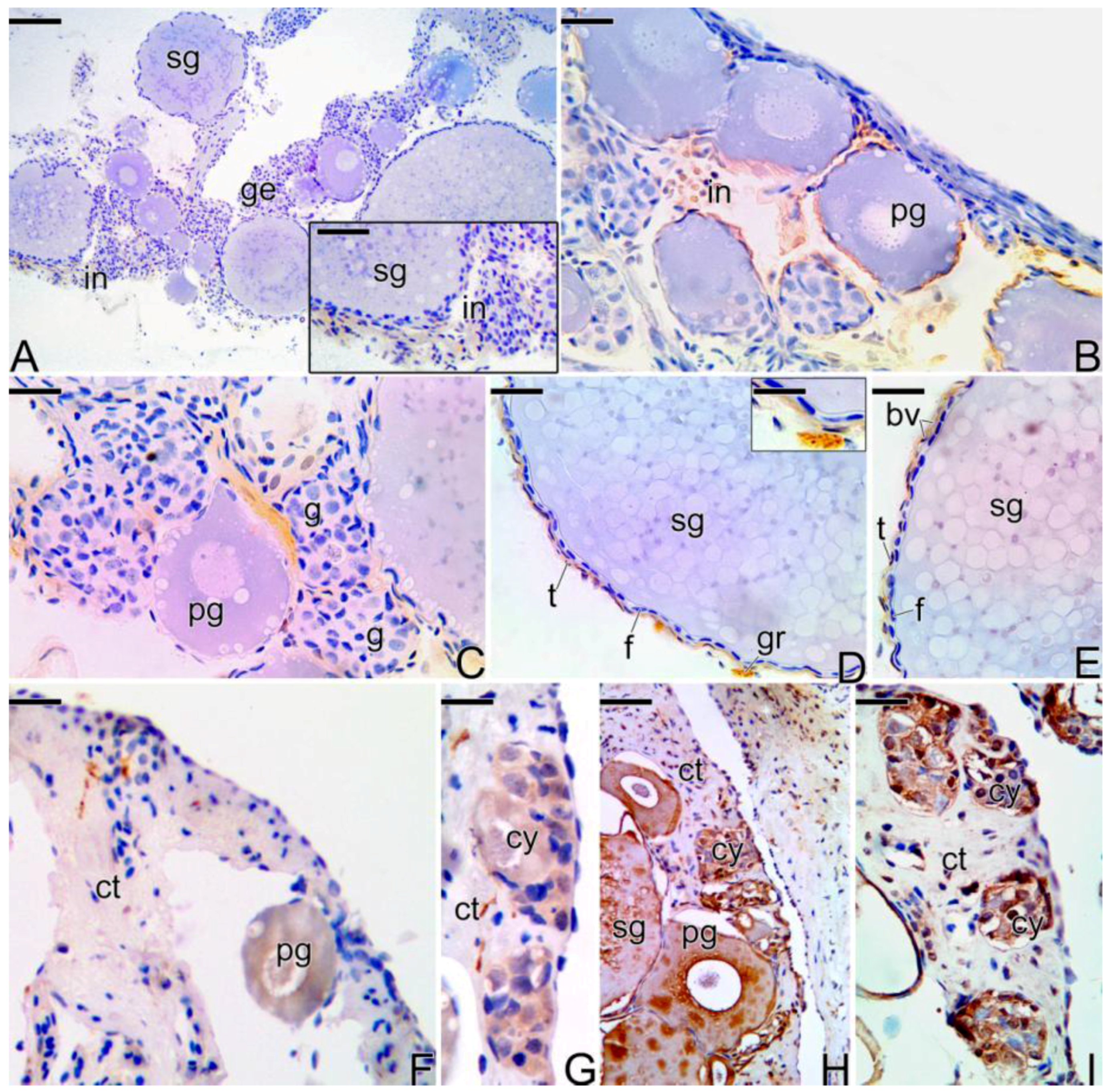
3.1. Gonodal Structure of Synbranchus marmoratus
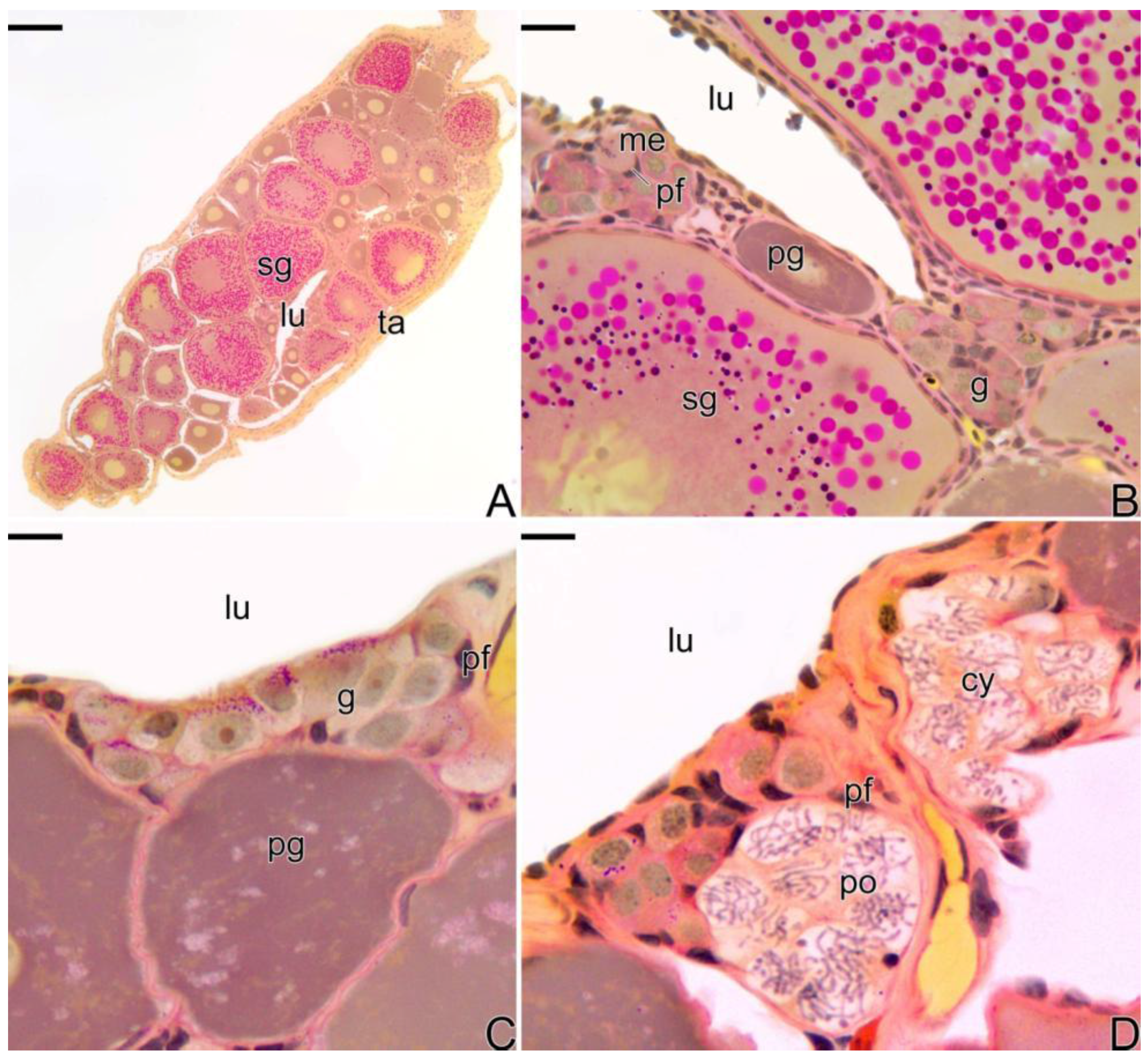
3.2. Female Gonad of Synbranchus marmoratus
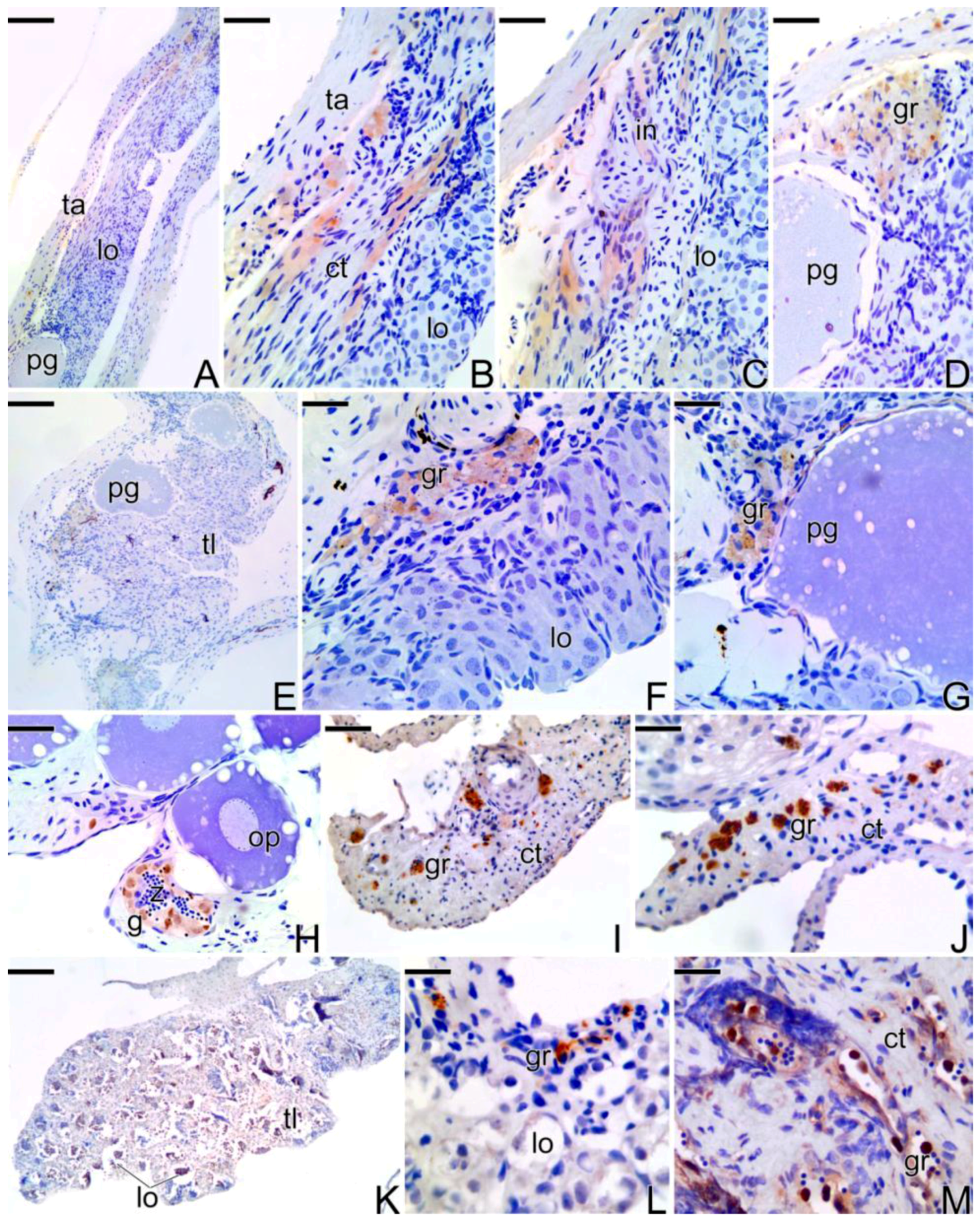
3.3. The Gonadal Remodeling during the Sex Reversal—Formation of the Gonad of the Secondary Male
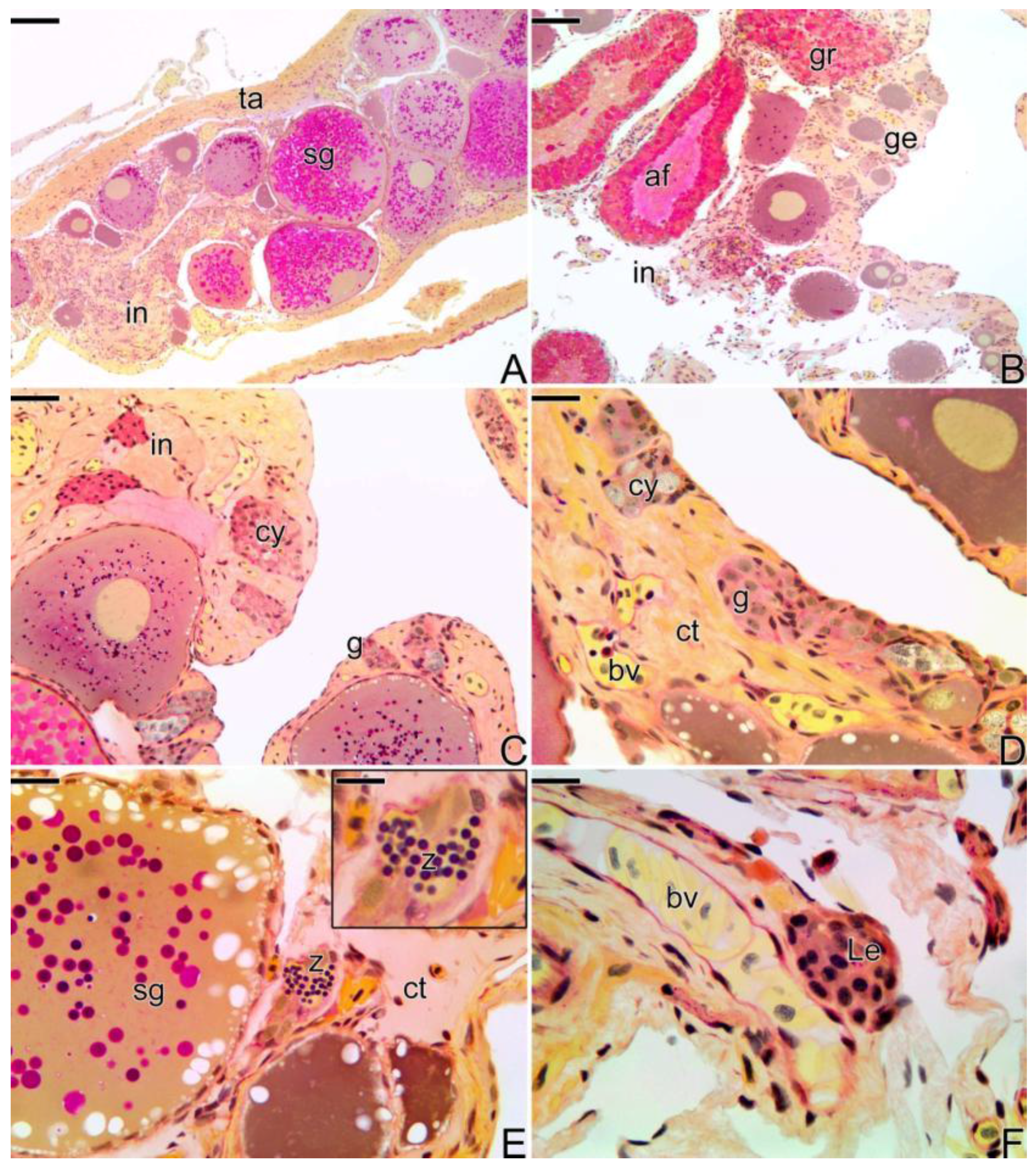
3.3.1. The Female Early Transitional Gonadal Tissue
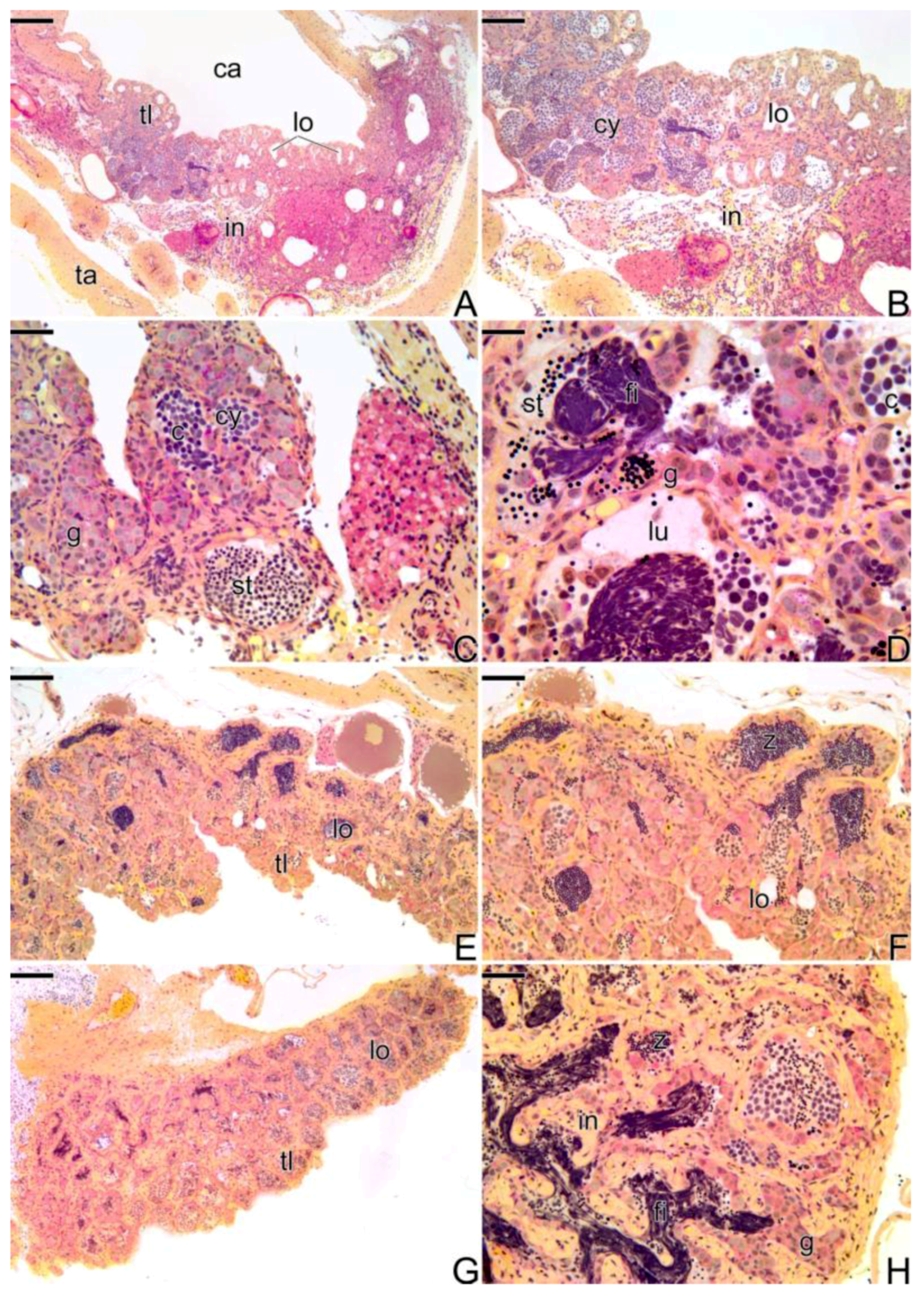
3.3.2. The Female Intermediary Transitional Gonad Tissue
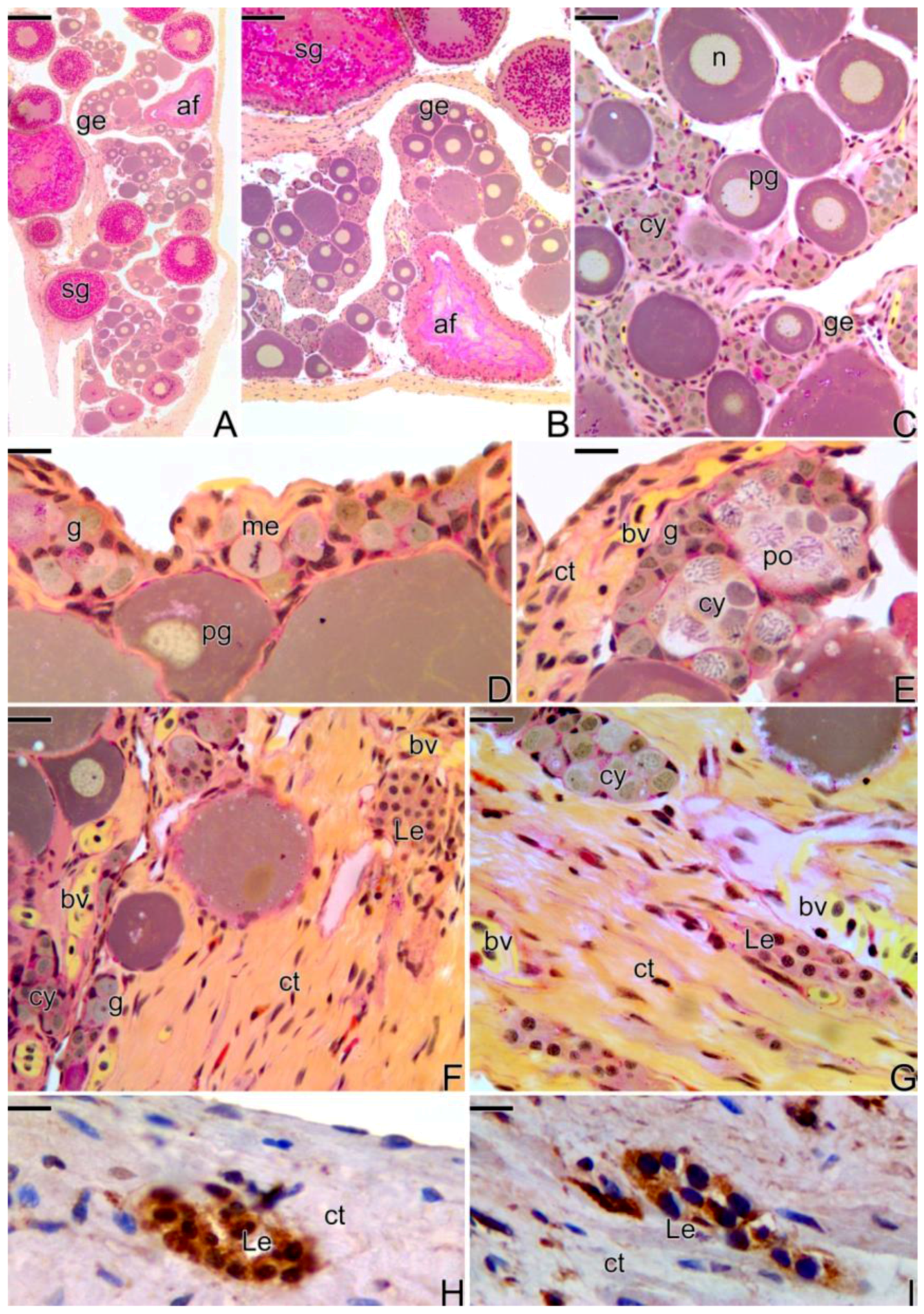
3.3.3. The Final Transitional Gonad—The Intersex
3.3.4. The Gonadal Tissue of the Secondary Male
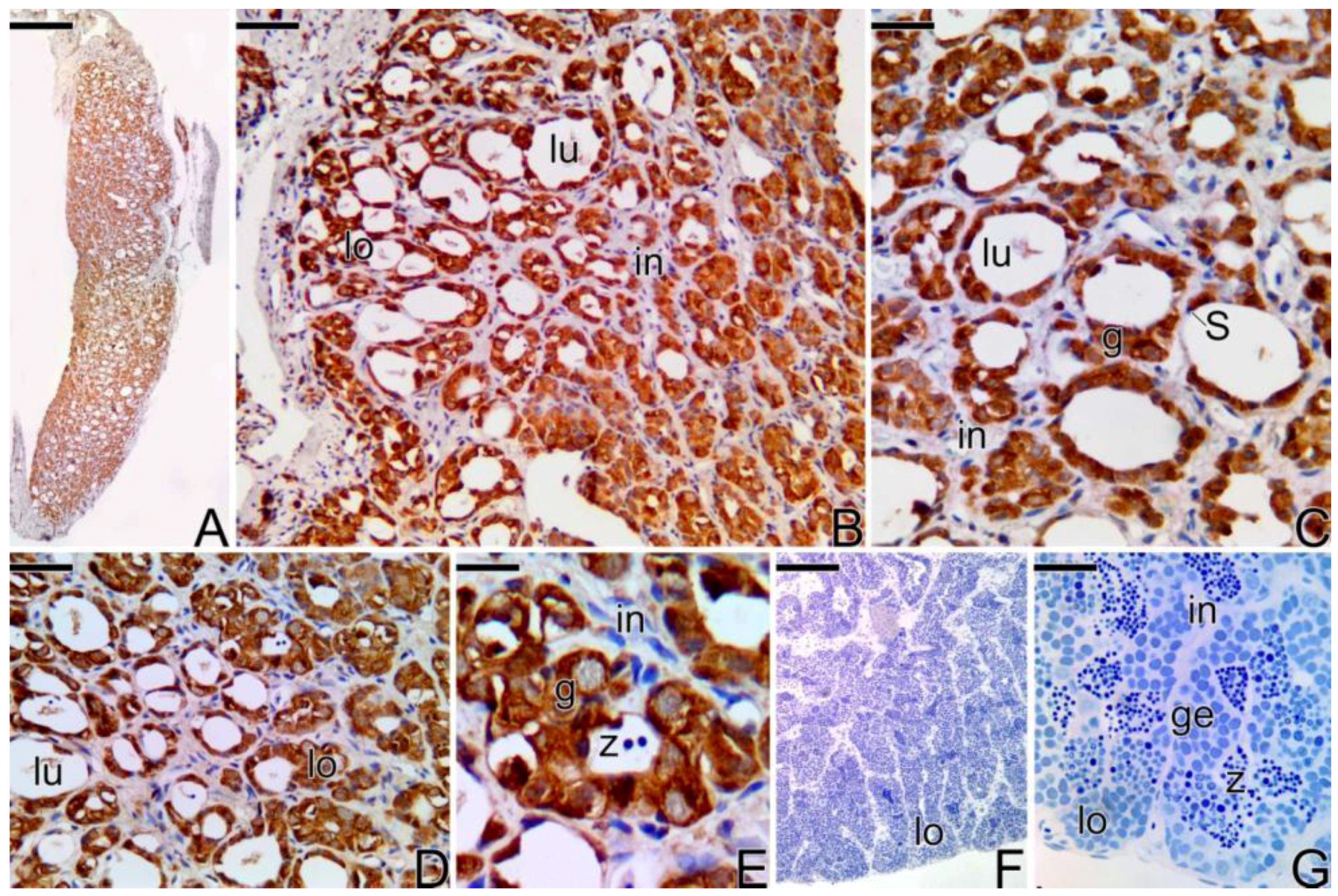
3.3.5. The Male Gonad of Synbranchus marmoratus—Primary Male
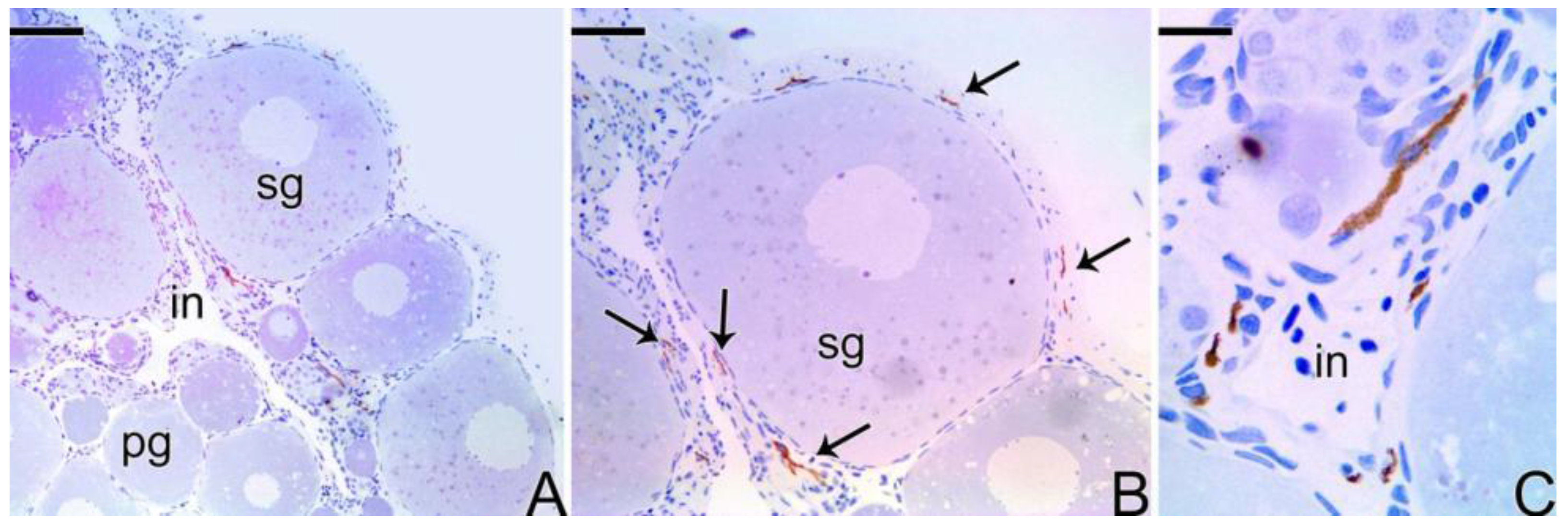
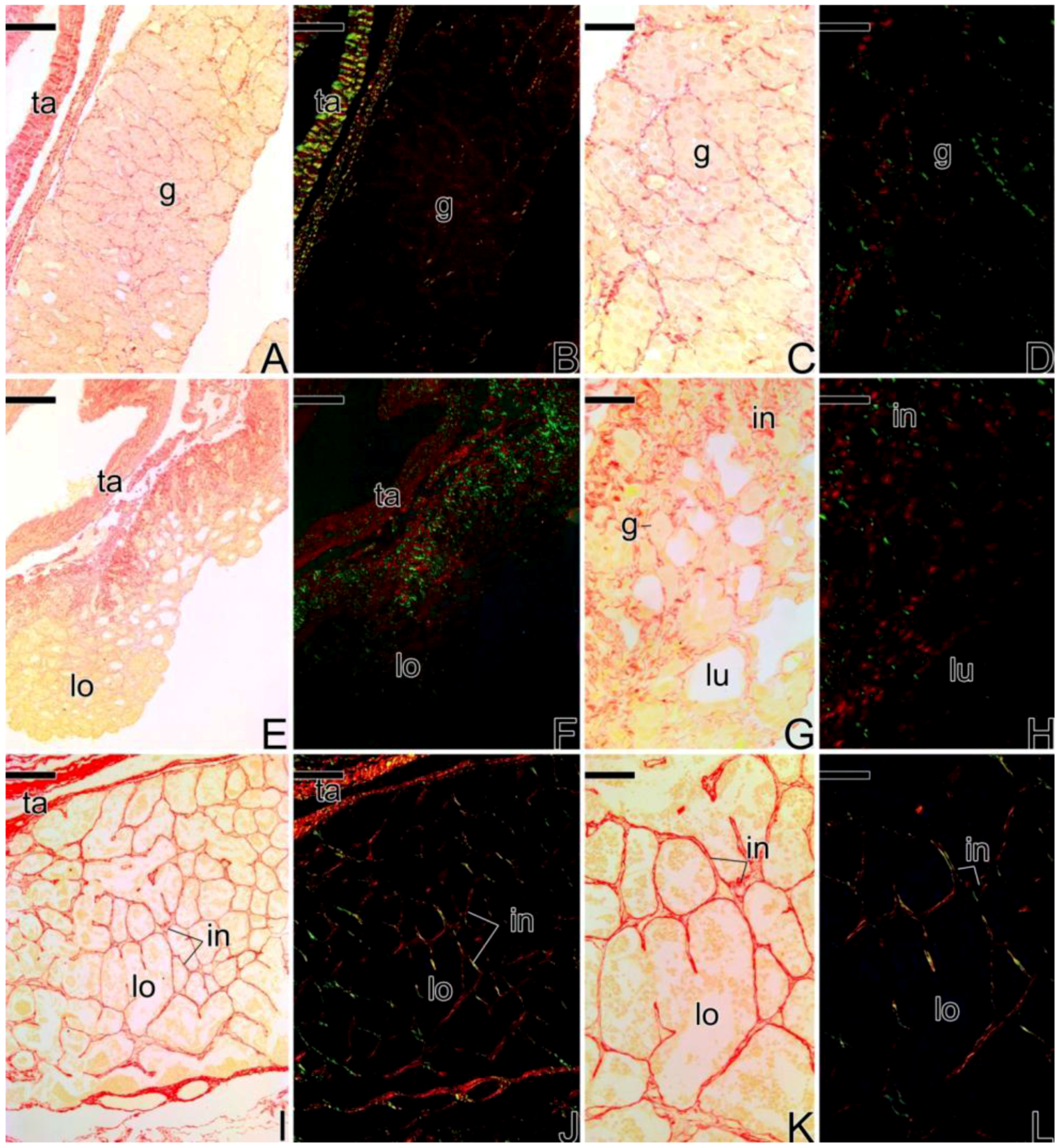
3.4. Detection of the Matrix Metalloproteinases MMP-9, MMP-2 and MMP-14 during the Sex Reversal
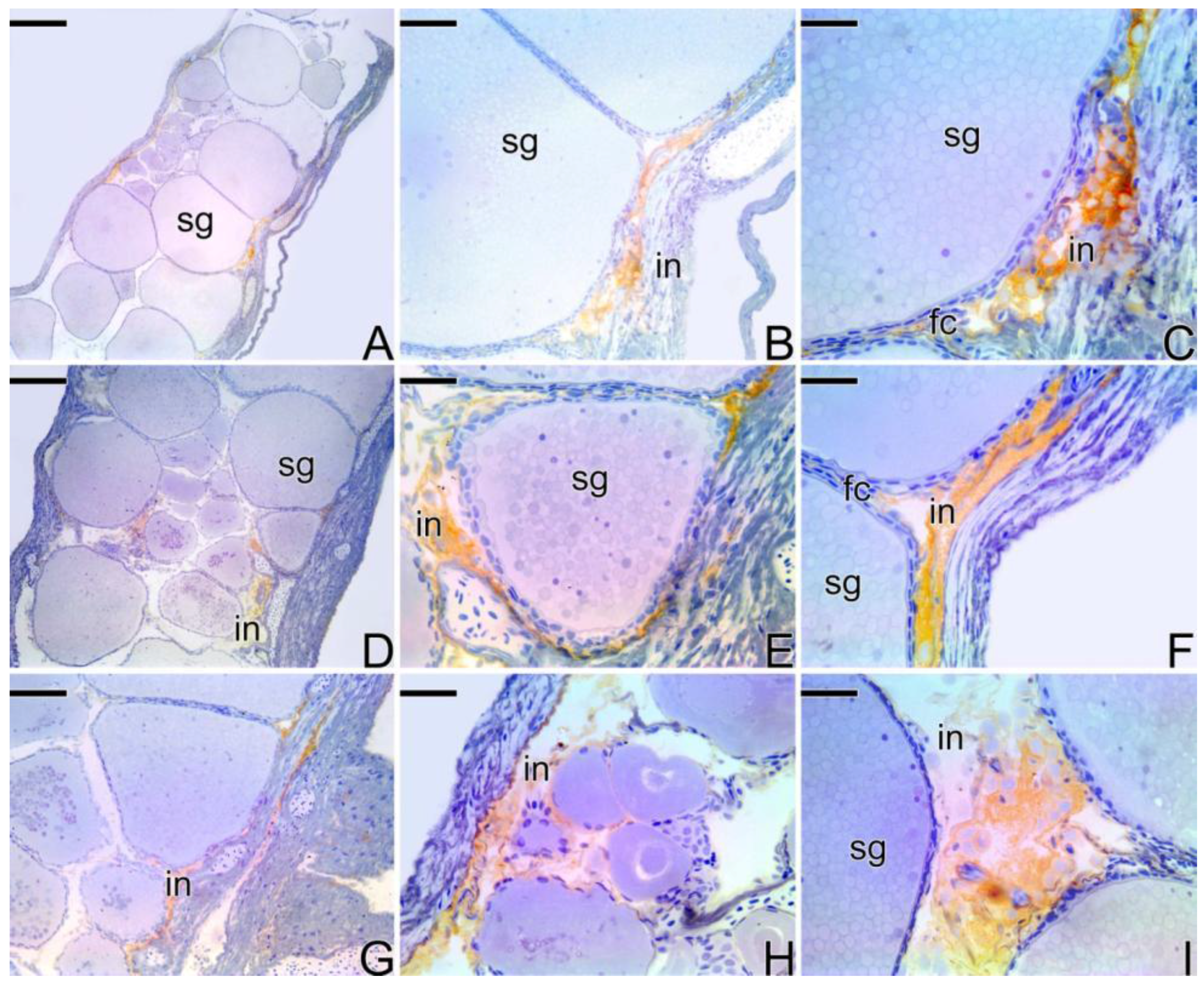
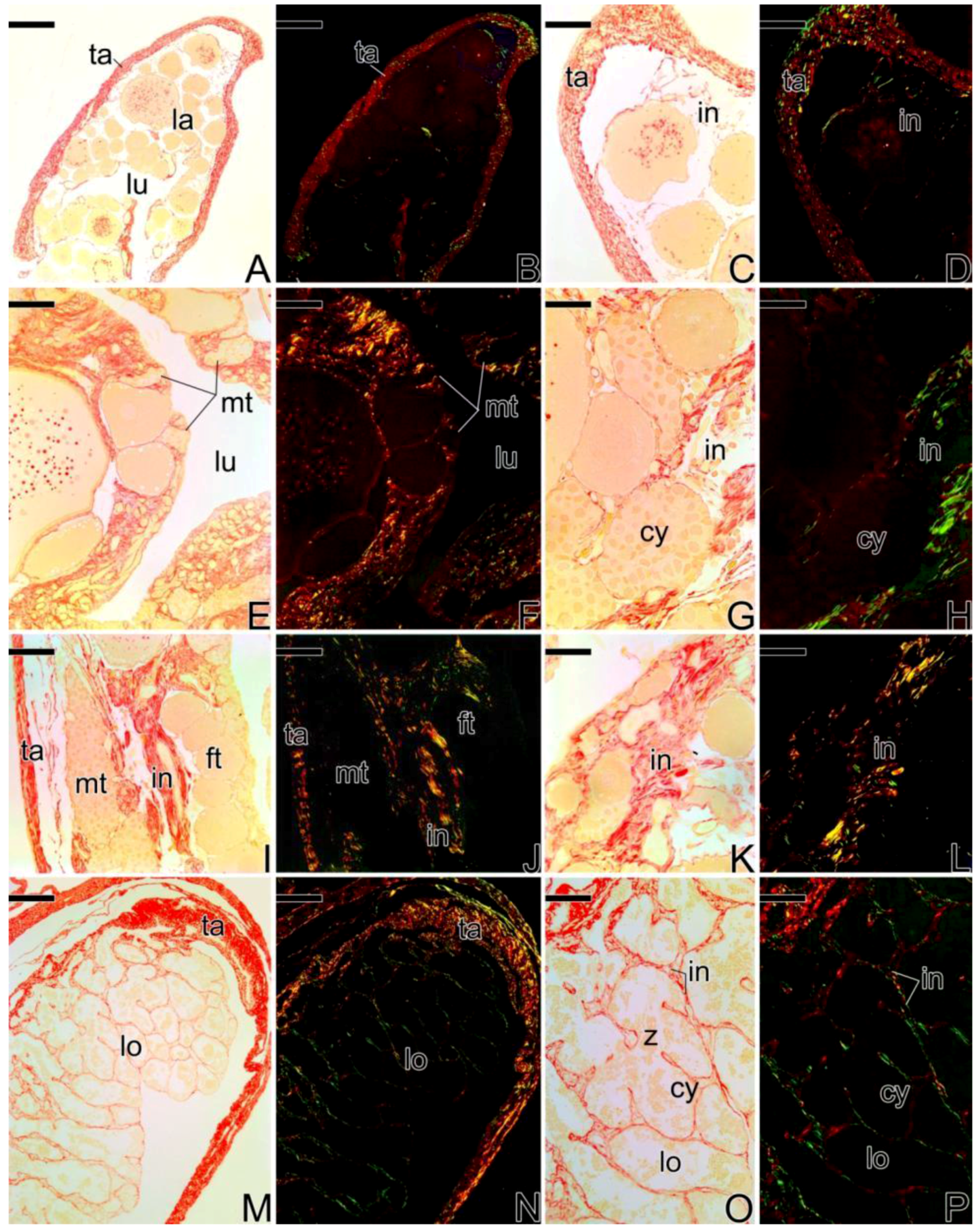
3.5. Remodelation of the Collagen Fibers in Synbranchus marmoratus
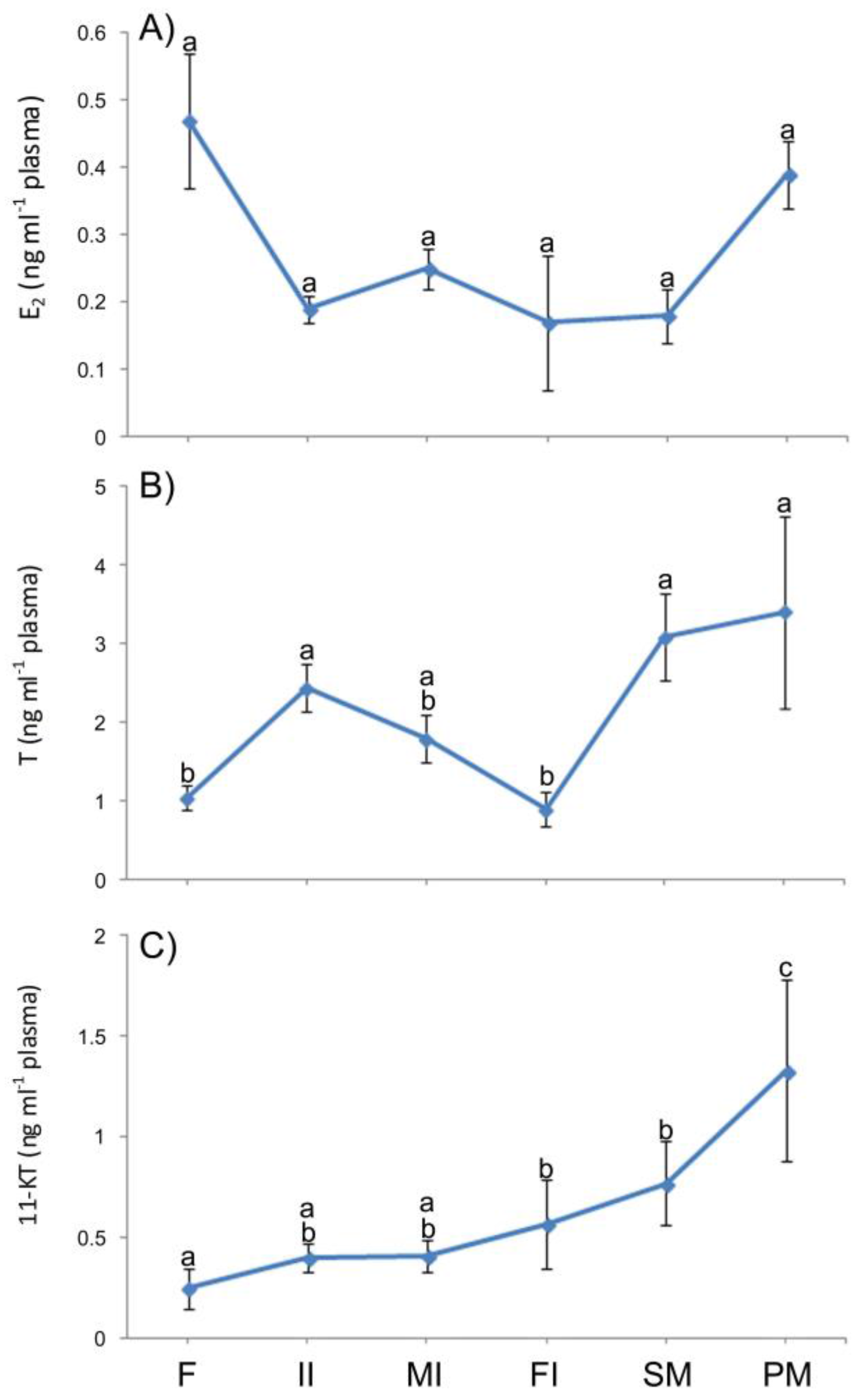
3.6. Plasma Levels of Sexual Steroids in Synbranchus marmoratus
4. Discussion
4.1. Gonadal Remodeling
4.2. The Basement Membrane in the Remodeling of the Germinal Epithelium
4.3. Expression of the Matrix Metalloproteinases Enzymes during the Sex Reversion
4.4. The Sexual Steroids and the Expression of the 3β-Hydroxyterid Dehydrognase (3β-HSD) during the Sex Reversal
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sadovy de Mitcheson, Y.; Liu, M. Functional hermaphroditism in teleosts. Fish Fish. 2008, 9, 1–43. [Google Scholar] [CrossRef]
- Francis, R.C. Sexual lability in teleosts: Developmental factors. Q. Rev. Biol. 1992, 67, 1–17. [Google Scholar] [CrossRef]
- Frisch, A. Sex change and gonadal steroids in sequentially hermaphroditic teleost fish. Rev. Fish Biol. Fish. 2004, 14, 481–499. [Google Scholar] [CrossRef]
- Wu, G.C.; Tomy, S.; Lee, M.F.; Lee, Y.H.; Yueh, W.S.; Lin, C.J.; Lau, E.L.; Chang, C.F. Sex differentiation and sex change in the protandrous black porgy, Acanthopagrus schlegeli. Gen. Comp. Endocrinol. 2010, 167, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.H.; Nagahama, Y. Sex determination and sex differentiation in fish: An overview of genetic, physiological and environmental influences. Aquaculture 2002, 208, 191–364. [Google Scholar] [CrossRef]
- Asoh, K. Gonadal development and diandric protogyny in two populations of Dascyllus reticulatus from Madang, Papua New Guinea. J. Fish Biol. 2005, 66, 1127–1148. [Google Scholar] [CrossRef]
- Liem, K.F. Sex Reversal as a Natural Process in the Synbranchiform Fish Monopterus albus. Copeia 1963, 2, 303–312. [Google Scholar] [CrossRef]
- Liem, K.F. Geographical and taxonomic variation in the pattern of natural sex reversal in the teleost fish order Synbranchiformes. J. Zool. 1968, 156, 225–238. [Google Scholar] [CrossRef]
- Lo Nostro, F.L.; Guerrero, G.A. Presence of primary and secondary males in a population of the protogynous Synbranchus marmoratus. J. Fish Biol. 1996, 49, 788–800. [Google Scholar] [CrossRef]
- Lo Nostro, F.L.; Grier, H.J.; Andreone, L.; Guerrero, G.A. Involvement of the gonadal germinal epithelium during sex reversal and seasonal testicular cycling in the protogynous swamp eel, Synbranchus marmoratus Bloch 1795 (Teleostei, Synbranchidae). J. Morphol. 2003, 257, 107–126. [Google Scholar] [CrossRef] [PubMed]
- Lo Nostro, F.L.; Grier, H.J.; Meijide, F.; Guerrero, G.A. Ultrastructure of the testis in Synbranchus marmoratus (Teleostei, Synbranchidae): The germinal compartment. Tissue Cell 2003, 35, 121–132. [Google Scholar] [CrossRef]
- Antoneli, F.N. Perfil Morfo-Funcional da Inversão de Sexo em Synbranchidae (Teleostei, Synbranchiformes, Synbranchidae). Ph.D. Thesis, Biblioteca do Instituto de Biologia, UNICAMP, Campinas, Brazil, 2006; 167p. [Google Scholar]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World, 5th ed.; Wiley: New York, NY, USA, 2011; 752p, ISBN 978-0471250319. [Google Scholar]
- Barros, N.H.C.; de Souza, A.A.; Peebles, E.B.; Chellappa, S. Dynamics of sex reversal in the marbled swamp eel (Synbranchus marmoratus Bloch, 1795), a diandric hermaphrodite from Marechal Dutra Reservoir, northeastern Brazil. J. Appl. Ichthyol. 2017, 33, 443–449. [Google Scholar] [CrossRef]
- Ravaglia, M.; Lo Nostro, F.L.; Maggese, M.; Guerrero, G.; Somoza, G. Characterization of molecular variants of GnRH, induction of spermiation and sex reversal using sGnRH-A and domperidone in the protogynous diandric fish, Synbranchus marmoratus Bloch, (Teleostei, Synbranchidae). Fish Physiol. Biochem. 1997, 5, 425–436. [Google Scholar] [CrossRef]
- Santana, J.C.; Quagio-Grassiotto, I. Extracellular matrix remodeling of the testes through the male reproductive cycle in Teleostei fish. Fish Physiol. Biochem. 2014, 40, 1863–1875. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, T.S.; Grier, H.J.; Quagio-Grassiotto, I. The basement membrane and the sex establishment in the juvenile hermaphroditism during gonadal differentiation of the Gymnocorymbus ternetzi (Teleostei: Characiformes: Characidae). Anat. Rec. 2015, 298, 1984–2010. [Google Scholar] [CrossRef] [PubMed]
- Hulboy, D.L.; Rudolph, L.A.; Matrisian, L.M. Matrix metalloproteinases as mediators of reproductive function. Mol. Hum. Reprod. 1997, 3, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Khokha, R.; Murthy, A.; Weiss, A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Birkedal-Hansen, H.; Moore, W.G.I.; Bodden, M.K. Matrix metalloproteinases: A review. Crit. Rev. Oral Biol. Med. 1993, 4, 197–250. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Martins, V.L.; Caley, M.; O’Toole, E.A. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 2013, 351, 55–268. [Google Scholar] [CrossRef] [PubMed]
- Longin, J.; Guillaumot, P.; Chauvin, M.A.; Morera, A.M.; Le Magueresse-Battistoni, B. MT1-MMP in rat testicular development and the control of Sertoli cell proMMP-2 activation. J. Cell Sci. 2001, 114, 2125–2134. [Google Scholar] [PubMed]
- Hägglund, A.C.; Ny, A.; Leonardsson, G.; Ny, T. Regulation and localization of matrix metalloproteinases and tissue inhibitors of metalloproteinases in the mouse ovary during gonadotropin-induced ovulation. Endocrinology 1999, 140, 4351–4358. [Google Scholar] [CrossRef] [PubMed]
- Piprek, R.P.; Kolasa, M.; Podkowa, D.; Kloc, M.; Kubiak, J.Z. Transcriptional profiling validates involvement of extracellular matrix and proteinases genes in mouse gonad development. Mech. Dev. 2018, 149, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Pandian, T.J. Endocrine Sex Differentiation in Fish; CRC Press: Boca Raton, FL, USA, 2013; 299p, ISBN 9781466575608. [Google Scholar]
- Pudney, J. Leydig and Sertoli Cells Nonmmamalian. In Encyclopedia of Reproduction; Knobil, E., Skinner, M.A., Neill, J.D., Eds.; Academic Press: London, UK, 1998; pp. 1008–1021. ISBN 9780122270208. [Google Scholar]
- Payne, A.H.; Hardy, M.P.; Russell, L.D. The Leydig Cell; Cache River Press: Vienna, Austria, 1996; 802p, ISBN 9781588297549. [Google Scholar]
- Guraya, S.S. Comparative Cellular and Molecular Biology of Testis in Vertebrates: Trends in Endocrine, Paracrine and Autocrine Regulation of Structure and Functions; Science Publishers Inc.: Enfield, Australia, 2001; 91p, ISBN 1578081653. [Google Scholar]
- Pudney, J. Comparative Cytology of the Leydig Cell. In The Leydig Cell; Payne, A.H., Hardy, M.P., Russel, L.D., Eds.; Cache River Press: Vienna, Austria, 1996; pp. 98–142. ISBN 9781588297549. [Google Scholar]
- Loir, M. Trout steroidogenic testicular cells in primary culture. II Steroidogenic activity of interstitial cells, Sertoli cells and spermatozoa. Gen. Comp. Endocrinol. 1990, 78, 388–398. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nakamura, M.; Kajiura-Kobayashi, H.; Young, G.; Nagahama, Y. Immunolocalization of steroidogenic enzymes (P450scc, P450arom and 3β-HSD) in immature and mature testes of rainbow trout (Oncorhynchus mykiss). Cell Tissue Res. 1998, 292, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Ijiri, S.; Takei, N.; Kazeto, Y.; Todo, T.; Adachi, S.; Yamauchi, K. Changes in localization of cytochrome P450 cholesterol side-chain cleavage (P450scc) in Japanese eel testis and ovary during gonadal development. Gen. Comp. Endocrinol. 2006, 145, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Nóbrega, R.H.; Quagio-Grassiotto, I. Morphofunctional changes in Leydig cells throughout the continuous spermatogenesis of the freshwater teleost fish, Serrasalmus spilopleura (Characiformes, Characidae): An ultrastructural and enzyme study. Cell Tissue Res. 2007, 329, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Lo Nostro, F.L.; Antoneli, F.N.; Quagio-Grassiotto, I.; Guerrero, G.A. Testicular interstitial cells and steroidogenic detection in the protogynous fish, Synbranchus marmoratus (Teleostei, Synbranchidae). Tissue Cell 2004, 36, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Hunter, I.; Grier, H.; Muscato, M. Enhancement of histological detail using Metanil Yellow as counterstain in periodic acid/Schiff’s hematoxylin staining of glycol methacrylate tissue sections. Biotech. Histochem. 1991, 66, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Vidal, B.C. Histochemical and anisotropical properties characteristics of silver impregnation: The differentiation of reticulin fibers from the other interstitial collagens. Zool. J. Anat. 1988, 117, 485–494. [Google Scholar]
- Junqueira, L.C.U.; Bignolas, G.; Brentani, R.R. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem. J. 1979, 11, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Yamashita, M.; Kitano, T.; Iguchi, T. Oocyte apoptosis during the transition from ovary-like tissue to testes during sex differentiation of juvenile zebrafish. J. Exp. Biol. 2002, 205, 711–718. [Google Scholar] [PubMed]
- Balch, G.C.; Mackenzie, C.A.; Metcalfe, C.D. Alterations to gonadal development and reproductive success in Japanese medaka (Oryzias latipes) exposed to 17alphaethinylestradiol. Environ. Toxicol. Chem. 2004, 23, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.R.; Krieger, H.O. Histological Analysis of Endocrine Disruptive Effects in Small Laboratory Fish; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2009; 319p, ISBN 9780471763581. [Google Scholar]
- Chaves-Pozo, E.; Liarte, S.; Mulero, I.; Abellán, E.; Meseguer, J.; García-Ayala, A. Early presence of immune cells in the developing gonad of the gilthead seabream (Sparus aurata Linnaeus, 1758). J. Reprod. Dev. 2009, 55, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Pozo, E.; Castillo-Briceño, P.; García-Alcázar, A.; Meseguer, J.; Mulero, V.; García-Ayala, A. A role for matrix metalloproteinases in granulocyte infiltration and testicular remodelation in a seasonal breeding teleost. Mol. Immunol. 2008, 45, 2820–2830. [Google Scholar] [CrossRef] [PubMed]
- Crouch, E.C.; Martin, G.R.; Brody, J.S.; Laurie, G.W. Basement membrane. In The Lung; Crystal, R.G., West, J.B., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1997; Volume 1, pp. 769–791. ISBN 0397516320. [Google Scholar]
- Domogatskaya, A.; Rodin, S.; Tryggvason, K. Functional diversity of laminins. Annu. Rev. Cell. Dev. Biol. 2012, 28, 523–553. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, T.S.; Grier, H.J.; Quagio-Grassiotto, I. Germline cysts and the formation of the germinal epithelium during the female gonadal morphogenesis in Cyprinus carpio (Teleostei: Ostariophysi). Anat. Rec. 2010, 293, 1581–1606. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y. The functional morphology of teleost gonads. In Fish Physiology; Hoar, W.S., Randall, D.J., Donaldson, E.M., Eds.; Academic Press: New York, NY, USA, 1983; Volume 9, pp. 223–275. ISBN 9780080585338. [Google Scholar]
- Pudney, J. Comparative cytology of the non-mammalian vertebrate Sertoli cell. In The Sertoli Cell; Russell, L.D., Griswold, M.D., Eds.; Cache River Press: Clearwater, FL, USA, 1993; pp. 612–657. [Google Scholar]
- Meijide, F.J.; Lo Nostro, F.; Guerrero, G.A. Gonadal Development and Sex Differentiation in the Cichlid Fish Cichlasoma dimerus (Teleostei, Perciformes): A Light- and Electron-Microscopic Study. J. Morphol. 2005, 264, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Schulz, R.W.; França, L.R.; Lareyre, J.J.; LeGac, F.; Chiarini-Garcia, H.; Nóbrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef] [PubMed]
- Grier, H.J.; Neidig, C.L.; Quagio-Grassiotto, I. Development and fate of the postovulatory follicle complex, postovulatory follicle and observations on folliculogenesis and oocyte atresia in ovulated common snook, Centropomus undecimalis (Bloch, 1792). J. Morphol. 2017, 278, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Piprek, R.P.; Kloc, M.; Tassane, J.P.; Kubiak, J.Z. Development of Xenopus laevis bipotential gonads into testis or ovary is driven by sex-specific cell-cell interactions, proliferation rate, cell migration and deposition of extracellular matrix. Dev. Biol. 2017, 432, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Lukashev, M.E.; Werb, Z. Ecmsignalling: Orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998, 8, 437–441. [Google Scholar] [CrossRef]
- Hipps, D.S.; Hembry, R.M.; Docherty, A.J.; Reynolds, J.J.; Murphy, G. Purification and characterization of human 72-kDa gelatinase (type IV collagenase). Use of immunolocalisation to demonstrate the non-coordinate regulation of the 72-kDa and 95-kDa gelatinases by human fibroblasts. Biol. Chem. Hoppe Seyler 1991, 372, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ge, R.S.; Zirkin, B.R. Leydig cells: From stem cells to aging. Mol. Cell. Endocrinol. 2009, 306, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Chang, X.T.; Nakamura, M.; Kajiura, H.; Nagahama, Y. Fish 3β-Hydroxysteroid Dehydrogenase/Δ5-Δ4Isomerase: Antibody Production and Their Use for the Immunohistochemical Detection of Fish Steroidogenic Tissues. Zool. Sci. 1996, 13, 909–914. [Google Scholar] [CrossRef]
- Mazzoni, T.S.; Grier, H.J.; Quagio-Grassiotto, I. Male gonadal differentiation and the paedomorphic evolution of the testis in Teleostei. Anat. Rec. 2014, 297, 1137–1162. [Google Scholar] [CrossRef] [PubMed]
- Nagahama, Y. Endocrine regulation of gametogenesis in fish. Int. J. Dev. Biol. 1994, 38, 217–229. [Google Scholar] [PubMed]
- Schulz, R.W.; Miura, T. Spermatogenesis and its endocrine regulation. Fish Physiol. Biochem. 2002, 26, 43–56. [Google Scholar] [CrossRef]
- Connaughton, M.A.; Aida, K. Female reproductive system fish. In Encyclopedia of Reproduction; Krobil, E., Neill, J.D., Eds.; Academic Press: San Diego, CA, USA, 1998; pp. 193–205. ISBN 9780122270208. [Google Scholar]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzoni, T.S.; Lo Nostro, F.L.; Antoneli, F.N.; Quagio-Grassiotto, I. Action of the Metalloproteinases in Gonadal Remodeling during Sex Reversal in the Sequential Hermaphroditism of the Teleostei Fish Synbranchus marmoratus (Synbranchiformes: Synbranchidae). Cells 2018, 7, 34. https://doi.org/10.3390/cells7050034
Mazzoni TS, Lo Nostro FL, Antoneli FN, Quagio-Grassiotto I. Action of the Metalloproteinases in Gonadal Remodeling during Sex Reversal in the Sequential Hermaphroditism of the Teleostei Fish Synbranchus marmoratus (Synbranchiformes: Synbranchidae). Cells. 2018; 7(5):34. https://doi.org/10.3390/cells7050034
Chicago/Turabian StyleMazzoni, Talita Sarah, Fabiana Laura Lo Nostro, Fernanda Natália Antoneli, and Irani Quagio-Grassiotto. 2018. "Action of the Metalloproteinases in Gonadal Remodeling during Sex Reversal in the Sequential Hermaphroditism of the Teleostei Fish Synbranchus marmoratus (Synbranchiformes: Synbranchidae)" Cells 7, no. 5: 34. https://doi.org/10.3390/cells7050034



