Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Primary Culture of MSC
2.2. Isolation of Extracellular Vesicles by Differential Centrifugation
2.3. Blood Sampling
2.4. Rotational Thromboelastometry (ROTEM)
2.5. Thrombodynamics Assay
2.6. Anti-Xa Assay
2.7. Transmission Electron Microscopy
2.8. Nanoparticle Tracking Analysis
2.9. Proteomic Analysis of MSCs and EVs
2.10. Flow Cytometry Analysis of Phosphatidylserine Exposure on MSC and EV Fractions
2.11. Western Blotting
2.12. Modulation of MSC Procoagulant Activity by Heparin and Annexin V
2.13. Statistical Analysis
3. Results
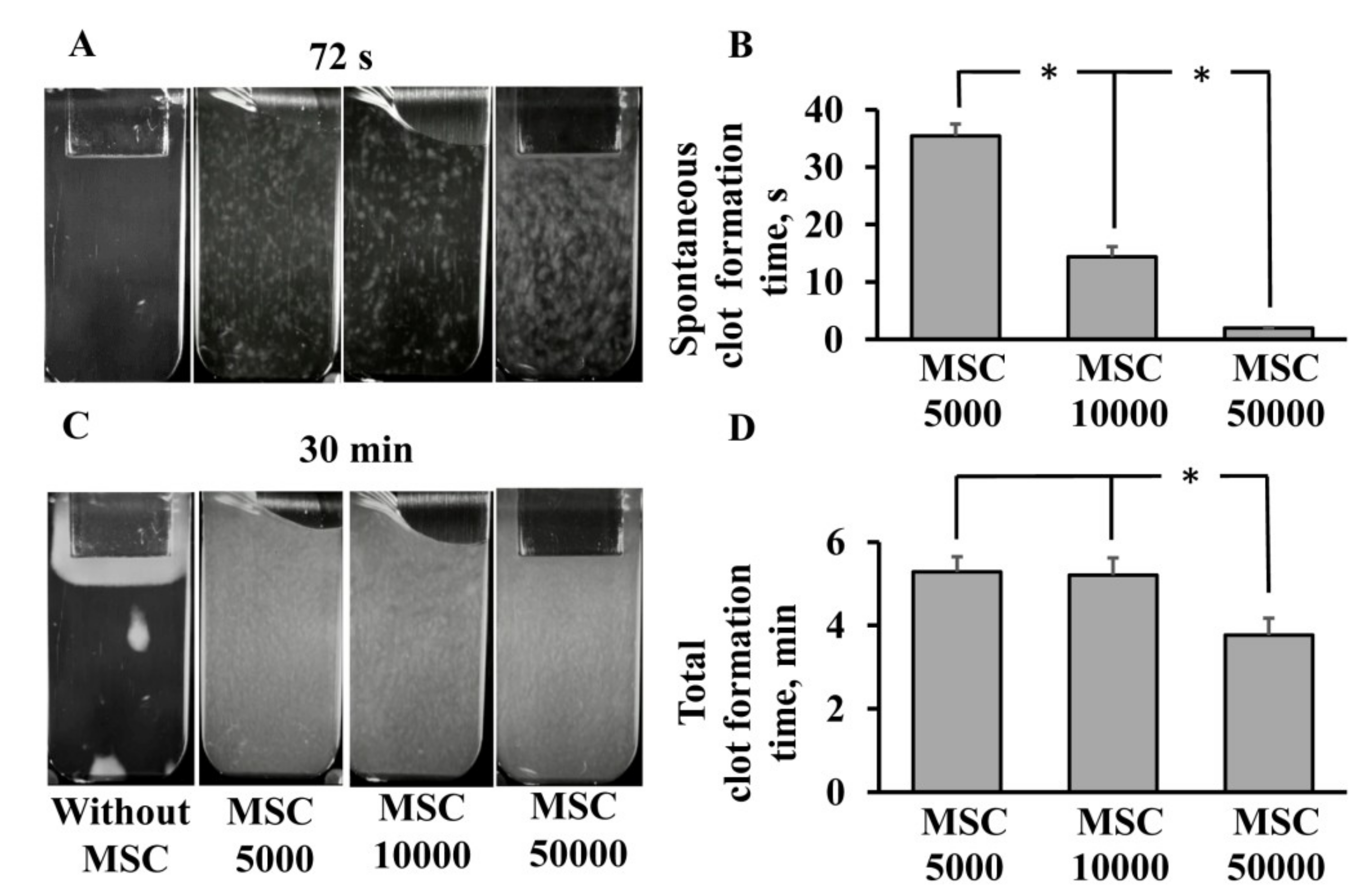
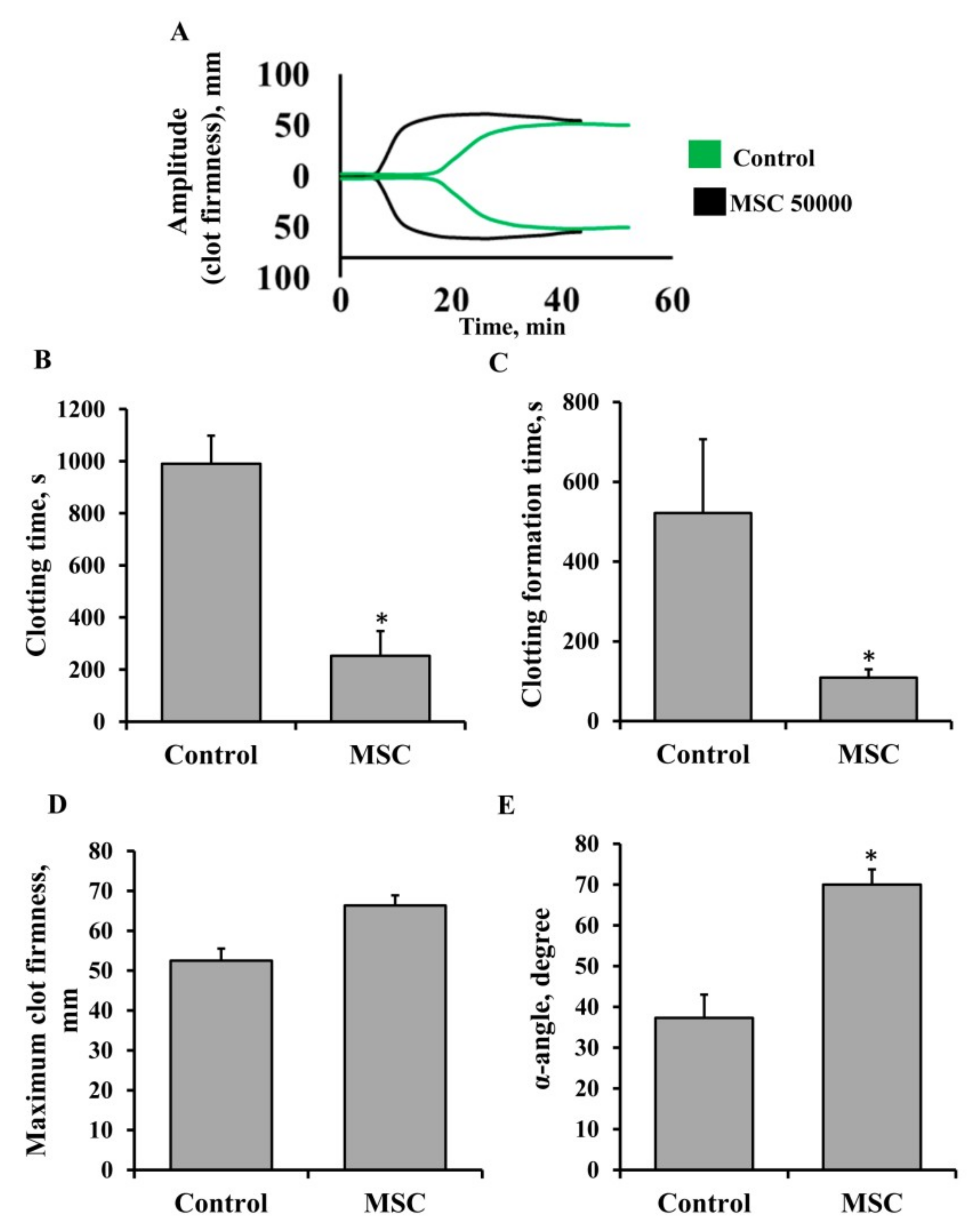
3.1. Procoagulant Properties of MSCs against Human Blood
3.2. Effect of Extracellular Vesicles on Blood Coagulation
3.3. Elucidation of Mechanisms Involved in MSC- and EV-Induced Coagulation
3.4. Analysis of Coagulation-Associated Proteins in MSCs and Extracellular Vesicles
3.5. Procoagulant Activity of MSCs in Patients Undergoing Anticoagulant Therapy with Heparin
3.6. Modulation of MSC Procoagulant Activity by Heparin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef]
- Horák, J.; Nalos, L.; Martínková, V.; Beneš, J.; Štengl, M.; Matějovič, M. Mesenchymal Stem Cells in Sepsis and Associated Organ Dysfunction: A Promising Future or Blind Alley? Stem Cells Int. 2017, 2017, 7304121. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, X.; Li, X. Exosomes derived from mesenchymal stem cells. Int. J. Mol. Sci. 2014, 15, 4142–4157. [Google Scholar] [CrossRef]
- Zhang, B.; Yin, Y.; Lai, R.C.; Lim, S.K. Immunotherapeutic potential of extracellular vesicles. Front. Immunol. 2014, 5, 518. [Google Scholar] [CrossRef]
- Börger, V.; Bremer, M.; Ferrer-Tur, R.; Gockeln, L.; Stambouli, O.; Becic, A.; Giebel, B. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. Int. J. Mol. Sci. 2017, 18, 1450. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials with Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Tatsumi, K.; Ohashi, K.; Matsubara, Y.; Kohori, A.; Ohno, T.; Kakidachi, H.; Horii, A.; Kanegae, K.; Utoh, R.; Iwata, T.; et al. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem. Biophys. Res. Commun. 2013, 431, 203–209. [Google Scholar] [CrossRef]
- Ozdemir, E.; Kansu, E. Deep Vein Thrombosis Following Non-myeloablative Allogeneic Stem Cell Transplantation: Presentation of Three Cases and Literature Review. Turk. J. Haematol. 2013, 30, 188–190. [Google Scholar] [CrossRef]
- Lim, R.; Malhotra, A.; Tan, J.; Chan, S.T.; Lau, S.; Zhu, D.; Mockler, J.C.; Wallace, E.M. First-In-Human Administration of Allogeneic Amnion Cells in Premature Infants With Bronchopulmonary Dysplasia: A Safety Study. Stem Cells Transl. Med. 2018, 7, 628–635. [Google Scholar] [CrossRef]
- Jung, J.W.; Kwon, M.; Choi, J.C.; Shin, J.W.; Park, I.W.; Choi, B.W.; Kim, J.Y. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med. J. 2013, 54, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Perlee, D.; van Vught, L.A.; Scicluna, B.P.; Maag, A.; Lutter, R.; Kemper, E.M.; van ’t Veer, C.; Punchard, M.A.; González, J.; Richard, M.P.; et al. Intravenous Infusion of Human Adipose Mesenchymal Stem Cells Modifies the Host Response to Lipopolysaccharide in Humans: A Randomized, Single-Blind, Parallel Group, Placebo Controlled Trial. Stem Cells 2018. [Google Scholar] [CrossRef] [PubMed]
- Stéphenne, X.; Vosters, O.; Najimi, M.; Beuneu, C.; Dung, K.N.; Wijns, W.; Goldman, M.; Sokal, E.M. Tissue factor-dependent procoagulant activity of isolated human hepatocytes: Relevance to liver cell transplantation. Liver Transpl. 2007, 13, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Beuneu, C.; Vosters, O.; Movahedi, B.; Remmelink, M.; Salmon, I.; Pipeleers, D.; Pradier, O.; Goldman, M.; Verhasselt, V. Human pancreatic duct cells exert tissue factor-dependent procoagulant activity: Relevance to islet transplantation. Diabetes 2004, 53, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Rasmusson-Duprez, I.; von Bahr, L.; Connolly-Andersen, A.-M.; Elgue, G.; Funke, L.; Hamad, O.A.; Lönnies, H.; Magnusson, P.U.; Sanchez, J.; et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells 2012, 30, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Christy, B.A.; Herzig, M.C.; Montgomery, R.K.; Delavan, C.; Bynum, J.A.; Reddoch, K.M.; Cap, A.P. Procoagulant activity of human mesenchymal stem cells. J. Trauma Acute Care Surg. 2017, 83, S164–S169. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Shi, B.; Chang, H.; Su, X.; Zhang, L.; Bi, C.; Shuai, Y.; Du, X.; Deng, Z.; Jin, Y. Heparin improves BMSC cell therapy: Anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics 2017, 7, 106–116. [Google Scholar] [CrossRef]
- Spronk, H.M.H.; ten Cate, H.; van der Meijden, P.E.J. Differential roles of tissue factor and phosphatidylserine in activation of coagulation. Thromb. Res. 2014, 133 (Suppl. 1), S54–S56. [Google Scholar] [CrossRef]
- Stephenne, X.; Nicastro, E.; Eeckhoudt, S.; Hermans, C.; Nyabi, O.; Lombard, C.; Najimi, M.; Sokal, E. Bivalirudin in combination with heparin to control mesenchymal cell procoagulant activity. PLoS ONE 2012, 7, e42819. [Google Scholar] [CrossRef]
- Makatsaria, A.D.; Bitsadze, V.O.; Akinshina, S.V. Severe forms of preeclampsia as a manifestation of thrombotic microangiopathy. Akusherstvo i Ginekologiya (Russian Federation) 2017, 21–26. [Google Scholar] [CrossRef]
- Shamshirsaz, A.A.; Paidas, M.; Krikun, G. Preeclampsia, hypoxia, thrombosis, and inflammation. J. Pregnancy 2012, 2012, 374047. [Google Scholar] [CrossRef] [PubMed]
- Saracco, P.; Bagna, R.; Gentilomo, C.; Magarotto, M.; Viano, A.; Magnetti, F.; Giordano, P.; Luciani, M.; Molinari, A.C.; Suppiej, A.; et al. Neonatal Working Group of Registro Italiano Trombosi Infantili (RITI) Clinical Data of Neonatal Systemic Thrombosis. J. Pediatr. 2016, 171, 60–66.e1. [Google Scholar] [CrossRef]
- Sukhikh, G.T.; Silachev, D.N.; Goryunov, K.V.; Volochaeva, M.V.; Shmakov, R.G. Role of stem cell dysfunction in the development of great obstetrical syndromes. Akusherstvo i Ginekologiya (Russian Federation) 2018, 5–11. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, L.; Collins, J.J.P.; Vadivel, A.; Cyr-Depauw, C.; Zhong, S.; Mense, L.; Möbius, M.A.; Thébaud, B. Human Umbilical Cord Mesenchymal Stromal Cells Improve Survival and Bacterial Clearance in Neonatal Sepsis in Rats. Stem Cells Dev. 2017, 26, 1054–1064. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Inflammation and coagulation. Crit. Care Med. 2010, 38, S26–S34. [Google Scholar] [CrossRef] [PubMed]
- Franquesa, M.; Hoogduijn, M.J.; Ripoll, E.; Luk, F.; Salih, M.; Betjes, M.G.H.; Torras, J.; Baan, C.C.; Grinyó, J.M.; Merino, A.M. Update on controls for isolation and quantification methodology of extracellular vesicles derived from adipose tissue mesenchymal stem cells. Front. Immunol. 2014, 5, 525. [Google Scholar] [CrossRef] [PubMed]
- Soshitova, N.P.; Karamzin, S.S.; Balandina, A.N.; Fadeeva, O.A.; Kretchetova, A.V.; Galstian, G.M.; Panteleev, M.A.; Ataullakhanov, F.I. Predicting prothrombotic tendencies in sepsis using spatial clot growth dynamics. Blood Coagul. Fibrinolysis 2012, 23, 498–507. [Google Scholar] [CrossRef]
- E56 Committee. Guide for Measurement of Particle Size Distribution of Nanomaterials in Suspension by Nanoparticle Tracking Analysis (NTA); ASTM International: Sydney, Australia, 2018. [Google Scholar]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Kononikhin, A.S.; Starodubtseva, N.L.; Chagovets, V.V.; Ryndin, A.Y.; Burov, A.A.; Popov, I.A.; Bugrova, A.E.; Dautov, R.A.; Tokareva, A.O.; Podurovskaya, Y.L.; et al. Exhaled breath condensate analysis from intubated newborns by nano-HPLC coupled to high resolution MS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2017, 1047, 97–105. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Csordas, A.; Bai, J.; Bernal-Llinares, M.; Hewapathirana, S.; Kundu, D.J.; Inuganti, A.; Griss, J.; Mayer, G.; Eisenacher, M.; et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019, 47, D442–D450. [Google Scholar] [CrossRef]
- Davies, J.E.; Walker, J.T.; Keating, A. Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Transl. Med. 2017, 6, 1620–1630. [Google Scholar] [CrossRef]
- Kim, D.-W.; Staples, M.; Shinozuka, K.; Pantcheva, P.; Kang, S.-D.; Borlongan, C.V. Wharton’s jelly-derived mesenchymal stem cells: Phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int. J. Mol. Sci. 2013, 14, 11692–11712. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Hochberg, F.H.; Jones, P.S. Extracellular vesicles: The growth as diagnostics and therapeutics; a survey. J. Extracell. Vesicles 2018, 7, 1438720. [Google Scholar] [CrossRef] [PubMed]
- György, B.; Hung, M.E.; Breakefield, X.O.; Leonard, J.N. Therapeutic applications of extracellular vesicles: Clinical promise and open questions. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 439–464. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.; Yan, I.K.; Parasramka, M.; Mohankumar, S.; Matsuda, A.; Patel, T. In vitro toxicology studies of extracellular vesicles. J. Appl. Toxicol. 2017, 37, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-S.; Choi, D.-Y.; Yun, S.J.; Choi, S.-M.; Kang, J.W.; Jung, J.W.; Hwang, D.; Kim, K.P.; Kim, D.-W. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J. Proteome Res. 2012, 11, 839–849. [Google Scholar] [CrossRef]
- Garnier, D.; Magnus, N.; Lee, T.H.; Bentley, V.; Meehan, B.; Milsom, C.; Montermini, L.; Kislinger, T.; Rak, J. Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J. Biol. Chem. 2012, 287, 43565–43572. [Google Scholar] [CrossRef]
- Gardiner, C.; Harrison, P.; Belting, M.; Böing, A.; Campello, E.; Carter, B.S.; Collier, M.E.; Coumans, F.; Ettelaie, C.; van Es, N.; et al. Extracellular vesicles, tissue factor, cancer and thrombosis—Discussion themes of the ISEV 2014 Educational Day. J. Extracell. Vesicles 2015, 4, 26901. [Google Scholar] [CrossRef]
- Moll, G.; Ignatowicz, L.; Catar, R.; Luecht, C.; Sadeghi, B.; Hamad, O.; Jungebluth, P.; Dragun, D.; Schmidtchen, A.; Ringdén, O. Different Procoagulant Activity of Therapeutic Mesenchymal Stromal Cells Derived from Bone Marrow and Placental Decidua. Stem Cells Dev. 2015, 24, 2269–2279. [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Marchetti, M.; Vignoli, A. Coagulation and cancer: Biological and clinical aspects. J. Thromb. Haemost. 2013, 11, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Geddings, J.E.; Mackman, N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood 2013, 122, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Bach, R.R.; Horton, R.; Konigsberg, W.H.; Todd, M.B. Procoagulant activity in cancer cells is dependent on tissue factor expression. Oncol. Res. 1994, 6, 321–327. [Google Scholar]
- Dvorak, H.F.; Van DeWater, L.; Bitzer, A.M.; Dvorak, A.M.; Anderson, D.; Harvey, V.S.; Bach, R.; Davis, G.L.; DeWolf, W.; Carvalho, A.C. Procoagulant activity associated with plasma membrane vesicles shed by cultured tumor cells. Cancer Res. 1983, 43, 4434–4442. [Google Scholar] [PubMed]
- Rao, L.V. Tissue factor as a tumor procoagulant. Cancer Metastasis Rev. 1992, 11, 249–266. [Google Scholar] [PubMed]
- Sugimura, M.; Donato, R.; Kakkar, V.V.; Scully, M.F. Annexin V as a probe of the contribution of anionic phospholipids to the procoagulant activity of tumour cell surfaces. Blood Coagul. Fibrinolysis 1994, 5, 365–373. [Google Scholar]
- Zhao, L.; Bi, Y.; Kou, J.; Shi, J.; Piao, D. Phosphatidylserine exposing-platelets and microparticles promote procoagulant activity in colon cancer patients. J. Exp. Clin. Cancer Res. 2016, 35, 54. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Lim, S.K. Membrane Lipids Define Small Extracellular Vesicle Subtypes Secreted by Mesenchymal Stromal Cell. J. Lipid Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- van der Poll, T.; Herwald, H. The coagulation system and its function in early immune defense. Thromb. Haemost. 2014, 112, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, B.M.; Martin, K.; Ali, M.T.; Kumar, A.H.S.; Pillai, M.G.-K.; Kumar, S.P.G.; O’Sullivan, J.F.; Whelan, D.; Stocca, A.; Khider, W.; et al. Bone Marrow-Derived Mesenchymal Stem Cells Have Innate Procoagulant Activity and Cause Microvascular Obstruction Following Intracoronary Delivery: Amelioration by Antithrombin Therapy. Stem Cells 2015, 33, 2726–2737. [Google Scholar] [CrossRef]
- Tripisciano, C.; Weiss, R.; Eichhorn, T.; Spittler, A.; Heuser, T.; Fischer, M.B.; Weber, V. Different Potential of Extracellular Vesicles to Support Thrombin Generation: Contributions of Phosphatidylserine, Tissue Factor, and Cellular Origin. Sci. Rep. 2017, 7, 6522. [Google Scholar] [CrossRef] [PubMed]
- Kapustin, A.N.; Schoppet, M.; Schurgers, L.J.; Reynolds, J.L.; McNair, R.; Heiss, A.; Jahnen-Dechent, W.; Hackeng, T.M.; Schlieper, G.; Harrison, P.; et al. Prothrombin Loading of Vascular Smooth Muscle Cell-Derived Exosomes Regulates Coagulation and Calcification. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e22–e32. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, F.; Ammollo, C.T.; Morrissey, J.H.; Dale, G.L.; Friese, P.; Esmon, N.L.; Esmon, C.T. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: Involvement of platelet TLR2 and TLR4. Blood 2011, 118, 1952–1961. [Google Scholar] [CrossRef]
- Deguchi, H.; Sinha, R.K.; Marchese, P.; Ruggeri, Z.M.; Zilberman-Rudenko, J.; McCarty, O.J.T.; Cohen, M.J.; Griffin, J.H. Prothrombotic skeletal muscle myosin directly enhances prothrombin activation by binding factors Xa and Va. Blood 2016, 128, 1870–1878. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-C.; Lin, Y.-W.; Huang, C.-Y.; Lin, C.-Y.; Tsai, Y.-T.; Shih, C.-M.; Lee, C.-Y.; Chen, Y.-H.; Li, C.-Y.; Chang, N.-C.; et al. The role of calpain-myosin 9-Rab7b pathway in mediating the expression of Toll-like receptor 4 in platelets: A novel mechanism involved in α-granules trafficking. PLoS ONE 2014, 9, e85833. [Google Scholar] [CrossRef]
- Griffith, L.; Slupsky, J.; Seehafer, J.; Boshkov, L.; Shaw, A.R. Platelet activation by immobilized monoclonal antibody: Evidence for a CD9 proximal signal. Blood 1991, 78, 1753–1759. [Google Scholar] [PubMed]
- Israels, S.J.; McMillan-Ward, E.M. Palmitoylation supports the association of tetraspanin CD63 with CD9 and integrin alphaIIbbeta3 in activated platelets. Thromb. Res. 2010, 125, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Mangin, P.H.; Kleitz, L.; Boucheix, C.; Gachet, C.; Lanza, F. CD9 negatively regulates integrin alphaIIbbeta3 activation and could thus prevent excessive platelet recruitment at sites of vascular injury. J. Thromb. Haemost. 2009, 7, 900–902. [Google Scholar] [CrossRef]
- Reutelingsperger, C.P.; van Heerde, W.L. Annexin V, the regulator of phosphatidylserine-catalyzed inflammation and coagulation during apoptosis. Cell. Mol. Life Sci. 1997, 53, 527–532. [Google Scholar] [CrossRef]
- Tsuda, T.; Yoshimura, H.; Hamasaki, N. Effect of phosphatidylcholine, phosphatidylethanolamine and lysophosphatidylcholine on the activated factor X-prothrombin system. Blood Coagul. Fibrinolysis 2006, 17, 465–469. [Google Scholar] [CrossRef]
- Zwaal, R.F.; Comfurius, P.; van Deenen, L.L. Membrane asymmetry and blood coagulation. Nature 1977, 268, 358–360. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Zhu, X.-Y.; Puranik, A.S.; Woollard, J.R.; Tang, H.; Dasari, S.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. Comparative proteomic analysis of extracellular vesicles isolated from porcine adipose tissue-derived mesenchymal stem/stromal cells. Sci. Rep. 2016, 6, 36120. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, Y.; Wang, Z.; Wang, H.; Wang, L.; Xiao, F.; Wang, H.; Tan, J.; Guo, Z. Thrombin promotes fibronectin secretion by bone marrow mesenchymal stem cells via the protease-activated receptor mediated signalling pathways. Stem Cell Res. Ther. 2014, 5, 36. [Google Scholar] [CrossRef] [PubMed]
- Shiratsuki, S.; Terai, S.; Murata, Y.; Takami, T.; Yamamoto, N.; Fujisawa, K.; Burganova, G.; Quintanilha, L.F.; Sakaida, I. Enhanced survival of mice infused with bone marrow-derived as compared with adipose-derived mesenchymal stem cells. Hepatol. Res. 2015, 45, 1353–1359. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensébé, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. 2011, 26, 1474–1483. [Google Scholar] [CrossRef]
- Bruno, S.; Grange, C.; Collino, F.; Deregibus, M.C.; Cantaluppi, V.; Biancone, L.; Tetta, C.; Camussi, G. Microvesicles derived from mesenchymal stem cells enhance survival in a lethal model of acute kidney injury. PLoS ONE 2012, 7, e33115. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.R.; Kim, H.Y.; Park, J.-Y.; Lee, T.Y.; Baik, C.S.; Chai, Y.G.; Jung, K.H.; Park, K.S.; Roh, W.; Kim, K.S.; et al. Selection of optimal passage of bone marrow-derived mesenchymal stem cells for stem cell therapy in patients with amyotrophic lateral sclerosis. Neurosci. Lett. 2010, 472, 94–98. [Google Scholar] [CrossRef]
- Barbosa da Fonseca, L.M.; Gutfilen, B.; Rosado de Castro, P.H.; Battistella, V.; Goldenberg, R.C.S.; Kasai-Brunswick, T.; Chagas, C.L.R.; Wajnberg, E.; Maiolino, A.; Salles Xavier, S.; et al. Migration and homing of bone-marrow mononuclear cells in chronic ischemic stroke after intra-arterial injection. Exp. Neurol. 2010, 221, 122–128. [Google Scholar] [CrossRef]
- Silachev, D.N.; Kondakov, A.K.; Znamenskii, I.A.; Kurashvili, Y.B.; Abolenskaya, A.V.; Antipkin, N.R.; Danilina, T.I.; Manskikh, V.N.; Gulyaev, M.V.; Pirogov, Y.A.; et al. The Use of Technetium-99m for Intravital Tracing of Transplanted Multipotent Stromal Cells. Bull. Exp. Biol. Med. 2016, 162, 153–159. [Google Scholar] [CrossRef]
- Hirsh, J.; Anand, S.S.; Halperin, J.L.; Fuster, V. American Heart Association Guide to anticoagulant therapy: Heparin: A statement for healthcare professionals from the American Heart Association. Circulation 2001, 103, 2994–3018. [Google Scholar] [CrossRef]
- Ling, L.; Camilleri, E.T.; Helledie, T.; Samsonraj, R.M.; Titmarsh, D.M.; Chua, R.J.; Dreesen, O.; Dombrowski, C.; Rider, D.A.; Galindo, M.; et al. Effect of heparin on the biological properties and molecular signature of human mesenchymal stem cells. Gene 2016, 576, 292–303. [Google Scholar] [CrossRef]






| Gene Name | UniProt ID | Protein Name | Normalized Intensity | |
|---|---|---|---|---|
| MSC | EV | |||
| YWHAZ | P63104 | 14-3-3 protein zeta/delta | 1.3 × 104 | 2.2 × 105 |
| ACTB | P60709 | Actin, cytoplasmic 1 | 9.9 × 106 | 8.4 × 107 |
| ACTG1 | P63261 | Actin, cytoplasmic 2 | 9.9 × 106 | 5.1 × 107 |
| A2MG | P01023 | Alpha-2-macroglobulin | 6.9 × 106 | 0 |
| ACTN1 | P12814 | Alpha-actinin-1 | 0 | 4.6 × 105 |
| ANXA5 | P08758 | Annexin A5 | 0 | 4.9 × 105 |
| CD59 | P13987 | CD59 glycoprotein | 4.1 × 105 | 1.8 × 104 |
| CD9 | P21926 | CD9 antigen | 2.1 × 105 | 4.7 × 104 |
| FA5 | P12259 | Coagulation factor V | 7.2 × 104 | 0 |
| CSRP1 | P21291 | Cysteine and glycine-rich protein 1 | 0 | 1.5 × 104 |
| FLNA | P21333 | Filamin-A | 0 | 3.7 × 106 |
| GNB1 | P62873 | Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 | 1.4 × 104 | 2.9 × 103 |
| HSPB1 | P04792 | Heat shock protein beta-1 | 0 | 2.1 × 105 |
| HBB | P68871 | Hemoglobin subunit beta | 3.0 × 105 | 0 |
| HIST1H3A | P68431 | Histone H3.1 | 1.0 × 105 | 1.8 × 106 |
| HIST2H3A | Q71DI3 | Histone H3.2 | 1.1 × 104 | 8.8 × 105 |
| H3F3A | P84243 | Histone H3.3 | 1.6 × 104 | 4.4 × 105 |
| MYL12A | P19105 | Myosin regulatory light chain 12A | 0 | 3.2 × 105 |
| MYH9 | P35579 | Myosin-9 | 2.0 × 105 | 4.9 × 106 |
| THRB | P00734 | Prothrombin | 7.8 × 105 | 0 |
| TLN1 | Q9Y490 | Talin-1 | 1.5 × 104 | 1.3 × 105 |
| VCL | P18206 | Vinculin | 0 | 3.5 × 105 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silachev, D.N.; Goryunov, K.V.; Shpilyuk, M.A.; Beznoschenko, O.S.; Morozova, N.Y.; Kraevaya, E.E.; Popkov, V.A.; Pevzner, I.B.; Zorova, L.D.; Evtushenko, E.A.; et al. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells 2019, 8, 258. https://doi.org/10.3390/cells8030258
Silachev DN, Goryunov KV, Shpilyuk MA, Beznoschenko OS, Morozova NY, Kraevaya EE, Popkov VA, Pevzner IB, Zorova LD, Evtushenko EA, et al. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells. 2019; 8(3):258. https://doi.org/10.3390/cells8030258
Chicago/Turabian StyleSilachev, Denis N., Kirill V. Goryunov, Margarita A. Shpilyuk, Olga S. Beznoschenko, Natalya Y. Morozova, Elizaveta E. Kraevaya, Vasily A. Popkov, Irina B. Pevzner, Ljubava D. Zorova, Ekaterina A. Evtushenko, and et al. 2019. "Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation" Cells 8, no. 3: 258. https://doi.org/10.3390/cells8030258
APA StyleSilachev, D. N., Goryunov, K. V., Shpilyuk, M. A., Beznoschenko, O. S., Morozova, N. Y., Kraevaya, E. E., Popkov, V. A., Pevzner, I. B., Zorova, L. D., Evtushenko, E. A., Starodubtseva, N. L., Kononikhin, A. S., Bugrova, A. E., Evtushenko, E. G., Plotnikov, E. Y., Zorov, D. B., & Sukhikh, G. T. (2019). Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells, 8(3), 258. https://doi.org/10.3390/cells8030258









