Nerve, Muscle, and Synaptogenesis
Abstract
1. Introduction
2. The Agrin Hypothesis
2.1. Historical Development of the Model
2.2. Challenge to the Model
2.3. Revised Model for Agrin
3. Synapse Formation: Two Mechanisms—Agrin and Prepatterning
3.1. Postsynaptic Prepatterning and Synapse Formation
Refinement of the Prepattern: Implications for A Unitary Molecular Mechanism
3.2. Agrin—Stabilizer, Inducer, or Both?
3.2.1. Agrin as Stabilizer
3.2.2. Agrin as Inducer
Early Studies: Issues of Interpretation
Agrin in the Amphibian In Vitro System
Neuronal Agrin as Basal Lamina Constituent: Implications for Function
Time Course of Agrin Deposition during Synaptogenesis in Living Cultures
Behavior of AChR Clusters after Denervation
The Amphibian Culture System: Conclusions, Caveats, and Recommendations
4. Synapse Induction: Alternatives to Agrin
4.1. Ligand Receptor Interactions
4.2. Wnt Signaling
4.3. Focal Pericellular Proteolysis
4.3.1. Proteinases as Modulators of Nervous System Processes
4.3.2. Proteolysis as Mediator of Synapse Maintenance
4.4. Integrin Signaling
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Langley, J.N. On the reaction of cells and of nerve-endings to certain poisons, chiefly as regards the reaction of striated muscle to nicotine and to curari. J. Physiol. 1905, 33, 374–413. [Google Scholar] [CrossRef] [PubMed]
- Dale, H.H.; Feldberg, W.; Vogt, M. Release of acetylcholine at voluntary motor nerve endings. J. Physiol. 1936, 86, 353–380. [Google Scholar] [CrossRef]
- Katz, B. The Release of Neural Transmitter Substances; Charles, C., Ed.; Thomas: Springfield, IL, USA, 1969. [Google Scholar]
- Twain, M. The Celebrated Jumping Frog of Calaveras County; Filter Press: Palmer Lake, CO, USA, 1965. [Google Scholar]
- Gwyn, D.G.; Aitken, J.T. The formation of new motor endplates in mammalian skeletal muscle. J. Anat. 1996, 100 Pt 1, 111–126. [Google Scholar]
- Fex, S.; Sonesson, B.; Thesleff, S.; Zelená, J. Nerve implants in botulinum poisoned mammalian muscle. J. Physiol. 1966, 184, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Miledi, R. Formation of extra nerve-muscle junctions in innervated muscle. Nature 1963, 199, 1191–1192. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Cohen, M.W. Nerve-induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J. Physiol. 1977, 268, 757–773. [Google Scholar] [CrossRef]
- Frank, E.; Fischbach, G.D. Early events in neuromuscular junction formation in vitro: Induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J. Cell Biol. 1979, 83, 143–158. [Google Scholar] [CrossRef]
- Ziskind-Conhaim, L.; Geffen, I.; Hall, Z.W. Redistribution of acetylcholine receptors on developing rat myotubes. J. Neurosci. 1984, 4, 2346–2349. [Google Scholar] [CrossRef]
- Sanes, J.R.; Marshall, L.M.; McMahan, U.J. Reinnervation of muscle fiber basal lamina after removal of myofibers. Differentiation of regenerating axons at original synaptic sites. J. Cell Biol. 1978, 78, 176–198. [Google Scholar] [CrossRef]
- Burden, S.J.; Sargent, P.B.; McMahan, U. Acetylcholine receptors in regenerating muscle accumulate at original synaptic sites in the absence of the nerve. J. Cell Biol. 1979, 182, 412–425. [Google Scholar] [CrossRef]
- McMahan, U.J. The agrin hypothesis. Cold Spring Harb. Symp. Quant. Biol. 1990, 55, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J. Nerve-induced remodeling of muscle basal lamina during synaptogenesis. J. Cell Biol. 1986, 102, 863–877. [Google Scholar] [CrossRef] [PubMed]
- Nitkin, R.M.; Smith, M.A.; Magill, C.; Fallon, J.R.; Yao, Y.M.; Wallace, B.G.; McMahan, U.J. Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J. Cell Biol. 1987, 105, 2471–2478. [Google Scholar] [CrossRef] [PubMed]
- Magill-Solc, C.; McMahan, U.J. Motor neurons contain agrin-like molecules. J. Cell Biol. 1988, 107, 1825–1833. [Google Scholar] [CrossRef]
- Magill-Solc, C.; McMahan, U.J. Synthesis and transport of agrin-like molecules in motor neurons. J. Exp. Biol. 1990, 153, 1–10. [Google Scholar]
- Rupp, F.; Payan, D.G.; Magill-Solc, C.; Cowan, D.M.; Scheller, R.H. Structure and expression of a rat agrin. Neuron 1991, 6, 811–823. [Google Scholar] [CrossRef]
- Tsim, K.W.; Ruegg, M.A.; Escher, G.; Kröger, S.; McMahan, U.J. cDNA that encodes active agrin. Neuron 1992, 8, 677–689. [Google Scholar] [CrossRef]
- Smith, M.A.; Magill-Solc, C.; Rupp, F.; Yao, Y.M.; Schilling, J.W.; Snow, P.; McMahan, U.J. Isolation and characterization of a cDNA that encodes an agrin homolog in the marine ray. Mol. Cell. Neurosci. 1992, 3, 406–417. [Google Scholar] [CrossRef]
- Jennings, C.G.; Dyer, S.M.; Burden, S.J. Muscle-specific trk-related receptor with a kringle domain defines a distinct class of receptor tyrosine kinases. Proc. Natl. Acad. Sci. USA 1993, 90, 2895–2899. [Google Scholar] [CrossRef]
- Valenzuela, D.M.; Stitt, T.N.; DiStefano, P.S.; Rojas, E.; Mattsson, K.; Compton, D.L.; Nuñez, L.; Park, J.S.; Stark, J.L.; Gies, D.R.; et al. Receptor tyrosine kinase specific for the skeletal muscle lineage: Expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron 1995, 15, 573–584. [Google Scholar] [CrossRef]
- Gautam, M.; Noakes, P.G.; Moscoso, L.; Rupp, F.; Scheller, R.H.; Merlie, J.P.; Sanes, J.R. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell 1996, 85, 525–535. [Google Scholar] [CrossRef]
- Burgess, R.W.; Nguyen, Q.T.; Son, Y.J.; Lichtman, J.W.; Sanes, J.R. Alternatively spliced isoforms of nerve- and muscle-derived agrin: Their roles at the neuromuscular junction. Neuron 1999, 23, 33–44. [Google Scholar] [CrossRef]
- DeChiara, T.M.; Bowen, D.C.; Valenzuela, D.M.; Simmons, M.V.; Poueymirou, W.T.; Thomas, S.; Kinetz, E.; Compton, D.L.; Rojas, E.; Park, J.S.; et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 1996, 85, 501–512. [Google Scholar] [CrossRef]
- Glass, D.J.; Bowen, D.C.; Stitt, T.N.; Radziejewski, C.; Bruno, J.; Ryan, T.E.; Gies, D.R.; Shah, S.; Mattsson, K.; Burden, S.J.; et al. Agrin acts via a MuSK receptor complex. Cell 1996, 85, 513–523. [Google Scholar] [CrossRef]
- Weatherbee, S.D.; Anderson, K.V.; Niswander, L.A. LDL-receptor-related protein 4 is crucial for formation of the neuromuscular junction. Development 2006, 133, 4993–5000. [Google Scholar] [CrossRef]
- Kim, N.; Stiegler, A.L.; Cameron, T.O.; Hallock, P.T.; Gomez, A.M.; Huang, J.H.; Hubbard, S.R.; Dustin, M.L.; Burden, S.J. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell 2008, 135, 334–342. [Google Scholar] [CrossRef]
- Zhang, B.; Luo, S.; Wang, Q.; Suzuki, T.; Xiong, W.C.; Mei, L. LRP4 serves as a coreceptor of agrin. Neuron 2008, 60, 285–297. [Google Scholar] [CrossRef]
- Kleiman, R.J.; Reichardt, L.F. Testing the agrin hypothesis. Cell 1996, 85, 461–464. [Google Scholar] [CrossRef]
- Blakeslee, S. How Nerve Meets Muscle and Begins to Talk. Available online: http://www.nytimes.com/1996/05/21/science/how-nerve-meets-muscle-and-begins-to-talk.html (accessed on 23 June 2019).
- Sanes, J.R.; Lichtman, J.W. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2001, 2, 791–805. [Google Scholar] [CrossRef]
- Yang, X.; Li, W.; Prescott, E.D.; Burden, S.J.; Wang, J.C. DNA topoisomerase IIbeta and neural development. Science 2000, 287, 131–134. [Google Scholar] [CrossRef]
- Yang, X.; Arber, S.; William, C.; Li, L.; Tanabe, Y.; Jessell, T.M.; Birchmeier, C.; Burden, S.J. Patterning of muscle acetylcholine receptor gene expression in the absence of motor innervation. Neuron 2001, 30, 399–410. [Google Scholar] [CrossRef]
- Lin, W.; Burgess, R.W.; Dominguez, B.; Pfaff, S.L.; Sanes, J.R.; Lee, K.F. Distinct roles of nerve and muscle in postsynaptic differentiation of the neuromuscular synapse. Nature 2001, 410, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Kummer, T.T.; Misgeld, T.; Lichtman, J.W.; Sanes, J.R. Nerve-independent formation of a topologically complex postsynaptic apparatus. J. Cell Biol. 2004, 164, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Misgeld, T.; Kummer, T.T.; Lichtman, J.W.; Sanes, J.R. Agrin promotes synaptic differentiation by counteracting an inhibitory effect of neurotransmitter. Proc. Natl. Acad. Sci. USA 2005, 102, 11088–11093. [Google Scholar] [CrossRef]
- Lin, W.; Dominguez, B.; Yang, J.; Aryal, P.; Brandon, E.P.; Gage, F.H.; Lee, K.F. Neurotransmitter acetylcholine negatively regulates neuromuscular synapse formation by a Cdk5-dependent mechanism. Neuron 2005, 46, 569–579. [Google Scholar] [CrossRef]
- Anderson, M.J.; Fambrough, D.M. Aggregates of acetylcholine receptors are associated with plaques of a basal lamina heparan sulfate proteoglycan on the surface of skeletal muscle fibers. J. Cell Biol. 1983, 97, 1396–1411. [Google Scholar] [CrossRef]
- Bayne, E.K.; Anderson, M.J.; Fambrough, D.M. Extracellular matrix organization in developing muscle: Correlation with acetylcholine receptor aggregates. J. Cell Biol. 1984, 99, 1486–1501. [Google Scholar] [CrossRef]
- Chen, F.; Qian, L.; Yang, Z.H.; Huang, Y.; Ngo, S.T.; Ruan, N.J.; Wang, J.; Schneider, C.; Noakes, P.G.; Ding, Y.Q.; et al. Rapsyn interaction with calpain stabilizes AChR clusters at the neuromuscular junction. Neuron 2007, 55, 247–260. [Google Scholar] [CrossRef]
- Wang, J.Y.; Chen, F.; Fu, X.Q.; Ding, C.S.; Zhou, L.; Zhang, X.H.; Luo, Z.G. Caspase-3 cleavage of dishevelled induces elimination of postsynaptic structures. Dev. Cell. 2014, 28, 670–684. [Google Scholar] [CrossRef]
- Burden, S.J. Building the vertebrate neuromuscular synapse. J. Neurobiol. 2002, 53, 501–511. [Google Scholar] [CrossRef]
- Kummer, T.T.; Misgeld, T.; Sanes, J.R. Assembly of the postsynaptic membrane at the neuromuscular junction: Paradigm lost. Curr. Opin. Neurobiol. 2006, 16, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Milton, J. Paradise Lost; A Poem in Twelve Books, 2nd ed.; S. Simmons: London, UK, 1674. [Google Scholar]
- Kuhn, T.S. The Structure of Scientific Revolutions; University of Chicago Press: Chicago, IL, USA, 1962. [Google Scholar]
- Kim, N.; Burden, S.J. MuSK controls where motor axons grow and form synapses. Nat. Neurosci. 2008, 11, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Escher, P.; Lacazette, E.; Courtet, M.; Blindenbacher, A.; Landmann, L.; Bezakova, G.; Lloyd, K.C.; Mueller, U.; Brenner, H.R. Synapses form in skeletal muscles lacking neuregulin receptors. Science 2005, 308, 1920–1923. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, A.; Burden, S.J. Neuromuscular synapse formation in mice lacking motor neuron- and skeletal muscle-derived neuregulin-1. J. Neurosci. 2006, 26, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Newbern, J.; Birchmeier, C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Semin. Cell Dev. Biol. 2010, 21, 922–928. [Google Scholar] [CrossRef]
- Wang, J.; Song, F.; Loeb, J.A. Neuregulin1 fine-tunes pre-, post-, and perisynaptic neuromuscular junction, development. Dev. Dyn. 2017, 246, 368–380. [Google Scholar] [CrossRef]
- Wu, S.; Huang, Y.; Xing, Y.; Chen, L.; Yang, M.; Li, S. Two Pathways Regulate Differential Expression of nAChRs Between the Orbicularis Oris and Gastrocnemius. J. Surg. Res. 2019, 243, 130–142. [Google Scholar] [CrossRef]
- Ohkawara, B.; Cabrera-Serrano, M.; Nakata, T.; Milone, M.; Asai, N.; Ito, K.; Ito, M.; Masuda, A.; Ito, Y.; Engel, A.G.; et al. LRP4 third β-propeller domain mutations cause novel congenital myasthenia by compromising agrin-mediated MuSK signaling in a position-specific manner. Hum. Mol. Genet. 2014, 23, 1856–1868. [Google Scholar] [CrossRef]
- Gomez, A.M.; Burden, S.J. The extracellular region of Lrp4 is sufficient to mediate neuromuscular synapse formation. Dev. Dyn. 2011, 240, 2626–2633. [Google Scholar] [CrossRef]
- Wu, H.; Xiong, W.C.; Mei, L. To build a synapse: Signaling pathways in neuromuscular junction assembly. Development 2010, 137, 1017–1033. [Google Scholar] [CrossRef]
- Zong, Y.; Jin, R. Structural mechanisms of the agrin-LRP4-MuSK signaling pathway in neuromuscular junction differentiation. Cell. Mol. Life Sci. 2013, 270, 3077–3088. [Google Scholar] [CrossRef] [PubMed]
- Burden, S.J.; Huijbers, M.G.; Remedio, L. Fundamental molecules and mechanisms for forming and maintaining neuromuscular synapses. Int. J. Mol. Sci. 2018, 19, 490. [Google Scholar] [CrossRef] [PubMed]
- Flanagan-Steet, H.; Fox, M.A.; Meyer, D.; Sanes, J.R. Neuromuscular synapses can form in vivo by incorporation of initially aneural postsynaptic specializations. Development 2005, 132, 4471–4481. [Google Scholar] [CrossRef] [PubMed]
- Panzer, J.A.; Song, Y.; Balice-Gordon, R.J. In vivo imaging of preferential motor axon outgrowth to and synaptogenesis at prepatterned acetylcholine receptor clusters in embryonic zebrafish skeletal muscle. J. Neurosci. 2006, 26, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Sanes, J.R.; Yamagata, M. Many paths to synaptic specificity. Annu. Rev. Cell Dev. Biol. 2009, 25, 161–195. [Google Scholar] [CrossRef] [PubMed]
- Burden, S.J. SnapShot: Neuromuscular Junction. Cell 2011, 144, 826. [Google Scholar] [CrossRef]
- Sainath, R.; Gallo, G. Cytoskeletal and signaling mechanisms of neurite formation. Cell Tissue Res. 2015, 359, 267–278. [Google Scholar] [CrossRef]
- Campagna, J.A.; Ruegg, M.A.; Bixby, J.L. Evidence that agrin directly influences presynaptic differentiation at neuromuscular junctions in vitro. Eur. J. Neurosci. 1997, 9, 2269–2283. [Google Scholar] [CrossRef]
- Dimitropoulou, A.; Bixby, J.L. Motor neurite outgrowth is selectively inhibited by cell surface MuSK and agrin. Mol. Cell. Neurosci. 2005, 28, 292–302. [Google Scholar] [CrossRef]
- Wu, H.; Lu, Y.; Shen, C.; Patel, N.; Gan, L.; Xiong, W.C.; Mei, L. Distinct roles of muscle and motoneuron LRP4 in neuromuscular junction formation. Neuron 2012, 75, 94–107. [Google Scholar] [CrossRef]
- Yumoto, N.; Kim, N.; Burden, S.J. Lrp4 is a retrograde signal for presynaptic differentiation at neuromuscular synapses. Nature 2012, 489, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Darabid, H.; Perez-Gonzalez, A.P.; Robitaille, R. Neuromuscular synaptogenesis: Coordinating partners with multiple functions. Nat. Rev. Neurosci. 2014, 15, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Li, X.X.; Yu, Y.; Chiu, A.P.; Lo, L.H.; To, J.C.; Rowlands, D.K.; Keng, V.W. Schwann cell-specific PTEN and EGFR dysfunctions affect neuromuscular junction development by impairing Agrin signaling and autophagy. Biochem. Biophys. Res. Commun. 2019, 515, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, A.W.; Harris, A.J. Neural influence on acetylcholine receptor clusters in embryonic development of skeletal muscles. Nature 1979, 279, 549–551. [Google Scholar] [CrossRef]
- Verschuuren, J.J.; Huijbers, M.G.; Plomp, J.J.; Niks, E.H.; Molenaar, P.C.; Martinez-Martinez, P.; Gomez, A.M.; De Baets, M.H.; Losen, M. Pathophysiology of myasthenia gravis with antibodies to the acetylcholine receptor, muscle-specific kinase and low-density lipoprotein receptor-related protein 4. Autoimmun. Rev. 2013, 12, 918–923. [Google Scholar] [CrossRef]
- Gasperi, C.; Melms, A.; Schoser, B.; Zhang, Y.; Meltoranta, J.; Risson, V.; Schaeffer, L.; Schalke, B.; Kröger, S. Anti-agrin autoantibodies in myasthenia gravis. Neurology 2014, 82, 1976–1983. [Google Scholar] [CrossRef]
- Zhang, W.; Coldefy, A.S.; Hubbard, S.R.; Burden, S.J. Agrin binds to the N-terminal region of Lrp4 protein and stimulates association between Lrp4 and the first immunoglobulin-like domain in muscle-specific kinase (MuSK). J. Biol. Chem. 2011, 286, 40624–40630. [Google Scholar] [CrossRef]
- Zong, Y.; Zhang, B.; Gu, S.; Lee, K.; Zhou, J.; Yao, G.; Figueiredo, D.; Perry, K.; Mei, L.; Jin, R. Structural basis of agrin-LRP4-MuSK signaling. Genes Dev. 2012, 26, 247–258. [Google Scholar] [CrossRef]
- Burden, S.J.; Yumoto, N.; Zhang, W. The role of MuSK in synapse formation and neuromuscular disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a009167. [Google Scholar] [CrossRef]
- Li, L.; Xiong, W.C.; Mei, L. Neuromuscular junction formation, aging, and disorders. Annu. Rev. Physiol. 2018, 80, 159–188. [Google Scholar] [CrossRef]
- Jing, L.; Lefebvre, J.L.; Gordon, L.R.; Granato, M. Wnt signals organize synaptic prepattern and axon guidance through the zebrafish unplugged/MuSK receptor. Neuron 2009, 61, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Remédio, L.; Gribble, K.D.; Lee, J.K.; Kim, N.; Hallock, P.T.; Delestrée, N.; Mentis, G.Z.; Froemke, R.C.; Granato, M.; Burden, S.J. Diverging roles for Lrp4 and Wnt signaling in neuromuscular synapse development during evolution. Genes Dev. 2016, 30, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.C.; Kuffler, S.W. Motor innervation of skeletal muscle: Multiple innervation of individual muscle fibres and motor unit function. J. Physiol. 1954, 126, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Ogata, T. Structure of motor endplates in the different fiber types of vertebrate skeletal muscles. Arch. Histol. Cytol. 1988, 51, 385–424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arber, S.; Burden, S.J.; Harris, A.J. Patterning of skeletal muscle. Curr. Opin. Neurobiol. 2001, 12, 100–103. [Google Scholar] [CrossRef]
- Sanes, J.R.; Lichtman, J.W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 1999, 22, 389–442. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Y.; Sugiura, Y.; Allen, P.D.; Gregg, R.G.; Lin, W. Neuromuscular synaptic patterning requires the function of skeletal muscle dihydropyridine receptors. Nat. Neurosci. 2011, 14, 570–577. [Google Scholar] [CrossRef]
- Sander, A.; Hesser, B.A.; Witzemann, V. MuSK induces in vivo acetylcholine receptor clusters in a ligand-independent manner. J. Cell Biol. 2001, 155, 1287–1296. [Google Scholar] [CrossRef]
- Tezuka, T.; Inoue, A.; Hoshi, T.; Weatherbee, S.D.; Burgess, R.W.; Ueta, R.; Yamanashi, Y. The MuSK activator agrin has a separate role essential for postnatal maintenance of neuromuscular synapses. Proc. Natl. Acad. Sci. USA 2014, 111, 16556–16561. [Google Scholar] [CrossRef]
- Cohen, M.W.; Weldon, P.R. Localization of acetylcholine receptors and synaptic ultrastructure at nerve-muscle contacts in culture: Dependence on nerve type. J. Cell Biol. 1980, 86, 388–401. [Google Scholar] [CrossRef]
- Ma, E.; Morgan, R.; Godfrey, E.W. Agrin mRNA variants are differentially regulated in developing chick embryo spinal cord and sensory ganglia. J. Neurobiol. 1995, 26, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Bezakova, G.; Helm, J.P.; Francolini, M.; Lømo, T. Effects of purified recombinant neural and muscle agrin on skeletal muscle fibers in vivo. J. Cell Biol. 2001, 153, 1441–1452. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Meier, T.; Lichtsteiner, M.; Witzemann, V.; Sakmann, B.; Brenner, H.R. Induction by agrin of ectopic and functional postsynaptic-like membrane in innervated muscle. Proc. Natl. Acad. Sci. USA 1997, 94, 2654–2659. [Google Scholar] [CrossRef] [PubMed]
- Bezakova, G.; Rabben, I.; Sefland, I.; Fumagalli, G.; Lømo, T. Neural agrin controls acetylcholine receptor stability in skeletal muscle fibers. Proc. Natl. Acad. Sci. USA 2001, 98, 9924–9929. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lugo, B.; Shah, S.; Godfrey, E.W.; Daniels, M.P. Synaptic localization and axonal targeting of agrin secreted by ventral spinal cord neurons in culture. J. Neurobiol. 2000, 43, 338–351. [Google Scholar] [CrossRef]
- Peng, H.B.; Baker, L.P.; Dai, Z. A role of tyrosine phosphorylation in the formation of acetylcholine receptor clusters induced by electric fields in cultured Xenopus muscle cells. J. Cell Biol. 1993, 120, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Peng, H.B. Mechanism of acetylcholine receptor cluster formation induced by DC electric field. PLoS ONE 2011, 6, e26805. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Champaneria, S.; Swenarchuk, L.E. Synaptic differentiation can be evoked by polymer microbeads that mimic localized pericellular proteolysis by removing proteins from adjacent surfaces. Dev. Biol. 1991, 147, 464–479. [Google Scholar] [CrossRef]
- Jones, G.; Herczeg, A.; Ruegg, M.A.; Lichtsteiner, M.; Kröger, S.; Brenner, H.R. Substrate-bound agrin induces expression of acetylcholine receptor epsilon-subunit gene in cultured mammalian muscle cells. Proc. Natl. Acad. Sci. USA 1996, 93, 5985–5990. [Google Scholar] [CrossRef]
- Kung, F.H.; Sillitti, D.; Shrirao, A.B.; Shreiber, D.I.; Firestein, B.L. Collagen nanofiber anisotropy induces myotube differentiation and acetylcholine receptor clustering. J. Tissue Eng. Regen. Med. 2018, 12, e2010–e2019. [Google Scholar] [CrossRef]
- Kidokoro, Y.; Anderson, M.J.; Gruener, R. Changes in synaptic potential properties during acetylcholine receptor accumulation and neurospecific interactions in Xenopus nerve-muscle cell culture. Dev. Biol. 1980, 78, 464–483. [Google Scholar] [CrossRef]
- Anderson, M.J.; Klier, F.G.; Tanguay, K.E. Acetylcholine receptor aggregation parallels the deposition of a basal lamina proteoglycan during development of the neuromuscular junction. J. Cell Biol. 1984, 99, 1769–1784. [Google Scholar] [CrossRef] [PubMed]
- Bruneau, E.; Sutter, D.; Hume, R.I.; Akaaboune, M. Identification of nicotinic acetylcholine receptor recycling and its role in maintaining receptor density at the neuromuscular junction in vivo. J. Neurosci. 2005, 25, 9949–9959. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.; Martin, P.T. Role of extracellular matrix proteins and their receptors in the development of the vertebrate neuromuscular junction. Dev. Neurobiol. 2011, 71, 982–1005. [Google Scholar] [CrossRef]
- Burgess, R.W.; Skarnes, W.C.; Sanes, J.R. Agrin isoforms with distinct amino termini: Differential expression, localization, and function. J. Cell Biol. 2000, 151, 41–52. [Google Scholar] [CrossRef]
- Denzer, A.J.; Brandenberger, R.; Gesemann, M.; Chiquet, M.; Ruegg, M.A. Agrin binds to the nerve-muscle basal lamina via laminin. J. Cell Biol. 1997, 137, 671–683. [Google Scholar] [CrossRef]
- Denzer, A.J.; Schulthess, T.; Fauser, C.; Schumacher, B.; Kammerer, R.A.; Engel, J.; Ruegg, M.A. Electron microscopic structure of agrin and mapping of its binding site in laminin-1. EMBO J. 1998, 17, 335–343. [Google Scholar] [CrossRef]
- Kammerer, R.A.; Schulthess, T.; Landwehr, R.; Schumacher, B.; Lustig, A.; Yurchenco, P.D.; Ruegg, M.A.; Engel, J.; Denzer, A.J. Interaction of agrin with laminin requires a coiled-coil conformation of the agrin-binding site within the laminin gamma1 chain. EMBO J. 1999, 18, 6762–6770. [Google Scholar] [CrossRef]
- Patton, B.L.; Miner, J.H.; Chiu, A.Y.; Sanes, J.R. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J. Cell Biol. 1997, 139, 1507–1521. [Google Scholar] [CrossRef]
- Patton, B.L. Laminins of the neuromuscular system. Microsc. Res. Tech. 2000, 51, 247–261. [Google Scholar] [CrossRef]
- Rogers, R.S.; Nishimune, H. The role of laminins in the organization and function of neuromuscular junctions. Matrix Biol. 2017, 57–58, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.J.; Shi, Z.Q.; Zackson, S.L. Proteolytic disruption of laminin-integrin complexes on muscle cells during synapse formation. Mol. Cell. Biol. 1996, 16, 4972–4984. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Swenarchuk, L.E.; Champaneria, S.; Anderson, M.J. Induction of a specialized muscle basal lamina at chimaeric synapses in culture. Development 1990, 110, 51–61. [Google Scholar] [PubMed]
- Nishimune, H.; Valdez, G.; Jarad, G.; Moulson, C.L.; Müller, U.; Miner, J.H.; Sanes, J.R. Laminins promote postsynaptic maturation by an autocrine mechanism at the neuromuscular junction. J. Cell Biol. 2008, 182, 1201–1215. [Google Scholar] [CrossRef] [PubMed]
- Patton, B.L. Basal lamina and the organization of neuromuscular synapses. J. Neurocytol. 2003, 32, 883–903. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.W.; Godfrey, E.W. Early appearance of and neuronal contribution to agrin-like molecules at embryonic frog nerve-muscle synapses formed in culture. J. Neurosci. 1992, 12, 2982–2992. [Google Scholar] [CrossRef]
- Anderson, M.J.; Shi, Z.Q.; Grawel, R.; Zackson, S.L. Erratic deposition of agrin during the formation of Xenopus neuromuscular junctions in culture. Dev. Biol. 1995, 170, 1–20. [Google Scholar] [CrossRef]
- Santos, A.F.; Caroni, P. Assembly, plasticity and selective vulnerability to disease of mouse neuromuscular junctions. J. Neurocytol. 2003, 32, 849–862. [Google Scholar] [CrossRef]
- Kuromi, H.; Kidokoro, Y. Denervation disperses acetylcholine receptor clusters at the neuromuscular junction in Xenopus cultures. Dev. Biol. 1984, 104, 421–427. [Google Scholar] [CrossRef]
- Champaneria, S.; Swenarchuk, L.E.; Anderson, M.J. Increases in pericellular proteolysis at developing neuromuscular junctions in culture. Dev. Biol. 1992, 149, 261–277. [Google Scholar] [CrossRef]
- Werle, M.J. Cell-to-cell signaling at the neuromuscular junction: The dynamic role of the extracellular matrix. Ann. N. Y. Acad. Sci. 2008, 1132, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.R.; Butler, G.; McFarlane, A.; Xie, I.; Overall, C.M.; Stetefeld, J. Site specific cleavage mediated by MMPs regulates function of agrin. PLoS ONE 2012, 7, e43669. [Google Scholar] [CrossRef] [PubMed]
- Reif, R.; Sales, S.; Hettwer, S.; Dreier, B.; Gisler, C.; Wolfel, J.; Luscher, D.; Zurlinden, A.; Stephan, A.; Ahmed, S.; et al. Specific cleavage of agrin by neurotrypsin, a synaptic protease linked to mental retardation. FASEB J. 2007, 21, 3468–3478. [Google Scholar] [CrossRef] [PubMed]
- Almenar-Queralt, A.; Kim, S.N.; Benner, C.; Herrera, C.M.; Kang, D.E.; Garcia-Bassets, I.; Goldstein, L.S. Presenilins regulate neurotrypsin gene expression and neurotrypsin-dependent agrin cleavage via cyclic AMP response element-binding protein (CREB) modulation. J. Biol. Chem. 2013, 288, 35222–35236. [Google Scholar] [CrossRef] [PubMed]
- Valm, A.M.; Cohen, S.; Legant, W.R.; Melunis, J.; Hershberg, U.; Wait, E.; Cohen, A.R.; Davidson, M.W.; Betzig, E.; Lippincott-Schwartz, J. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nature 2017, 546, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Yogev, S.; Shen, K. Cellular and molecular mechanisms of synaptic specificity. Annu. Rev. Cell Dev. Biol. 2014, 30, 417–437. [Google Scholar] [CrossRef] [PubMed]
- De Wit, J.; Ghosh, A. Specification of synaptic connectivity by cell surface interactions. Nat. Rev. Neurosci. 2016, 17, 22–35. [Google Scholar] [CrossRef]
- Hata, K.; Maeno-Hikichi, Y.; Yumoto, N.; Burden, S.J.; Landmesser, L.T. Distinct roles of different presynaptic and postsynaptic NCAM isoforms in early motoneuron-myotube interactions required for functional synapse formation. J. Neurosci. 2018, 38, 498–510. [Google Scholar] [CrossRef]
- Moscoso, L.M.; Cremer, H.; Sanes, J.R. Organization and reorganization of neuromuscular junctions in mice lacking neural cell adhesion molecule, tenascin-C, or fibroblast growth factor-5. J. Neurosci. 1998, 18, 1465–1477. [Google Scholar] [CrossRef]
- Rafuse, V.F.; Polo-Parada, L.; Landmesser, L.T. Structural and functional alterations of neuromuscular junctions in NCAM-deficient mice. J. Neurosci. 2000, 20, 6529–6539. [Google Scholar] [CrossRef]
- Wang, P.; Yang, G.; Mosier, D.R.; Chang, P.; Zaidi, T.; Gong, Y.D.; Zhao, N.M.; Dominguez, B.; Lee, K.F.; Gan, W.B.; et al. Defective neuromuscular synapses in mice lacking amyloid precursor protein (APP) and APP-Like protein 2. J. Neurosci. 2005, 25, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Klevanski, M.; Saar, M.; Baumkötter, F.; Weyer, S.W.; Kins, S.; Müller, U.C. Differential role of APP and APLPs for neuromuscular synaptic morphology and function. Mol. Cell. Neurosci. 2014, 61, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Liu, Y.; Tennert, C.; Sugiura, Y.; Karakatsani, A.; Kröger, S.; Johnson, E.B.; Hammer, R.E.; Lin, W.; Herz, J. APP interacts with LRP4 and agrin to coordinate the development of the neuromuscular junction in mice. Elife 2013, 2, e00220. [Google Scholar] [CrossRef] [PubMed]
- Herms, J.; Anliker, B.; Heber, S.; Ring, S.; Fuhrmann, M.; Kretzschmar, H.; Sisodia, S.; Müller, U. Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members. EMBO J. 2004, 23, 4106–4115. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, J.H.; Klevanski, M.; Saar, M.; Müller, U.C. Roles of the amyloid precursor protein family in the peripheral nervous system. Mech. Dev. 2013, 130, 433–446. [Google Scholar] [CrossRef]
- Luo, Z.G.; Wang, Q.; Zhou, J.Z.; Wang, J.; Luo, Z.; Liu, M.; He, X.; Wynshaw-Boris, A.; Xiong, W.C.; Lu, B.; et al. Regulation of AChR clustering by Dishevelled interacting with MuSK and PAK1. Neuron 2002, 35, 489–505. [Google Scholar] [CrossRef]
- Barik, A.; Zhang, B.; Sohal, G.S.; Xiong, W.C.; Mei, L. Crosstalk between Agrin and Wnt signaling pathways in development of vertebrate neuromuscular junction. Dev. Neurobiol. 2014, 74, 828–838. [Google Scholar] [CrossRef]
- He, C.-W.; Liao, C.-P.; Pan, C.-L. Wnt signalling in the development of axon, dendrites and synapses. Open Biol. 2018, 8, 180116. [Google Scholar] [CrossRef]
- Shen, C.; Li, L.; Zhao, K.; Bai, L.; Wang, A.; Shu, X.; Xiao, Y.; Zhang, J.; Zhang, K.; Hui, T.; et al. Motoneuron Wnts regulate neuromuscular junction development. Elife 2018, 7, e34625. [Google Scholar] [CrossRef]
- Messéant, J.; Dobbertin, A.; Girard, E.; Delers, P.; Manuel, M.; Mangione, F.; Schmitt, A.; Le Denmat, D.; Molgó, J.; Zytnicki, D.; et al. MuSK frizzled-like domain is critical for mammalian neuromuscular junction formation and maintenance. J. Neurosci. 2015, 35, 4926–4941. [Google Scholar] [CrossRef]
- Almonte, A.G.; Sweatt, J.D. Serine proteases, serine protease inhibitors, and protease-activated receptors: Roles in synaptic function and behavior. Brain Res. 2011, 1407, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Baranger, K.; Rivera, S.; Liechti, F.D.; Grandgirard, D.; Bigas, J.; Seco, J.; Tarrago, T.; Leib, S.L.; Khrestchatisky, M. Endogenous and synthetic MMP inhibitors in CNS physiopathology. Prog. Brain. Res. 2014, 214, 313–351. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef] [PubMed]
- Tsilibary, E.; Tzinia, A.; Radenovic, L.; Stamenkovic, V.; Lebitko, T.; Mucha, M.; Pawlak, R.; Frischknecht, R.; Kaczmarek, L. Neural ECM proteases in learning and synaptic plasticity. Prog. Brain Res. 2014, 214, 135–157. [Google Scholar] [CrossRef]
- De Stefano, M.E.; Herrero, M.T. The multifaceted role of metalloproteinases in physiological and pathological conditions in embryonic and adult brains. Prog. Neurobiol. 2017, 155, 36–56. [Google Scholar] [CrossRef]
- Wang, X.B.; Bozdagi, O.; Nikitczuk, J.S.; Zhai, Z.W.; Zhou, Q.; Huntley, G.W. Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc. Natl. Acad. Sci. USA 2008, 105, 19520–19525. [Google Scholar] [CrossRef]
- Attwood, B.; Bourgognon, J.M.; Patel, S.; Mucha, M.; Schiavon, E.; Skrzypiec, A.E.; Young, K.W.; Shiosaka, S.; Korostynski, M.; Piechota, M.; et al. Neuropsin cleaves EphB2 in the amygdala to control anxiety. Nature 2011, 473, 372–375. [Google Scholar] [CrossRef]
- Huntley, G.W. Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat. Rev. Neurosci. 2012, 13, 743–757. [Google Scholar] [CrossRef]
- Lin, K.T.; Sloniowski, S.; Ethell, D.W.; Ethell, I.M. Ephrin-B2-induced cleavage of EphB2 receptor is mediated by matrix metalloproteinases to trigger cell repulsion. J. Biol. Chem. 2008, 283, 28969–28979. [Google Scholar] [CrossRef]
- Valente, M.M.; Allen, M.; Bortolotto, V.; Lim, S.T.; Conant, K.; Grilli, M. The MMP-1/PAR-1 axis enhances proliferation and neuronal differentiation of adult hippocampal neural progenitor cells. Neural Plast. 2015, 2015, 646595. [Google Scholar] [CrossRef]
- Yin, K.J.; Cirrito, J.R.; Yan, P.; Hu, X.; Xiao, Q.; Pan, X.; Bateman, R.; Song, H.; Hsu, F.F.; Turk, J.; et al. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J. Neurosci. 2006, 26, 10939–10948. [Google Scholar] [CrossRef] [PubMed]
- Ridnour, L.A.; Dhanapal, S.; Hoos, M.; Wilson, J.; Lee, J.; Cheng, R.Y.; Brueggemann, E.E.; Hines, H.B.; Wilcock, D.M.; Vitek, M.P.; et al. Nitric oxide-mediated regulation of β-amyloid clearance via alterations of MMP-9/TIMP-1. J. Neurochem. 2012, 123, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Andries, L.; Van Hove, I.; Moons, L.; De Groef, L. Matrix metalloproteinases during axonal regeneration, a multifactorial role from start to finish. Mol. Neurobiol. 2017, 54, 2114–2125. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, D.; Morrison, C.J.; Overall, C.M. Matrix metalloproteinases: What do they not do? New substrates and biological roles identified by murine models and proteomics. Biochim. Biophys. Acta 2010, 1803, 39–54. [Google Scholar] [CrossRef]
- Mason, S.D.; Joyce, J.A. Proteolytic networks in cancer. Trends Cell Biol. 2011, 21, 228–237. [Google Scholar] [CrossRef]
- Atapattu, L.; Lackmann, M.; Janes, P.W. The role of proteases in regulating Eph/ephrin signaling. Cell Adhes. Migr. 2014, 8, 294–307. [Google Scholar] [CrossRef]
- Austin, K.M.; Covic, L.; Kuliopulos, A. Matrix metalloproteases and PAR1 activation. Blood 2013, 121, 431–439. [Google Scholar] [CrossRef]
- Ramachandran, R.; Altier, C.; Oikonomopoulou, K.; Hollenberg, M.D. Proteinases, their extracellular targets, and jnflammatory signaling. Pharm. Rev. 2016, 68, 1110–11142. [Google Scholar] [CrossRef]
- Dufour, A.; Overall, C.M. Subtracting Matrix out of the Equation: New Key Roles of Matrix Metalloproteinases in Innate Immunity and Disease. In Matrix Metalloproteinase Biology; Sagi, I., Gaffney, J.P., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 131–152. [Google Scholar]
- Kuhn, P.H.; Colombo, A.V.; Schusser, B.; Dreymueller, D.; Wetzel, S.; Schepers, U.; Herber, J.; Ludwig, A.; Kremmer, E.; Montag, D.; et al. Systematic substrate identification indicates a central role for the metalloprotease ADAM10 in axon targeting and synapse function. Elife 2016, 5, e12748. [Google Scholar] [CrossRef]
- Weber, S.; Saftig, P. Ectodomain shedding and ADAMs in development. Development 2012, 139, 3693–3709. [Google Scholar] [CrossRef]
- Saftig, P.; Lichtenthaler, S.F. The alpha secretase ADAM10: A metalloprotease with multiple functions in the brain. Prog. Neurobiol. 2015, 135, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wilkins-Port, C.E.; Higgins, S.P.; Higgins, C.E.; Kobori-Hotchkiss, I.; Higgins, P.J. Complex regulation of the pericellular proteolytic microenvironment during tumor progression and wound repair: Functional interactions between the serine protease and matrix metalloproteinase cascades. Biochem. Res. Int. 2012, 2012, 454368. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, J.; Solomonov, I.; Zehorai, E.; Sagi, I. Multilevel regulation of matrix metalloproteinases in tissue homeostasis indicates their molecular specificity in vivo. Matrix Biol. 2015, 44, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Ben Shimon, M.; Lenz, M.; Ikenberg, B.; Becker, D.; Shavit Stein, E.; Chapman, J.; Tanne, D.; Pick, C.G.; Blatt, I. Thrombin regulation of synaptic transmission and plasticity: Implications for health and disease. Front. Cell. Neurosci. 2015, 9, 151. [Google Scholar] [CrossRef]
- Danø, K.; Rømer, J.; Nielsen, B.S.; Bjørn, S.; Pyke, C.; Rygaard, J.; Lund, L.R. Cancer invasion and tissue remodeling—Cooperation of protease systems and cell types. APMIS 1999, 107, 120–127. [Google Scholar] [CrossRef]
- He, C.S.; Wilhelm, S.M.; Pentland, A.P.; Marmer, B.L.; Grant, G.A.; Eisen, A.Z.; Goldberg, G.I. Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc Natl. Acad. Sci. USA 1989, 86, 2632–2636. [Google Scholar] [CrossRef]
- Crawford, H.C.; Stack, M.S. Matrix Metalloproteinase Modification of Extracellular Matrix-Mediated Signaling. In Matrix Metalloproteinase Biology; Sagi, I., Gaffney, J.P., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2015; pp. 103–113. [Google Scholar]
- Loeb, J.A.; Fischbach, G.D. ARIA can be released from extracellular matrix through cleavage of a heparin-binding domain. J. Cell Biol. 1995, 130, 127–135. [Google Scholar] [CrossRef]
- Li, Q.; Loeb, J.A. Neuregulin-heparan-sulfate proteoglycan interactions produce sustained erbB receptor activation required for the induction of acetylcholine receptors in muscle. J. Biol. Chem. 2001, 276, 38068–38075. [Google Scholar] [CrossRef]
- Ruiz de Almodovar, C.; Lambrechts, D.; Mazzone, M.; Carmeliet, P. Role and therapeutic potential of VEGF in the nervous system. Physiol. Rev. 2009, 289, 607–648. [Google Scholar] [CrossRef]
- Poesen, K.; Lambrechts, D.; Van Damme, P.; Dhondt, J.; Bender, F.; Frank, N.; Bogaert, E.; Claes, B.; Heylen, L.; Verheyen, A.; et al. Novel role for vascular endothelial growth factor (VEGF) receptor-1 and its ligand VEGF-B in motor neuron degeneration. J. Neurosci. 2008, 28, 10451–10459. [Google Scholar] [CrossRef]
- Bry, M.; Kivelä, R.; Leppänen, V.M.; Alitalo, K. Vascular endothelial growth factor-B in physiology and disease. Physiol. Rev. 2014, 94, 779–794. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, A.; Aoki, M.; Nagai, M.; Warita, H.; Kato, S.; Kato, M.; Nakamura, T.; Funakoshi, H.; Itoyama, Y. Intrathecal delivery of hepatocyte growth factor from amyotrophic lateral sclerosis onset suppresses disease progression in rat amyotrophic lateral sclerosis model. J. Neuropathol. Exp. Neurol. 2007, 66, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Shute, J. Glycosaminoglycan and chemokine/growth factor interactions. Handb. Exp. Pharm. 2012, 207, 307–324. [Google Scholar] [CrossRef]
- Deuel, T.F.; Zhang, N.; Yeh, H.J.; Silos-Santiago, I.; Wang, Z.Y. Pleiotrophin: A cytokine with diverse functions and a novel signaling pathway. Arch. Biochem. Biophys. 2002, 397, 162–171. [Google Scholar] [CrossRef]
- Mi, R.; Chen, W.; Höke, A. Pleiotrophin is a neurotrophic factor for spinal motor neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 4664–4669. [Google Scholar] [CrossRef]
- Henderson, C.E.; Phillips, H.S.; Pollock, R.A.; Davies, A.M.; Lemeulle, C.; Armanini, M.; Simmons, L.; Moffet, B.; Vandlen, R.A.; Koliatsos, V.E.; et al. GDNF: A potent survival factor for motoneurons present in peripheral nerve and muscle. Science 1994, 266, 1062–1064. [Google Scholar] [CrossRef]
- Alfano, I.; Vora, P.; Mummery, R.S.; Mulloy, B.; Rider, C.C. The major determinant of the heparin binding of glial cell-line-derived neurotrophic factor is near the N-terminus and is dispensable for receptor binding. Biochem. J. 2007, 404, 131–140. [Google Scholar] [CrossRef]
- Chou, H.J.; Lai, D.M.; Huang, C.W.; McLennan, I.S.; Wang, H.D.; Wang, P.Y. BMP4 is a peripherally-derived factor for motor neurons and attenuates glutamate-induced excitotoxicity in vitro. PLoS ONE 2013, 8, e58441. [Google Scholar] [CrossRef]
- Mercier, F.; Douet, V. Bone morphogenetic protein-4 inhibits adult neurogenesis and is regulated by fractone-associated heparan sulfates in the subventricular zone. J. Chem. Neuroanat. 2014, 57, 54–61. [Google Scholar] [CrossRef]
- Russo, V.C.; Schütt, B.S.; Andaloro, E.; Ymer, S.I.; Hoeflich, A.; Ranke, M.B.; Bach, L.A.; Werther, G.A. Insulin-like growth factor binding protein-2 binding to extracellular matrix plays a critical role in neuroblastoma cell proliferation, migration, and invasion. Endocrinology 2005, 146, 4445–4455. [Google Scholar] [CrossRef]
- Miyamoto, S.; Nakamura, M.; Yano, K.; Ishii, G.; Hasebe, T.; Endoh, Y.; Sangai, T.; Maeda, H.; Shi-Chuang, Z.; Chiba, T.; et al. Matrix metalloproteinase-7 triggers the matricrine action of insulin-like growth factor-II via proteinase activity on insulin-like growth factor binding protein 2 in the extracellular matrix. Cancer Sci. 2007, 98, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, K.A.; Kim, B.; Feldman, E.L. Insulin-like growth factors in the peripheral nervous system. Endocrinology 2008, 149, 5963–5971. [Google Scholar] [CrossRef] [PubMed]
- Allodi, I.; Comley, L.; Nichterwitz, S.; Nizzardo, M.; Simone, C.; Benitez, J.A.; Cao, M.; Corti, S.; Hedlund, E. Differential neuronal vulnerability identifies IGF-2 as a protective factor in ALS. Sci. Rep. 2016, 6, 25960. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadou, I.; Manolaras, I.; Irvine, E.E.; Dieringer, M.; Trabalza, A.; Mazarakis, N.D. αCAR IGF-1 vector targeting of motor neurons ameliorates disease progression in ALS mice. Ann. Clin. Transl. Neurol. 2016, 3, 752–768. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.A.; Sanes, J.R.; Borza, D.B.; Eswarakumar, V.P.; Fässler, R.; Hudson, B.G.; John, S.W.; Ninomiya, Y.; Pedchenko, V.; Pfaff, S.L.; et al. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell 2007, 129, 179–193. [Google Scholar] [CrossRef]
- Kaplan, A.; Spiller, K.J.; Towne, C.; Kanning, K.C.; Choe, G.T.; Geber, A.; Akay, T.; Aebischer, P.; Henderson, C.E. Neuronal matrix metalloproteinase-9 is a determinant of selective neurodegeneration. Neuron 2014, 81, 333–348. [Google Scholar] [CrossRef]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef]
- Wolfenson, H.; Lavelin, I.; Geiger, B. Dynamic regulation of the structure and functions of integrin adhesions. Dev. Cell 2013, 24, 447–458. [Google Scholar] [CrossRef]
- Yamada, M.; Sekiguchi, K. Molecular Basis of laminin-integrin interactions. Curr. Top. Membr. 2015, 76, 197–229. [Google Scholar] [CrossRef]
- Park, Y.K.; Goda, Y. Integrins in synapse regulation. Nat. Rev. Neurosci. 2016, 17, 745–756. [Google Scholar] [CrossRef]
- Michaluk, P.; Wawrzyniak, M.; Alot, P.; Szczot, M.; Wyrembek, P.; Mercik, K.; Medvedev, N.; Wilczek, E.; De Roo, M.; Zuschratter, W.; et al. Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J. Cell Sci. 2011, 124 Pt 19, 3369–3380. [Google Scholar] [CrossRef]
- Wong, K.C.; Meyer, T.; Harding, D.I.; Dick, J.R.; Vrbová, G.; Greensmith, L. Integrins at the neuromuscular junction are important for motoneuron survival. Eur. J. Neurosci. 1999, 11, 3287–3292. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.T.; Sanes, J.R. Integrins mediate adhesion to agrin and modulate agrin signaling. Development 1997, 124, 3909–3917. [Google Scholar] [PubMed]
- Burkin, D.J.; Gu, M.; Hodges, B.L.; Campanelli, J.T.; Kaufman, S.J. A functional role for specific spliced variants of the alpha7beta1 integrin in acetylcholine receptor clustering. J. Cell Biol. 1998, 143, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Burkin, D.J.; Kim, J.E.; Gu, M.; Kaufman, S.J. Laminin and alpha7beta1 integrin regulate agrin-induced clustering of acetylcholine receptors. J. Cell Sci. 2000, 113, 2877–2886. [Google Scholar] [PubMed]
- Anderson, M.J.; Shi, Z.Q.; Zackson, S.L. Nerve-induced disruption and reformation of beta1-integrin aggregates during development of the neuromuscular junction. Mech. Dev. 1997, 67, 125–139. [Google Scholar] [CrossRef]
- Ross, J.A.; Webster, R.G.; Lechertier, T.; Reynolds, L.E.; Turmaine, M.; Bencze, M.; Jamshidi, Y.; Cetin, H.; Muntoni, F.; Beeson, D.; et al. Multiple roles of integrin-α3 at the neuromuscular junction. J. Cell Sci. 2017, 130, 1772–1784. [Google Scholar] [CrossRef]
- Sun, C.C.; Qu, X.J.; Gao, Z.H. Arginine-Glycine-Aspartate-Binding Integrins as Therapeutic and Diagnostic Targets. Am. J. Ther. 2016, 23, e198–e207. [Google Scholar] [CrossRef]
- Miller, L.M.; Pritchard, J.M.; Macdonald, S.J.F.; Jamieson, C.; Watson, A.J.B. Emergence of small-molecule non-RGD-mimetic inhibitors for RGD integrins. J. Med. Chem. 2017, 60, 3241–3251. [Google Scholar] [CrossRef]
- Schwander, M.; Shirasaki, R.; Pfaff, S.L.; Müller, U. Beta1 integrins in muscle, but not in motor neurons, are required for skeletal muscle innervation. J. Neurosci. 2004, 24, 8181–8191. [Google Scholar] [CrossRef]
- Chakraborty, S.; Lakshmanan, M.; Swa, H.L.; Chen, J.; Zhang, X.; Ong, Y.S.; Loo, L.S.; Akıncılar, S.C.; Gunaratne, J.; Tergaonkar, V.; et al. An oncogenic role of agrin in regulating focal adhesion integrity in hepatocellular carcinoma. Nat. Commun. 2015, 6, 6184. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Njah, K.; Pobbati, A.V.; Lim, Y.B.; Raju, A.; Lakshmanan, M.; Tergaonkar, V.; Lim, C.T.; Hong, W. Agrin as a mechanotransduction signal regulating YAP through the Hippo pathway. Cell Rep. 2017, 18, 2464–2479. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Hong, W. Linking Extracellular Matrix Agrin to the Hippo Pathway in Liver Cancer and Beyond. Cancers 2018, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.C.; Mei, L. Agrin to YAP in cancer and neuromuscular junctions. Trends Cancer 2017, 3, 247–248. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Shen, C.; Lu, Y.; Huang, Z.; Li, L.; Rand, C.D.; Pan, J.; Sun, X.D.; Tan, Z.; Wang, H.; et al. Muscle Yap Is a regulator of neuromuscular junction formation and regeneration. J. Neurosci. 2017, 37, 3465–3477. [Google Scholar] [CrossRef] [PubMed]
- Njah, K.; Chakraborty, S.; Qiu, B.; Arumugam, S.; Raju, A.; Pobbati, A.V.; Lakshmanan, M.; Tergaonkar, V.; Thibault, G.; Wang, X.; et al. A role of Agrin in Maintaining the Stability of Vascular Endothelial Growth Factor Receptor-2 during Tumor Angiogenesis. Cell Rep. 2019, 28, 949–965. [Google Scholar] [CrossRef] [PubMed]




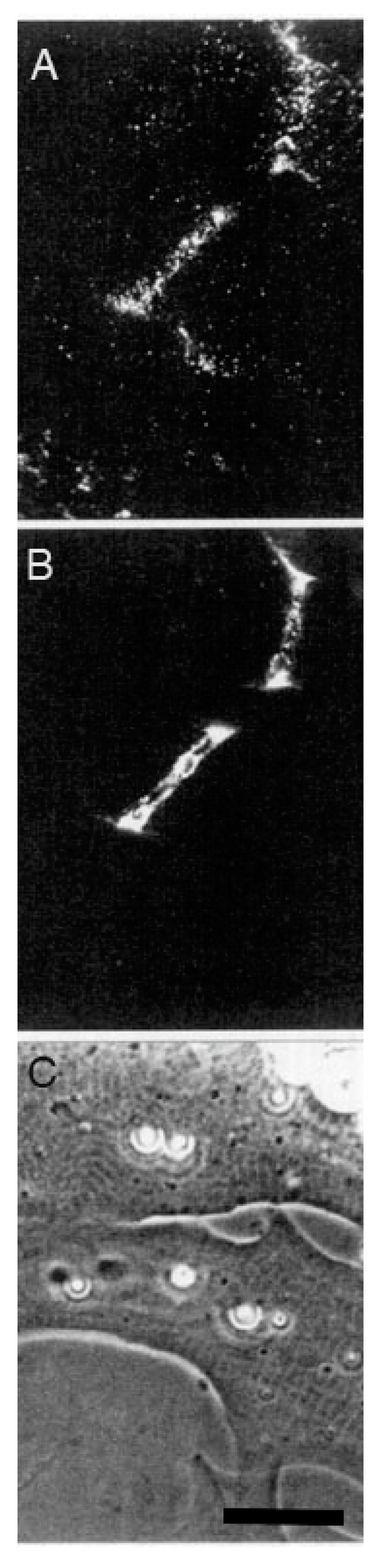
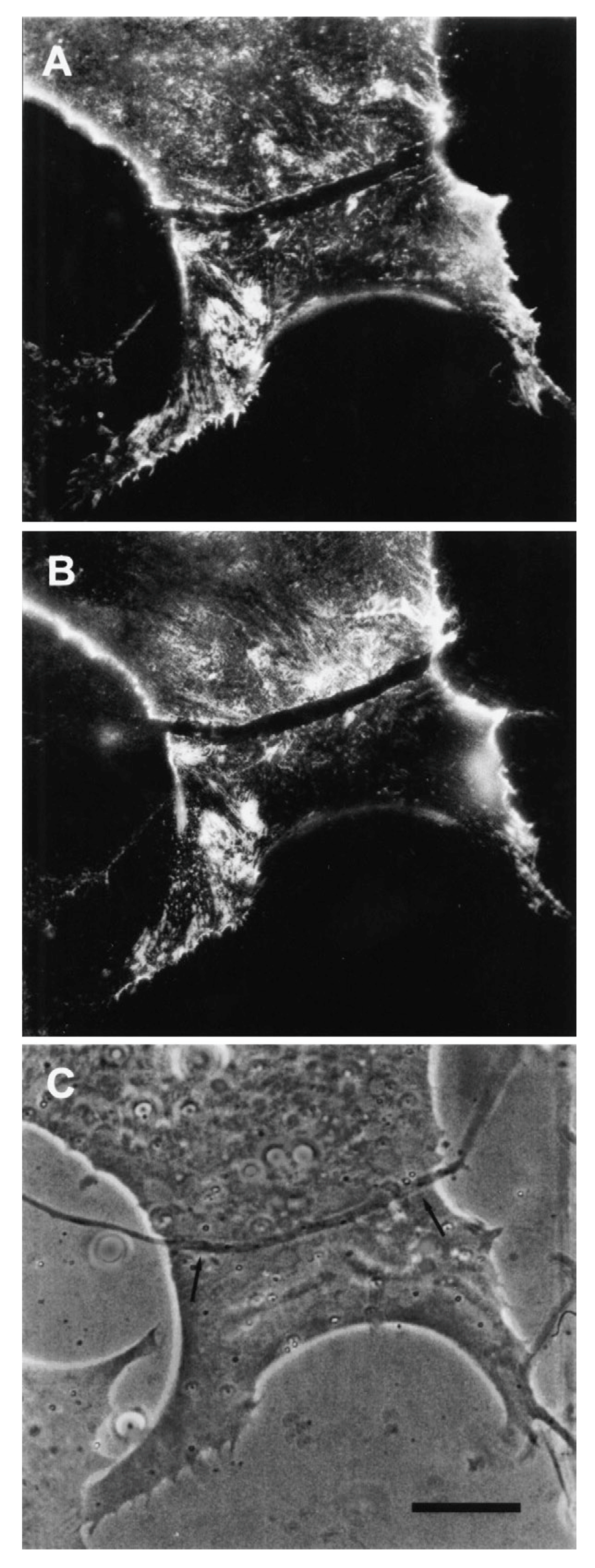

© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swenarchuk, L.E. Nerve, Muscle, and Synaptogenesis. Cells 2019, 8, 1448. https://doi.org/10.3390/cells8111448
Swenarchuk LE. Nerve, Muscle, and Synaptogenesis. Cells. 2019; 8(11):1448. https://doi.org/10.3390/cells8111448
Chicago/Turabian StyleSwenarchuk, Lauren Eric. 2019. "Nerve, Muscle, and Synaptogenesis" Cells 8, no. 11: 1448. https://doi.org/10.3390/cells8111448
APA StyleSwenarchuk, L. E. (2019). Nerve, Muscle, and Synaptogenesis. Cells, 8(11), 1448. https://doi.org/10.3390/cells8111448



