Direct Single-Cell Analysis of Human Polar Bodies and Cleavage-Stage Embryos Reveals No Evidence of the Telomere Theory of Reproductive Ageing in Relation to Aneuploidy Generation
Abstract
:1. Introduction
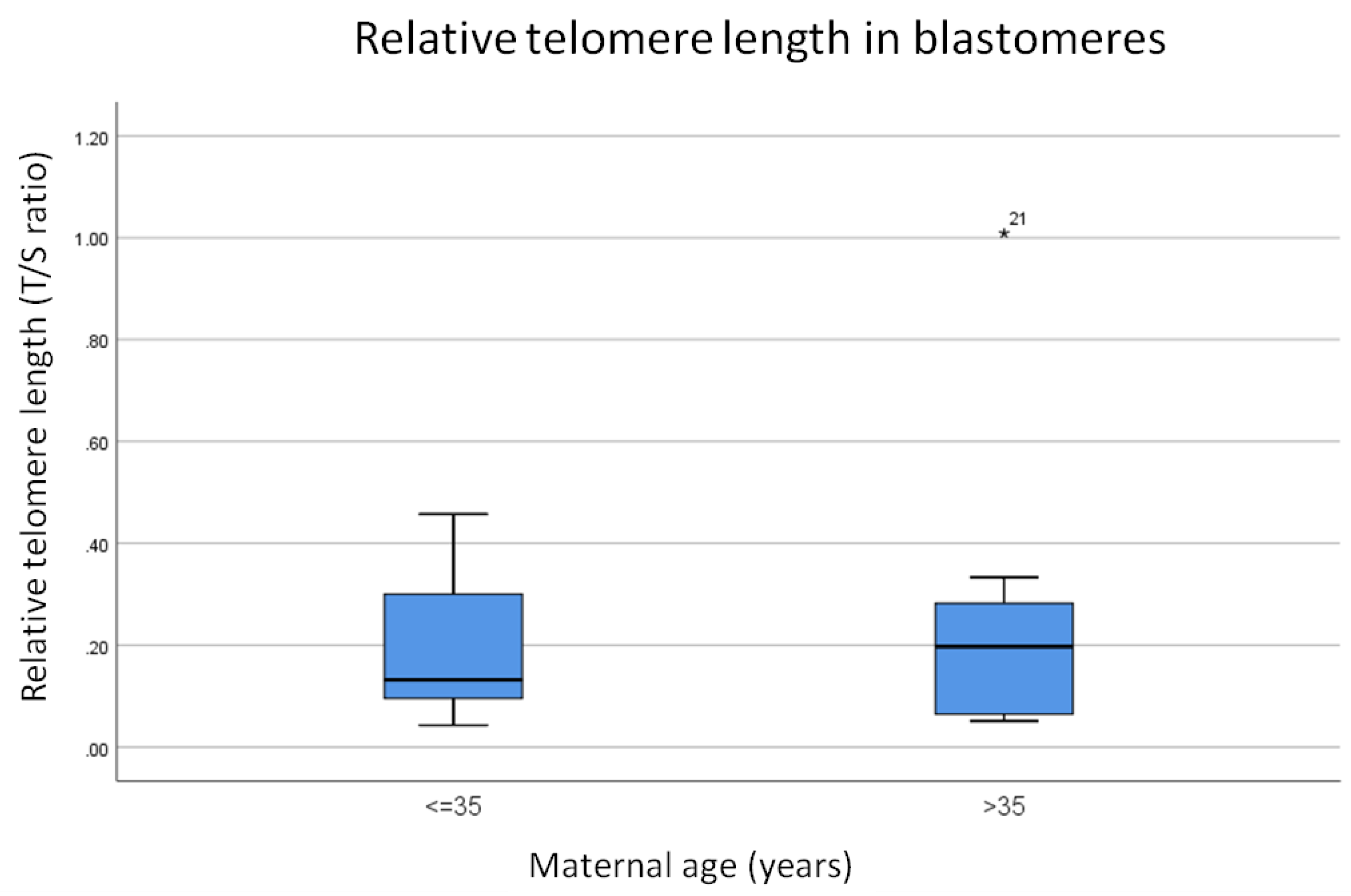
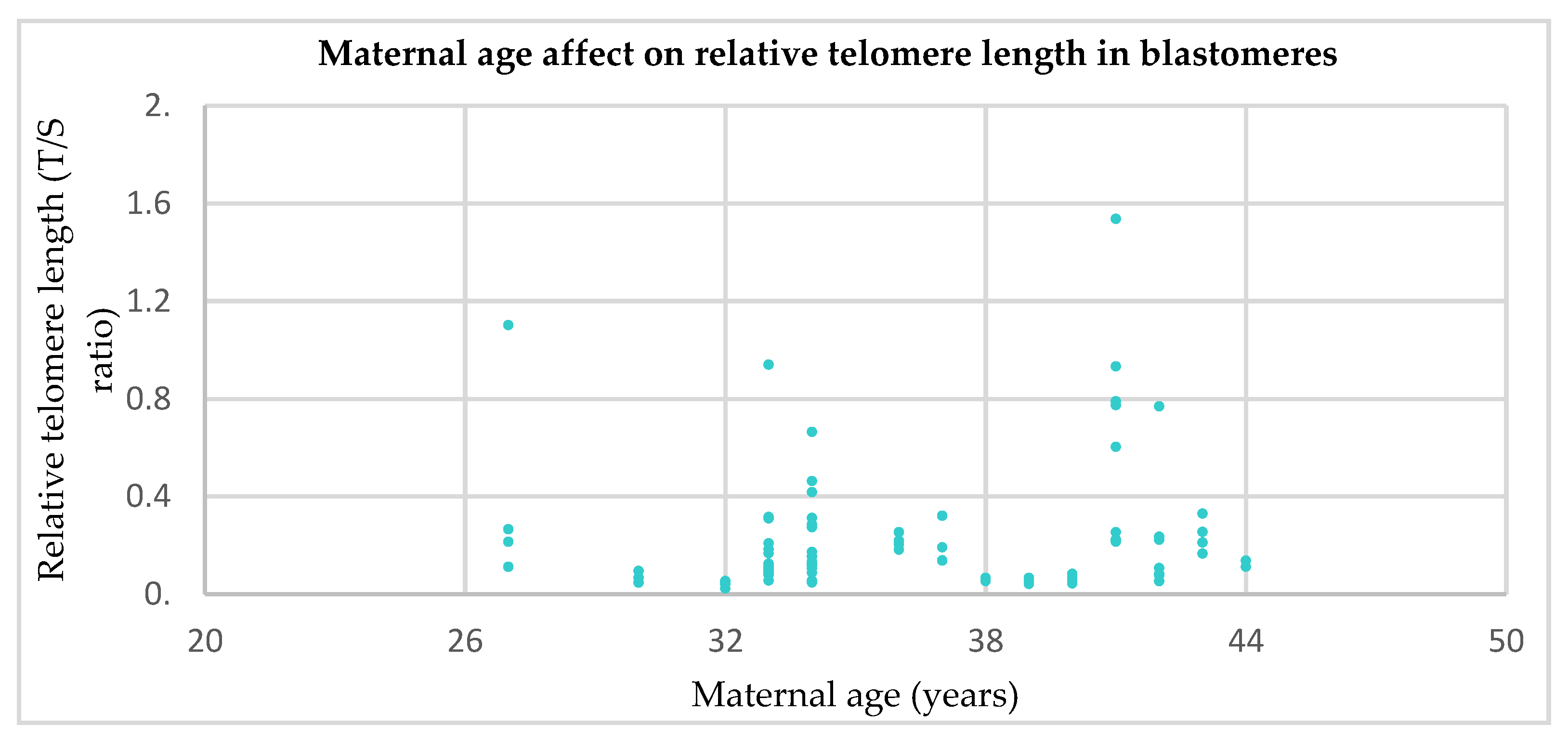
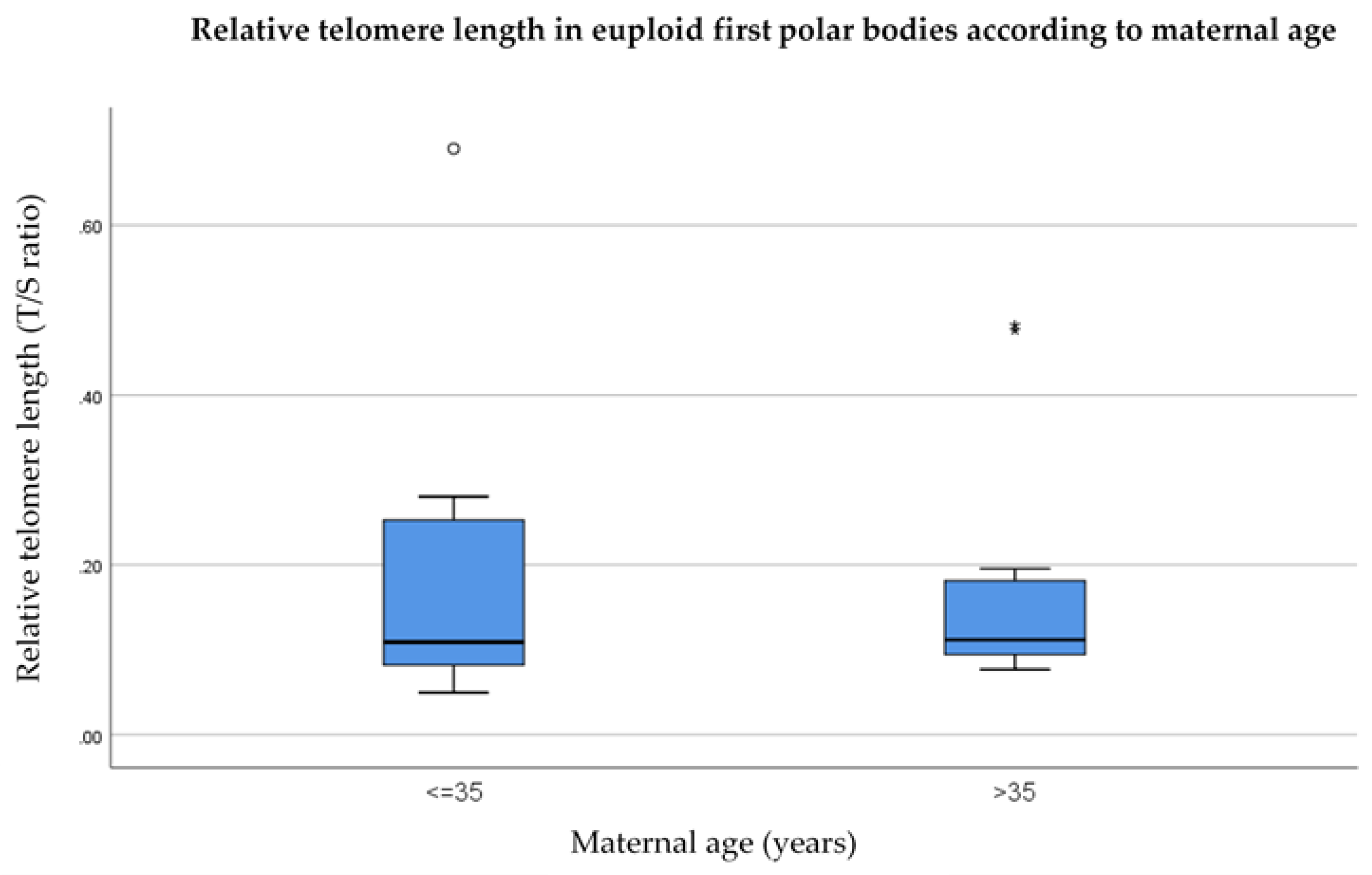
- Telomere length is significantly shorter in (a) the first polar bodies and (b) blastomeres from women of advanced maternal age (over the age of 35) compared to their younger counterparts.
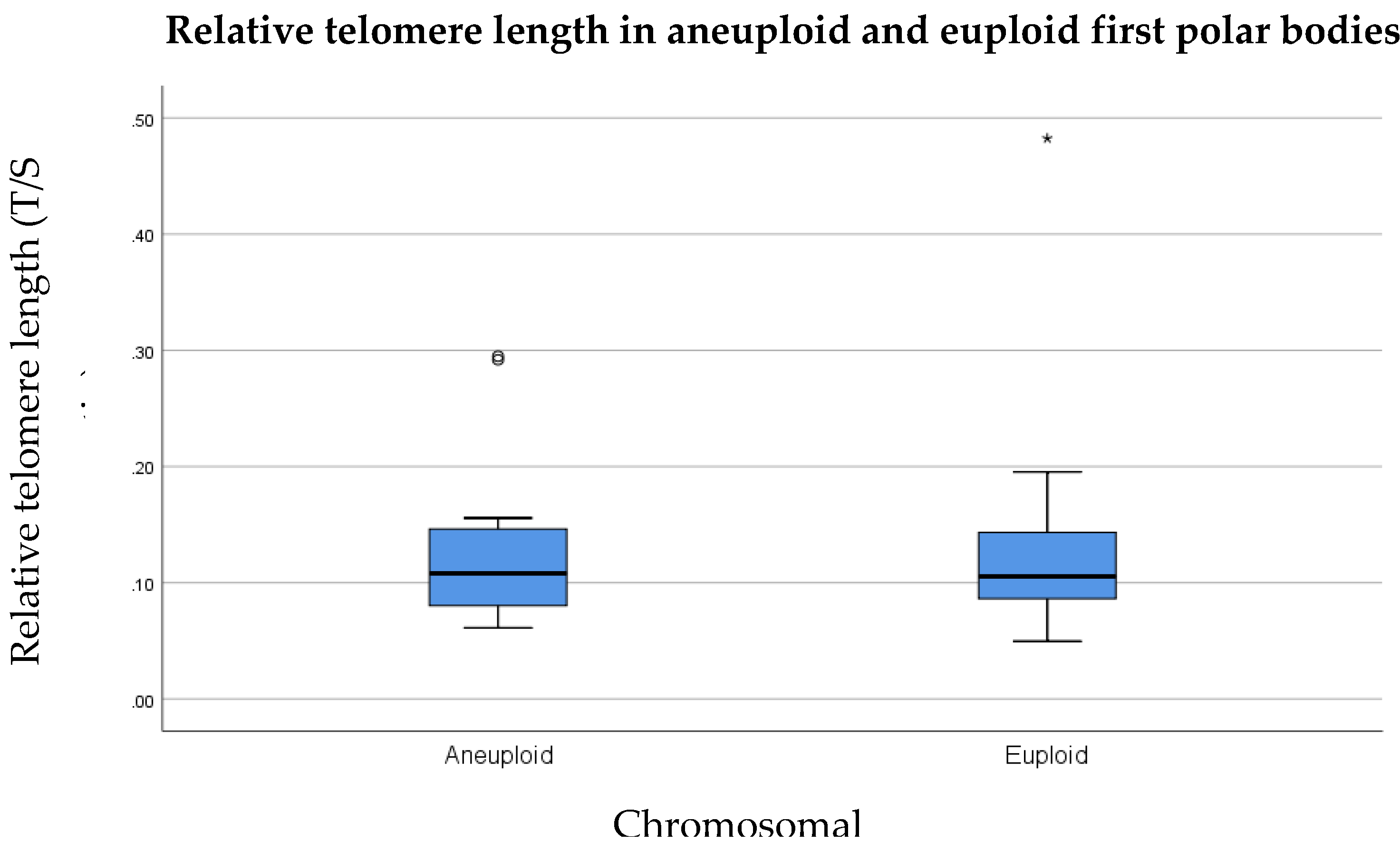
- Telomere length is significantly shorter in (a) first polar bodies, and (b) blastomeres that are involved in chromosome segregation errors compared to sibling euploid first polar bodies and embryos.
2. Materials and Methods
2.1. Whole Genome Amplification and Aneuploidy Screening
2.2. Whole Genome Amplification Product Purification
2.3. Quantitative Real-Time Polymerase Chain Reaction Analysis of Relative Telomere Length
2.4. Statistical Analyses
3. Results
3.1. Relative Telomere Length in the First Polar Bodies of Younger (≤35) Versus Older (>35) Donors
3.2. Relative Telomere Length in Cleavage Stage Embryos From Younger (≤35) Versus Older (>35) Mothers
3.3. Relative Telomere Length in Aneuploid Versus Euploid Polar Bodies
3.4. Relative Telomere Length in Aneuploid Versus Euploid Blastomeres
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cimadomo, D.; Fabozzi, G.; Vaiarelli, A.; Ubaldi, N.; Ubaldi, F.M.; Rienzi, L. Impact of maternal age on oocyte and embryo competence. Front. Endocrin. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Hassold, T.; Hunt, P. To err (meiotically) is human: The genesis of human aneuploidy. Nat. Rev. Genet. 2001, 2, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Aymé, S.; Lippman-Hand, A. Maternal-age effect in aneuploidy: Does altered embryonic selection play a role? Am. J. Hum. Genet. 1982, 34, 558. [Google Scholar] [PubMed]
- Capalbo, A.; Hoffmann, E.R.; Cimadomo, D.; Maria Ubaldi, F.; Rienzi, L. Human female meiosis revised: New insights into the mechanisms of chromosome segregation and aneuploidies from advanced genomics and time-lapse imaging. Hum. Reprod. Update 2017, 23, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, D.; Hornak, M.; Horak, J.; Navratil, R.; Tauwinklova, G.; Rubes, J.; Vesela, K. Incidence and origin of meiotic whole and segmental chromosomal aneuploidies detected by karyomapping. Reprod. Bio. Med. Online 2018. [Google Scholar] [CrossRef]
- Dorland, M.; Van Kooij, R.; Te Velde, E. General ageing and ovarian ageing. Maturitas 1998, 30, 113–118. [Google Scholar] [CrossRef]
- Freeman, S.B.; Yang, Q.; Allran, K.; Taft, L.F.; Sherman, S.L. Women with a reduced ovarian complement may have an increased risk for a child with Down syndrome. Am. J. Hum. Genet. 2000, 66, 1680–1683. [Google Scholar] [CrossRef]
- Munné, S.; Chen, S.; Fischer, J.; Colls, P.; Zheng, X.; Stevens, J.; Escudero, T.; Oter, M.; Schoolcraft, B.; Simpson, J.L. Preimplantation genetic diagnosis reduces pregnancy loss in women aged 35 years and older with a history of recurrent miscarriages. Fertil. Steril. 2005, 84, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Navot, D.; Bergh, R.; Williams, M.A.; Garrisi, G.J.; Guzman, I.; Sandler, B.; Grunfeld, L. Poor oocyte quality rather than implantation failure as a cause of age-related decline in female fertility. Lancet 1991, 337, 1375–1377. [Google Scholar] [CrossRef]
- Lamb, N.E.; Freeman, S.B.; Savage-Austin, A.; Pettay, D.; Taft, L.; Hersey, J.; Gu, Y.; Shen, J.; Saker, D.; May, K.M. Susceptible chiasmate configurations of chromosome 21 predispose to non–disjunction in both maternal meiosis I and meiosis II. Nat. Genet. 1996, 14, 400–405. [Google Scholar] [CrossRef]
- Keefe, D.L.; Marquard, K.; Liu, L. The telomere theory of reproductive senescence in women. Curr. Opin. Obstet. Gynecol. 2006, 18, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R. Are oocytes formed and used sequentially in the mammalian ovary? Philos. Trans. R. Soc. Lond. B Biol. Sci. 1970, 259, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Polani, P.E.; Crolla, J.A. A test of the production line hypothesis of mammalian oogenesis. Hum. Genet. 1991, 88, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Passos, J.o.F.; von Zglinicki, T. Mitochondria, telomeres and cell senescence. Exp. Gerontol. 2005, 40, 466–472. [Google Scholar] [CrossRef]
- Cooper, J.P.; Watanabe, Y.; Nurse, P. Fission yeast Taz1 protein is required for meiotic telomere clustering and recombination. Nature 1998, 392, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Dernburg, A.F.; Sedat, J.W.; Cande, W.Z.; Bass, H.W. Cytology of Telomeres; Cold Spring Harbor Laboratory: New York, NY, USA, 1995; Volume 29, pp. 295–338. [Google Scholar]
- Keefe, D.L.; Franco, S.; Liu, L.; Trimarchi, J.; Cao, B.; Weitzen, S.; Agarwal, S.; Blasco, M.A. Telomere length predicts embryo fragmentation after in vitro fertilization in women—toward a telomere theory of reproductive aging in women. Am. J. Obstet. Gynecol. 2005, 192, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Franco, S.; Spyropoulos, B.; Moens, P.B.; Blasco, M.A.; Keefe, D.L. Irregular telomeres impair meiotic synapsis and recombination in mice. Proc. Natl. Acad. Sci. USA 2004, 101, 6496–6501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimmo, E.R.; Pidoux, A.L.; Perry, P.E.; Allshire, R.C. Defective meiosis in telomere-silencing mutants of Schizosaccharomyces pombe. Nature 1998, 392, 825–828. [Google Scholar] [CrossRef]
- Liu, L.; Blasco, M.A.; Keefe, D.L. Requirement of functional telomeres for metaphase chromosome alignments and integrity of meiotic spindles. EMBO Rep. 2002, 3, 230–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Blasco, M.A.; Trimarchi, J.R.; Keefe, D.L. An essential role for functional telomeres in mouse germ cells during fertilization and early development. Dev. Biol. 2002, 249, 74–84. [Google Scholar] [CrossRef]
- Ghosh, S.; Feingold, E.; Chakraborty, S.; Dey, S.K. Telomere length is associated with types of chromosome 21 nondisjunction: A new insight into the maternal age effect on Down syndrome birth. Hum. Genet. 2010, 127, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Hartshorne, G.M. Telomere lengths in human pronuclei, oocytes and spermatozoa. Mol. Hum. Reprod. 2013. [Google Scholar] [CrossRef] [PubMed]
- Treff, N.R.; Su, J.; Taylor, D.; Scott, R.T., Jr. Telomere DNA deficiency is associated with development of human embryonic aneuploidy. PLoS Genet. 2011, 7, e1002161. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Schoolcraft, W.B.; Katz-Jaffe, M.G.; Stevens, J.; Rawlins, M.; Munne, S. Preimplantation aneuploidy testing for infertile patients of advanced maternal age: A randomized prospective trial. Fertil. Steril. 2009, 92, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere length measurement by a novel mon°Chrome multiplex quantitative PCR method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Wong, H.P.; Rai, J.; Hartshorne, G.M. Telomere lengths in human oocytes, cleavage stage embryos and blastocysts. Mol. Hum. Reprod. 2010, 16, 685–694. [Google Scholar] [CrossRef] [Green Version]
- Mania, A.; Mantzouratou, A.; Delhanty, J.D.; Baio, G.; Serhal, P.; Sengupta, S.B. Telomere length in human blastocysts. Reprod. Biomed. Online 2014, 28, 624–637. [Google Scholar] [CrossRef]
- Vasu, V.; Turner, K.J.; George, S.; Greenall, J.; Slijepcevic, P.; Griffin, D.K. Preterm infants have significantly longer telomeres than their term born counterparts. PLoS ONE 2017, 12, e0180082. [Google Scholar] [CrossRef]
- Youngren, K.; Jeanclos, E.; Aviv, H.; Kimura, M.; Stock, J.; Hanna, M.; Skurnick, J.; Bardeguez, A.; Aviv, A. Synchrony in telomere length of the human fetus. Hum. Genet. 1998, 102, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.K.; Bellantuono, I.; Walkinshaw, S.A.; Alfirevic, Z.; Johnston, T.A.; Subhedar, N.V.; Chittick, R.; Swindell, R.; Wynn, R.F. Telomere length dynamics differ in foetal and early post-natal human leukocytes in a longitudinal study. Biogerontology 2009, 10, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Bardeguez, A.; Gardner, J.P.; Rodriguez, P.; Ganesh, V.; Kimura, M.; Skurnick, J.; Awad, G.; Aviv, A. Telomere length in the newborn. Pediatr. Res. 2002, 52, 377. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W.; Blackburn, E.H.; Shannon, K.M. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl. Acad. Sci. USA 1998, 95, 5607–5610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rufer, N.; Dragowska, W.; Thornbury, G.; Roosnek, E.; Lansdorp, P.M. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat. Biotechnol. 1998, 16, 743. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, P.M. Telomeres, stem cells, and hematology. Blood 2008, 111, 1759–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, D.L.; Jones, E.L.; Mayer, J.F.; Oehninger, S.; Gibbons, W.E.; Lanzendorf, S.E. Characterization of telomerase activity in the human oocyte and preimplantation embryo. Mol. Hum. Reprod. 2001, 7, 947–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, D.H.; King, W.A. Telomerase activity and telomere detection during early bovine development. Dev. Genet. 1999, 25, 397–403. [Google Scholar] [CrossRef]
- Eisenhauer, K.M.; Gerstein, R.M.; Chiu, C.-P.; Conti, M.; Hsueh, A. Telomerase activity in female and male rat germ cells undergoing meiosis and in early embryos. Biol. Reprod. 1997, 56, 1120–1125. [Google Scholar] [CrossRef] [Green Version]
- Allsopp, R.C.; Vaziri, H.; Patterson, C.; Goldstein, S.; Younglai, E.V.; Futcher, A.B.; Greider, C.W.; Harley, C.B. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA 1992, 89, 10114–10118. [Google Scholar] [CrossRef]
- Nordfjäll, K.; Svenson, U.; Norrback, K.-F.; Adolfsson, R.; Lenner, P.; Roos, G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009, 5, e1000375. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.M.; Britt-Compton, B.; Rowson, J.; Amso, N.N.; Gregory, L.; Kipling, D. Telomere instability in the male germline. Hum. Mol. Genet. 2006, 15, 45–51. [Google Scholar] [CrossRef]
- Kimura, M.; Cherkas, L.F.; Kato, B.S.; Demissie, S.; Hjelmborg, J.B.; Brimacombe, M.; Cupples, A.; Hunkin, J.L.; Gardner, J.P.; Lu, X.; et al. Offspring’s Leukocyte Telomere Length, Paternal Age, and Telomere Elongation in Sperm. PLoS Genet. 2008, 4, e37. [Google Scholar] [CrossRef]
- Njajou, O.T.; Cawthon, R.M.; Damcott, C.M.; Wu, S.H.; Ott, S.; Garant, M.J.; Blackburn, E.H.; Mitchell, B.D.; Shuldiner, A.R.; Hsueh, W.C. Telomere length is paternally inherited and is associated with parental lifespan. Proc. Natl. Acad. Sci. USA 2007, 104, 12135–12139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unryn, B.M.; Cook, L.S.; Riabowol, K.T. Paternal age is positively linked to telomere length of children. Aging Cell 2005, 4, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Bailey, S.M.; Okuka, M.; Munoz, P.; Li, C.; Zhou, L.; Wu, C.; Czerwiec, E.; Sandler, L.; Seyfang, A.; et al. Telomere lengthening early in development. Nat. Cell Biol. 2007, 9, 1436–1441. [Google Scholar] [CrossRef]
- Hemann, M.T.; Strong, M.A.; Hao, L.-Y.; Greider, C.W. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 2001, 107, 67–77. [Google Scholar] [CrossRef]
- Lansdorp, P.M.; Verwoerd, N.P.; Van De Rijke, F.M.; Dragowska, V.; Little, M.-T.; Dirks, R.W.; Raap, A.K.; Tanke, H.J. Heterogeneity in telomere length of human chromosomes. Human Mol. Genet. 1996, 5, 685–691. [Google Scholar] [CrossRef] [Green Version]
- Burgoyne, P.S.; Mahadevaiah, S.K.; Turner, J.M. The consequences of asynapsis for mammalian meiosis. Nat. Rev. Genet. 2009, 10, 207–216. [Google Scholar] [CrossRef]
- Wang, H.; Höög, C. Structural damage to meiotic chromosomes impairs DNA recombination and checkpoint control in mammalian oocytes. J. Cell Biol. 2006, 173, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Colls, P.; Escudero, T.; Cekleniak, N.; Sadowy, S.; Cohen, J.; Munné, S. Increased efficiency of preimplantation genetic diagnosis for infertility using “no result rescue”. Fertil. Steril. 2007, 88, 53–61. [Google Scholar] [CrossRef] [PubMed]
- DeUgarte, C.M.; Li, M.; Surrey, M.; Danzer, H.; Hill, D.; DeCherney, A.H. Accuracy of FISH analysis in predicting chromosomal status in patients undergoing preimplantation genetic diagnosis. Fertil. Steril. 2008, 90, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Harton, G.; Magli, M.; Lundin, K.; Montag, M.; Lemmen, J.; Harper, J. ESHRE PGD Consortium/Embryology Special Interest Group—best practice guidelines for polar body and embryo biopsy for preimplantation genetic diagnosis/screening (PGD/PGS). Hum. Reprod. 2011, 26, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, R.M. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002, 30, e47. [Google Scholar] [CrossRef] [PubMed]
- Treff, N.R.; Su, J.; Tao, X.; Northrop, L.E.; Scott, R.T. Single-cell whole-genome amplification technique impacts the accuracy of SNP microarray-based genotyping and copy number analyses. Mol. Hum. Reprod. 2011, 17, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, J.A.; Buel, E. Simultaneous Determination of Total Human and Male DNA Using a Duplex Real-Time PCR Assay. J. Forensic. Sci. 2006, 51, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Otieno, A.C.; Carter, A.B.; Hedges, D.J.; Walker, J.A.; Ray, D.A.; Garber, R.K.; Anders, B.A.; Stoilova, N.; Laborde, M.E.; Fowlkes, J.D. Analysis of the Human Alu Ya-lineage. J. Mol. Biol. 2004, 342, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Gelbaya, T.A.; Potdar, N.; Jeve, Y.B.; Nardo, L.G. Definition and epidemiology of unexplained infertility. Obstet Gynecol. Surv. 2014, 69, 109–115. [Google Scholar] [CrossRef]
- Artandi, S.E.; Chang, S.; Lee, S.-L.; Alson, S.; Gottlieb, G.J.; Chin, L.; DePinho, R.A. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 2000, 406, 641–645. [Google Scholar] [CrossRef]
- Scherthan, H. Telomere attachment and clustering during meiosis. Cell Mol. Life Sci. 2007, 64, 117–124. [Google Scholar] [CrossRef]




| Name | Design | Reference |
|---|---|---|
| TelG | ACACTAAGGTTTGGGTTTGGGTTTGGGTTTGGGTTAGTGT | [27] |
| TelC | TGTTAGGTATCCCTATCCCTATCCCTATCCCTATCCCTAACA | |
| AluF | GACCATCCCGGCTAAAACG | [24] |
| AluR | CGGGTTCACGCCATTCTC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, K.; Lynch, C.; Rouse, H.; Vasu, V.; Griffin, D.K. Direct Single-Cell Analysis of Human Polar Bodies and Cleavage-Stage Embryos Reveals No Evidence of the Telomere Theory of Reproductive Ageing in Relation to Aneuploidy Generation. Cells 2019, 8, 163. https://doi.org/10.3390/cells8020163
Turner K, Lynch C, Rouse H, Vasu V, Griffin DK. Direct Single-Cell Analysis of Human Polar Bodies and Cleavage-Stage Embryos Reveals No Evidence of the Telomere Theory of Reproductive Ageing in Relation to Aneuploidy Generation. Cells. 2019; 8(2):163. https://doi.org/10.3390/cells8020163
Chicago/Turabian StyleTurner, Kara, Colleen Lynch, Hannah Rouse, Vimal Vasu, and Darren K. Griffin. 2019. "Direct Single-Cell Analysis of Human Polar Bodies and Cleavage-Stage Embryos Reveals No Evidence of the Telomere Theory of Reproductive Ageing in Relation to Aneuploidy Generation" Cells 8, no. 2: 163. https://doi.org/10.3390/cells8020163





