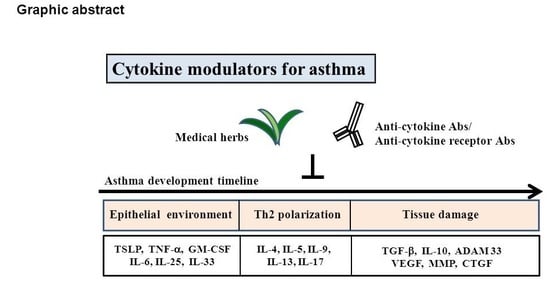
Advanced Molecular Knowledge of Therapeutic Drugs and Natural Products Focusing on Inflammatory Cytokines in Asthma
Abstract
:1. Introduction to Asthma
1.1. The Molecular and Cellular Basis for Asthma
1.2. Signaling Pathways Involved in Cytokine Activity during Asthma Development
1.3. Cytokines at Different Stages Play Crucial Roles in the Pathogenesis of Asthma
2. Therapeutic Drugs for Asthma
3. Clinical and Investigational Cytokine-Targeting Therapy for Asthma
4. Cytokine Immunomodulatory Effects of Natural Formula, Herbs, and Natural Compounds on Asthma
5. The Side Effect and Specific Outcomes in Asthma of Herbal Compounds
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Kim, D.Y.; Park, J.W.; Jeoung, D.; Ro, J.Y. Celastrol suppresses allergen-induced airway inflammation in a mouse allergic asthma model. Eur. J. Pharmacol. 2009, 612, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Abajobir, A.A.; Abate, K.H.; Abera, S.F.; Agrawal, A.; Ahmed, M.B.; Aichour, A.N.; Aichour, I.; Aichour, M.T.E.; Alam, K.; et al. A Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study. Lancet Respirat. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef]
- Loftus, P.A.; Wise, S.K. Epidemiology of asthma. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Serebrisky, D.; Wiznia, A. Pediatric Asthma: A Global Epidemic. Ann. Glob. Health 2019, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinbami, L.J.; Moorman, J.E.; Liu, X. Asthma Prevalence, Health Care Use, and Mortality; United States, 2005–2009; DHHS publication: Atlanta, GA, USA, 2011. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). Prevention, Vital signs: Asthma prevalence, disease characteristics, and self-management education: United States, 2001–2009. MMWR. Morb. Mortal. Wkly. Rep. 2011, 60, 547. [Google Scholar]
- Lin, S.C.; Lin, H.W.; Chiang, B.L. Association of croup with asthma in children: A cohort study. Medicine 2017, 96, e7667. [Google Scholar] [CrossRef] [PubMed]
- Cohn, L.; Elias, J.A.; Chupp, G.L. Asthma: Mechanisms of disease persistence and progression. Annu. Rev. Immunol. 2004, 22, 789–815. [Google Scholar] [CrossRef]
- Peebles, R.S., Jr.; Aronica, M.A. Proinflammatory Pathways in the Pathogenesis of Asthma. Clin. Chest Med. 2019, 40, 29–50. [Google Scholar] [CrossRef]
- Boulet, L.P.; Sterk, P.J. Airway remodelling: The future. Eur. Respir. J. 2007, 30, 831–834. [Google Scholar] [CrossRef]
- Mauad, T.; Bel, E.H.; Sterk, P.J. Asthma therapy and airway remodeling. J. Allergy Clin. Immunol. 2007, 120, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, P.K. Remodeling in asthma and chronic obstructive lung disease. Am. J. Respir. Crit. Care Med. 2001, 164, S28–S38. [Google Scholar] [CrossRef] [PubMed]
- Bai, T.R.; Cooper, J.; Koelmeyer, T.; Pare, P.D.; Weir, T.D. The effect of age and duration of disease on airway structure in fatal asthma. Am. J. Respir. Crit. Care Med. 2000, 162, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Royce, S.G.; Lim, C.X.F.; Patel, K.P.; Wang, B.; Samuel, C.S.; Tang, M.L.K. Intranasally administered serelaxin abrogates airway remodelling and attenuates airway hyperresponsiveness in allergic airways disease. Clin. Exp. Allergy 2014, 44, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.; Bai, T.R.; Bates, J.H.; Black, J.L.; Brown, R.H.; Brusasco, V.; Chitano, P.; Deng, L.; Dowell, M.; Eidelman, D.H.; et al. Airway smooth muscle dynamics: A common pathway of airway obstruction in asthma. Eur. Respir. J. 2007, 29, 834–860. [Google Scholar] [CrossRef] [PubMed]
- Holgate, S.T. Innate and adaptive immune responses in asthma. Nat. Med. 2012, 18, 673–683. [Google Scholar] [CrossRef] [PubMed]
- McWilliam, A.S. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J. Exp. Med. 1994, 179, 1331–1336. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; Hammad, H. The airway epithelium in asthma. Nat. Med. 2012, 18, 684–692. [Google Scholar] [CrossRef]
- Evans, S.E.; Xu, Y.; Tuvim, M.J.; Dickey, B.F. Inducible Innate Resistance of Lung Epithelium to Infection. Annu. Rev. Physiol. 2010, 72, 413–435. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.L.; Chen, S.H.; Wang, J.Y. Critical role of IL-6 in dendritic cell-induced allergic inflammation of asthma. J. Mol. Med. 2016, 94, 51–59. [Google Scholar] [CrossRef]
- Liew, F.Y.; Girard, J.-P.; Turnquist, H.R. Interleukin-33 in health and disease. Nat. Rev. Immunol. 2016, 16, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Kouzaki, H.; Tojima, I.; Kita, H.; Shimizu, T. Transcription of Interleukin-25 and Extracellular Release of the Protein Is Regulated by Allergen Proteases in Airway Epithelial Cells. Am. J. Respir. Cell Mol. Boil. 2013, 49, 741–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreakos, E.; Papadopoulos, N.G. IL-25: The Missing Link Between Allergy, Viral Infection, and Asthma? Sci. Transl. Med. 2014, 6, 256. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-C.; Cheng, F.-Y.; Liu, J.-J.; Ye, Y.-L. Expression and Regulation of Thymic Stromal Lymphopoietin and Thymic Stromal Lymphopoietin Receptor Heterocomplex in the Innate–Adaptive Immunity of Pediatric Asthma. Int. J. Mol. Sci. 2018, 19, 1231. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.K.; Hu, S.; Cheung, P.F.; Lam, C.W. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: Implications in allergic inflammation. Am. J. Respir. Cell Mol. Biol. 2010, 43, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Comeau, M.R.; Ziegler, S.F. The influence of TSLP on the allergic response. Mucosal. Immunol. 2010, 3, 138–147. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Liu, Y.-J.; Wang, Y.-H.; Liu, Y.-J.; Wang, Y.; Liu, Y. Thymic stromal lymphopoietin, OX40-ligand, and interleukin-25 in allergic responses. Clin. Exp. Allergy 2009, 39, 798–806. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, H.; Iwamoto, I.; Tomoe, S.; Matsumura, R.; Tomioka, H.; Takatsu, K.; Yoshida, S. CD4+ T-lymphocytes and interleukin-5 mediate antigen-induced eosinophil infiltration into the mouse trachea. Am. Rev. Respir. Dis. 1992, 146, 374–377. [Google Scholar] [CrossRef]
- Chung, K.F.; Barnes, P.J. Cytokines in asthma. Thorax 1999, 54, 825–857. [Google Scholar] [CrossRef] [Green Version]
- Masinovsky, B.; Urdal, D.; Gallatin, W.M. IL-4 acts synergistically with IL-1 beta to promote lymphocyte adhesion to microvascular endothelium by induction of vascular cell adhesion molecule-1. J. Immunol. 1990, 145, 2886–2895. [Google Scholar]
- Bossios, A.; Sjostrand, M.; Dahlborn, A.K.; Samitas, K.; Malmhall, C.; Gaga, M.; Lotvall, J. IL-5 expression and release from human CD34 cells in vitro; ex vivo evidence from cases of asthma and Churg-Strauss syndrome. Allergy 2010, 65, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Townsend, M.J.; Fallon, P.G.; Matthews, D.J.; Smith, P.; E Jolin, H.; McKenzie, A.N. IL-9-Deficient Mice Establish Fundamental Roles for IL-9 in Pulmonary Mastocytosis and Goblet Cell Hyperplasia but Not T Cell Development. Immun. 2000, 13, 573–583. [Google Scholar] [CrossRef] [Green Version]
- Fajt, M.L.; Wenzel, S.E. Development of New Therapies for Severe Asthma. Allergy, Asthma Immunol. Res. 2017, 9, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Al-Alawi, M.; Hassan, T.; Chotirmall, S.H. Transforming growth factor beta and severe asthma: A perfect storm. Respir. Med. 2014, 108, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Chou, H.C.; Chiang, B.L.; Chen, C.M. CTGF upregulation correlates with MMP-9 level in airway remodeling in a murine model of asthma. Arch. Med. Sci. 2017, 13, 670–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neill, D.R.; Wong, S.H.; Bellosi, A.; Flynn, R.J.; Daly, M.; Langford, T.K.A.; Bucks, C.; Kane, C.M.; Fallon, P.G.; Pannell, R.; et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464, 1367–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, A.E.; Liang, H.-E.; Sullivan, B.M.; Reinhardt, R.L.; Eisley, C.J.; Erle, D.J.; Locksley, R.M. Systemically dispersed innate IL-13–expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. 2010, 107, 11489–11494. [Google Scholar] [CrossRef]
- Ito, T.; Liu, Y.J.; Arima, K. Cellular and molecular mechanisms of TSLP function in human allergic disorders—TSLP programs the” Th2 code” in dendritic cells. Allergol. Int. 2012, 61, 35–43. [Google Scholar] [CrossRef]
- Kurowska-Stolarska, M.; Kewin, P.; Murphy, G.; Russo, R.C.; Stolarski, B.; Garcia, C.C.; Komai-Koma, M.; Pitman, N.; Li, Y.; Niedbala, W.; et al. IL-33 induces antigen-specific IL-5+ T cells and promotes allergic-induced airway inflammation independent of IL-4. J. Immunol. 2008, 181, 4780–4790. [Google Scholar] [CrossRef]
- Elder, M.J.; Webster, S.J.; Williams, D.L.; Gaston, J.S.; Goodall, J.C. TSLP production by dendritic cells is modulated by IL-1beta and components of the endoplasmic reticulum stress response. Eur. J. Immunol. 2016, 46, 455–463. [Google Scholar] [CrossRef]
- Schuliga, M. NF-kappaB Signaling in Chronic Inflammatory Airway Disease. Biomol. 2015, 5, 1266–1283. [Google Scholar] [CrossRef] [PubMed]
- Kool, M.; Van Loo, G.; Waelput, W.; De Prijck, S.; Muskens, F.; Sze, M.; Van Praet, J.; Branco-Madeira, F.; Janssens, S.; Reizis, B.; et al. The Ubiquitin-Editing Protein A20 Prevents Dendritic Cell Activation, Recognition of Apoptotic Cells, and Systemic Autoimmunity. Immun. 2011, 35, 82–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.G.A.; Muehling, L.M.; Eccles, J.D.; Woodfolk, J.A. T cells in severe childhood asthma. Clin. Exp. Allergy 2019, 49, 564–581. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y. GATA3: A master of many trades in immune regulation. Trends Immunol. 2014, 35, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Shrine, N.; Portelli, M.A.; John, C.; Soler Artigas, M.; Bennett, N.; Hall, R.; Lewis, J.; Henry, A.P.; Billington, C.K.; Ahmad, A.; et al. Moderate-to-severe asthma in individuals of European ancestry: A genome-wide association study. Lancet Respir. Med. 2019, 7, 20–34. [Google Scholar] [CrossRef]
- Vale, K. Targeting the JAK-STAT pathway in the treatment of ‘Th2-high’ severe asthma. Futur. Med. Chem. 2016, 8, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Pernis, A.B.; Rothman, P.B. JAK-STAT signaling in asthma. J. Clin. Investig. 2002, 109, 1279–1283. [Google Scholar] [CrossRef]
- Wisniewski, J.A.; Muehling, L.M.; Eccles, J.D.; Capaldo, B.J.; Agrawal, R.; Shirley, D.A.; Patrie, J.T.; Workman, L.J.; Schuyler, A.J.; Lawrence, M.G.; et al. TH1 signatures are present in the lower airways of children with severe asthma, regardless of allergic status. J. Allergy Clin. Immunol. 2018, 141, 2048–2060. [Google Scholar] [CrossRef]
- Redington, A.E.; Madden, J.; Frew, A.J.; Djukanović, R.; Roche, W.R.; Holgate, S.T.; Howarth, P.H. Transforming Growth Factor- β 1 in Asthma. Am. J. Respir. Crit. Care Med. 1997, 156, 642–647. [Google Scholar] [CrossRef]
- Moffatt, M.F.; Gut, I.G.; Demenais, F.; Strachan, D.P.; Bouzigon, E.; Heath, S.; Von Mutius, E.; Farrall, M.; Lathrop, M.; Cookson, W.O. A Large-Scale, Consortium-Based Genomewide Association Study of Asthma. New Engl. J. Med. 2010, 363, 1211–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigas, D.; Lewis, G.; Aron, J.L.; Wang, B.; Banie, H.; Sankaranarayanan, I.; Galle-Treger, L.; Maazi, H.; Lo, R.; Freeman, G.J.; et al. Type 2 innate lymphoid cell suppression by regulatory T cells attenuates airway hyperreactivity and requires inducible T-cell costimulator-inducible T-cell costimulator ligand interaction. J. Allergy Clin. Immunol. 2017, 139, 1468–1477. [Google Scholar] [CrossRef]
- A Patente, T.; Pelgrom, L.R.; Everts, B. Dendritic cells are what they eat: how their metabolism shapes T helper cell polarization. Curr. Opin. Immunol. 2019, 58, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hoch, H.E.; Szefler, S.J. Intermittent steroid inhalation for the treatment of childhood asthma. Expert Rev. Clin. Immunol. 2016, 12, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Horvath, G.; Wanner, A. Inhaled corticosteroids: effects on the airway vasculature in bronchial asthma. Eur. Respir. J. 2006, 27, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Inhaled Corticosteroids. Pharmaceuticals 2010, 3, 514–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotshall, R.W. Exercise-Induced Bronchoconstriction. Drugs 2002, 62, 1725–1739. [Google Scholar] [CrossRef] [PubMed]
- Raissy, H.H.; Blake, K. Does Use of Inhaled Corticosteroid for Management of Asthma in Children Make Them Shorter Adults? Pediatr. Allergy, Immunol. Pulmonol. 2013, 26, 99–101. [Google Scholar] [CrossRef] [Green Version]
- Chee, C.; Sellahewa, L.; Pappachan, J.M. Inhaled Corticosteroids and Bone Health. Open Respir. Med. J. 2014, 8, 85–92. [Google Scholar] [CrossRef]
- Sannarangappa, V.; Jalleh, R. Inhaled Corticosteroids and Secondary Adrenal Insufficiency. Open Respir. Med. J. 2014, 8, 93–100. [Google Scholar] [CrossRef]
- Egbuonu, F.; A Antonio, F.; Edavalath, M. Effect of Inhaled Corticosteroids on Glycemic Status. Open Respir. Med. J. 2014, 8, 101–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, M.; Hirota, T.; Jodo, A.I.; Hitomi, Y.; Sakashita, M.; Tsunoda, T.; Miyagawa, T.; Doi, S.; Kameda, M.; Fujita, K.; et al. Thymic Stromal Lymphopoietin Gene Promoter Polymorphisms Are Associated with Susceptibility to Bronchial Asthma. Am. J. Respir. Cell Mol. Boil. 2011, 44, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Ohtawa, J. Effects of Adding Omalizumab, an Anti-Immunoglobulin E Antibody, on Airway Wall Thickening in Asthma. Respir. 2012, 83, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Tamm, M. The effects of omalizumab on IgE-induced cytokine synthesis by asthmatic airway smooth muscle cells. Ann. Allergy, Asthma Immunol. 2010, 104, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Wenzel, S.; Rabe, K.F.; Bourdin, A.; Lugogo, N.L.; Kuna, P.; Barker, P.; Sproule, S.; Ponnarambil, S.; Goldman, M.; et al. Oral Glucocorticoid-Sparing Effect of Benralizumab in Severe Asthma. New Engl. J. Med. 2017, 376, 2448–2458. [Google Scholar] [CrossRef]
- Potaczek, D.P.; Garn, H.; Unger, S.D.; Renz, H. Antisense molecules: A new class of drugs. J. Allergy Clin. Immunol. 2016, 137, 1334–1346. [Google Scholar] [CrossRef] [Green Version]
- Kabata, H.; Moro, K.; Fukunaga, K.; Suzuki, Y.; Miyata, J.; Masaki, K.; Betsuyaku, T.; Koyasu, S.; Asano, K. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat. Commun. 2013, 4, 2675. [Google Scholar] [CrossRef] [Green Version]
- Gauvreau, G.M.; O’Byrne, P.M.; Boulet, L.P.; Wang, Y.; Cockcroft, D.; Bigler, J.; FitzGerald, J.M.; Boedigheimer, M.; Davis, B.E.; Dias, C.; et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N. Engl. J. Med. 2014, 370, 2102–2110. [Google Scholar] [CrossRef]
- Lin, S.-C.; Chou, H.-C.; Chen, C.-M.; Chiang, B.-L. Anti-thymic stromal lymphopoietin antibody suppresses airway remodeling in asthma through reduction of MMP and CTGF. Pediatr. Res. 2018, 1. [Google Scholar] [CrossRef]
- Lawrence, M.G.; Steinke, J.W.; Borish, L. Cytokine-targeting biologics for allergic diseases. Ann. Allergy, Asthma Immunol. 2018, 120, 376–381. [Google Scholar] [CrossRef] [Green Version]
- Holgado, A.; Braun, H.; Van Nuffel, E.; Detry, S.; Schuijs, M.J.; Deswarte, K.; Vergote, K.; Haegman, M.; Baudelet, G.; Haustraete, J.; et al. IL-33trap is a novel IL-33-neutralizing biologic that inhibits allergic airway inflammation. J. Allergy Clin. Immunol. 2019. [Google Scholar] [CrossRef]
- Ballantyne, S.J.; Barlow, J.L.; Jolin, H.E.; Nath, P.; Williams, A.S.; Chung, K.F.; Sturton, G.; Wong, S.H.; McKenzie, A.N. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J. Allergy Clin. Immunol. 2007, 120, 1324–1331. [Google Scholar] [CrossRef]
- Chu, D.K.; Al-Garawi, A.; Llop-Guevara, A.; A Pillai, R.; Radford, K.; Shen, P.; Walker, T.D.; Goncharova, S.; Calhoun, W.J.; Nair, P.; et al. Therapeutic potential of anti-IL-6 therapies for granulocytic airway inflammation in asthma. Allergy, Asthma Clin. Immunol. 2015, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, S.; Castro, M.; Corren, J.; Maspero, J.; Wang, L.; Zhang, B.; Pirozzi, G.; Sutherland, E.R.; Evans, R.R.; Joish, V.N.; et al. Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting beta2 agonist: A randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016, 388, 31–44. [Google Scholar] [CrossRef]
- Castro, M.; Corren, J.; Pavord, I.D.; Maspero, J.; Wenzel, S.; Rabe, K.F.; Busse, W.W.; Ford, L.; Sher, L.; Fitzgerald, J.M.; et al. Dupilumab Efficacy and Safety in Moderate-to-Severe Uncontrolled Asthma. New Engl. J. Med. 2018, 378, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Pizzichini, M.M.; Inman, M.D.; Efthimiadis, A.; Hargreave, F.E.; O’Byrne, P.M.; Nair, P.; Kjarsgaard, M.; Pizzichini, E. Mepolizumab for Prednisone-Dependent Asthma with Sputum Eosinophilia. New Engl. J. Med. 2009, 360, 985–993. [Google Scholar]
- Liu, A.H.; Anderson, W.C., 3rd; Dutmer, C.M.; Searing, D.A.; Szefler, S.J. Advances in asthma 2015: Across the lifespan. J. Allergy Clin. Immunol. 2016, 138, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FitzGerald, J.M.; Bleecker, E.R.; Nair, P.; Korn, S.; Ohta, K.; Lommatzsch, M.; Ferguson, G.T.; Busse, W.W.; Barker, P.; Sproule, S.; et al. Benralizumab, an anti-interleukin-5 receptor a monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016, 388, 2128–2141. [Google Scholar] [CrossRef]
- Piper, E.; Brightling, C.; Niven, R.; Oh, C.; Faggioni, R.; Poon, K.; She, D.; Kell, C.; May, R.D.; Geba, G.P.; et al. A phase II placebo-controlled study of tralokinumab in moderate-to-severe asthma. Eur. Respir. J. 2013, 41, 330–338. [Google Scholar] [CrossRef] [PubMed]
- A Hanania, N.; Noonan, M.; Corren, J.; Korenblat, P.; Zheng, Y.; Fischer, S.K.; Cheu, M.; Putnam, W.S.; Murray, E.; Scheerens, H.; et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax 2015, 70, 748–756. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Cho, K.-A.; Cho, Y.J.; Woo, S.-Y. Effects of Interleukin-9 Blockade on Chronic Airway Inflammation in Murine Asthma Models. Allergy, Asthma Immunol. Res. 2013, 5, 197–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, C.K.; Leigh, R.; McLaurin, K.K.; Kim, K.; Hultquist, M.; A Molfino, N. A randomized, controlled trial to evaluate the effect of an anti-interleukin-9 monoclonal antibody in adults with uncontrolled asthma. Respir. Res. 2013, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Camargo, L.D.N.; Righetti, R.F.; Aristoteles, L.; Dos Santos, T.M.; de Souza, F.C.R.; Fukuzaki, S.; Cruz, M.M.; Alonso-Vale, M.I.C.; Saraiva-Romanholo, B.M.; Prado, C.M.; et al. Effects of Anti-IL-17 on Inflammation, Remodeling, and Oxidative Stress in an Experimental Model of Asthma Exacerbated by LPS. Front. Immunol. 2017, 8, 1835. [Google Scholar] [CrossRef] [PubMed]
- Busse, W.W.; Holgate, S.; Kerwin, E.; Chon, Y.; Feng, J.; Lin, J.; Lin, S.L. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am. J. Respir. Crit. Care Med. 2013, 188, 1294–1302. [Google Scholar] [CrossRef] [PubMed]
- McMillan, S.J.; Xanthou, G.; Lloyd, C.M. Manipulation of allergen-induced airway remodeling by treatment with anti-TGF-beta antibody: effect on the Smad signaling pathway. J. Immunol. 2005, 174, 5774–5780. [Google Scholar] [CrossRef] [PubMed]
- Krug, N.; Hohlfeld, J.M.; Kirsten, A.-M.; Kornmann, O.; Beeh, K.M.; Kappeler, D.; Korn, S.; Ignatenko, S.; Timmer, W.; Rogon, C.; et al. Allergen-Induced Asthmatic Responses Modified by a GATA3-Specific DNAzyme. New Engl. J. Med. 2015, 372, 1987–1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauvreau, G.M.; Pageau, R.; Seguin, R.; Carballo, D.; Gauthier, J.; D’Anjou, H.; Campbell, H.; Watson, R.; Mistry, M.; Parry-Billings, M.; et al. Dose-response effects of TPI ASM8 in asthmatics after allergen. Allergy 2011, 66, 1242–1248. [Google Scholar] [CrossRef]
- Wu, B.-Y.; Liu, C.-T.; Hung, Y.-C.; Hu, W.-L. Complementary therapy with traditional Chinese medicine for childhood asthma. In Asthma—From Childhood Asthma to ACOS Phenotypes; Books on Demand: Norderstedt, Germany, 2016. [Google Scholar]
- Liu, L.; Wang, L.P.; He, S.; Ma, Y. Immune Homeostasis: Effects of Chinese Herbal Formulae and Herb-Derived Compounds on Allergic Asthma in Different Experimental Models. Chin. J. Integr. Med. 2018, 24, 390–398. [Google Scholar] [CrossRef]
- Srivastava, K.D.; Dunkin, D.; Liu, C.; Yang, N.; Miller, R.L.; Sampson, H.A.; Li, X.M. Effect of Antiasthma Simplified Herbal Medicine Intervention on neutrophil predominant airway inflammation in a ragweed sensitized murine asthma model. Ann. Allergy Asthma Immunol. 2014, 112, 339–347. [Google Scholar] [CrossRef]
- Busse, P.J.; Schofield, B.; Birmingham, N.; Yang, N.; Wen, M.-C.; Zhang, T.; Srivastava, K.; Li, X.-M. The traditional Chinese herbal formula ASHMI inhibits allergic lung inflammation in antigen-sensitized and antigen-challenged aged mice. Ann. Allergy, Asthma Immunol. 2010, 104, 236–246. [Google Scholar] [CrossRef]
- Srivastava, K.; Zhang, T.; Yang, N.; Sampson, H.; Li, X.M. Anti-Asthma Simplified Herbal Medicine Intervention-induced long-lasting tolerance to allergen exposure in an asthma model is interferon-gamma, but not transforming growth factor-beta dependent. Clinical and experimental allergy. J. B. Soc. Allergy Clin. Immunol. 2010, 40, 1678–1688. [Google Scholar]
- Li, X.-M.; Huang, C.-K.; Zhang, T.-F.; Teper, A.A.; Srivastava, K.; Schofield, B.H.; Sampson, H.A. The Chinese herbal medicine formula MSSM-002 suppresses allergic airway hyperreactivity and modulates TH1/TH2 responses in a murine model of allergic asthma. J. Allergy Clin. Immunol. 2000, 106, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.; Xin-sheng, F.; Jing-hua, Y.; Li, X.; Shan-shan, W. CD4+ CD25+ FOXP3+ T cells, Foxp3 gene and protein expression contribute to antiasthmatic effects of San’ao decoction in mice model of asthma. Phytomedicine 2014, 21, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Luo, Q.L.; Sun, J.; Chen, M.X.; Liu, F.; Dong, J.C. Bu-Shen-Yi-Qi formulae suppress chronic airway inflammation and regulate Th17/Treg imbalance in the murine ovalbumin asthma model. J. Ethnopharmacol. 2015, 164, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Jiu-lue, H. Effect of Xiaoqinglong Decoction on Airway Inflammation and IL-4, IFN-γ in the BALF of Mouse Asthmatic Model. Chin. J. Exp. Tradit. Med. Formul. 2012, 10, 265–267. [Google Scholar]
- Kuo, Y.-C.; Tsai, W.-J.; Wang, J.-Y.; Chang, S.-C.; Lin, C.-Y.; Shiao, M.-S. Regulation of bronchoalveolar lavage fluids cell function by the immunomodulatory agents from Cordyceps sinensis. Life Sci. 2001, 68, 1067–1082. [Google Scholar] [CrossRef]
- Li, L.; Hou, X.; Xu, R.; Liu, C.; Tu, M. Research review on the pharmacological effects of astragaloside IV. Fundam. Clin. Pharmacol. 2017, 31, 17–36. [Google Scholar] [CrossRef]
- Abe, Y.; Hashimoto, S.; Horie, T. Curcumin Inhibition of Inflammatory Cytokine Production by Human Peripheral Blood Monocytes and Alveolar Macrophages. Pharmacol. Res. 1999, 39, 41–47. [Google Scholar] [CrossRef]
- Yuan, S.; Cao, S.; Jiang, R.; Liu, R.; Bai, J.; Hou, Q. FLLL31, a derivative of curcumin, attenuates airway inflammation in a multi-allergen challenged mouse model. Int. Immunopharmacol. 2014, 21, 128–136. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, H.J.; Kim, S.M.; Park, K.R.; Jang, H.J.; Lee, E.H.; Jung, S.H.; Ahn, K.S. Methylene chloride fraction of the leaves of Thuja orientalis inhibits in vitro inflammatory biomarkers by blocking NF-kappaB and p38 MAPK signaling and protects mice from lethal endotoxemia. J. Ethnopharmacol. 2011, 133, 687–695. [Google Scholar] [CrossRef]
- Jung, H.S.; Kim, M.H.; Gwak, N.G.; Im, Y.S.; Lee, K.Y.; Sohn, Y.; Choi, H.; Yang, W.M. Antiallergic effects of Scutellaria baicalensis on inflammation in vivo and in vitro. J. Ethnopharmacol. 2012, 141, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.L.; Shang, J.H.; Pu, S.B.; Wang, H.S.; Wang, B.; Liu, L.; Liu, Y.P.; Shen, H.M.; Luo, X.D. Effect of total alkaloids from Alstonia scholaris on airway inflammation in rats. J. Ethnopharmacol. 2016, 178, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.H.; Wang, K.; Li, W.; Ying, Y.H.; Gao, G.X.; Li, X.B.; Huang, H.Q. Astragalus Membranaceus prevents airway hyperreactivity in mice related to Th2 response inhibition. J. Ethnopharmacol. 2008, 116, 363–369. [Google Scholar] [CrossRef]
- Qi, Y.; Gao, F.; Hou, L.; Wan, C. Anti-Inflammatory and Immunostimulatory Activities of Astragalosides. Am. J. Chin. Med. 2017, 45, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, L.; Wang, F.; Song, G. Astragaloside IV attenuates allergic inflammation by regulation Th1/Th2 cytokine and enhancement CD4(+)CD25(+)Foxp3 T cells in ovalbumin-induced asthma. Immunobiology 2014, 219, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.Y.; Zhu, J.X.; Bian, T.; Gao, F.; Qian, X.F.; Du, Q.; Yuan, M.Y.; Sun, H.; Shi, L.Z.; Yu, M.H. Protective effects of astragaloside IV against ovalbumin-induced lung inflammation are regulated/mediated by T-bet/GATA-3. Pharmacology 2014, 94, 51–59. [Google Scholar] [CrossRef]
- Sung, J.E.; Lee, H.A.; Kim, J.E.; Yun, W.B.; An, B.S.; Yang, S.Y.; Kim, D.S.; Lee, C.Y.; Lee, H.S.; Bae, C.J.; et al. Saponin-enriched extract of Asparagus cochinchinensis alleviates airway inflammation and remodeling in ovalbumin-induced asthma model. Int. J. Mol. Med. 2017, 40, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.Y.; Wu, F.H.; Wang, J.S.; Li, J.; Kong, L.Y. Attenuation of airway hyperreactivity and T helper cell type 2 responses by coumarins from Peucedanum praeruptorum Dunn in a murine model of allergic airway inflammation. J. Ethnopharmacol. 2012, 141, 314–321. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, J.; Wu, F.; Li, J.; Zhou, L.; Kong, L. Effects of (+/-)-praeruptorin A on airway inflammation, airway hyperresponsiveness and NF-kappaB signaling pathway in a mouse model of allergic airway disease. Eur. J. Pharmacol. 2012, 683, 316–324. [Google Scholar] [CrossRef]
- Xiong, Y.Y.; Wang, J.S.; Wu, F.H.; Li, J.; Kong, L.Y. The effects of (+/-)-Praeruptorin A on airway inflammation, remodeling and transforming growth factor-beta1/Smad signaling pathway in a murine model of allergic asthma. Int. Immunopharmacol. 2012, 14, 392–400. [Google Scholar] [CrossRef]
- Park, S.; Park, M.-S.; Jung, K.-H.; Song, J.; Kim, Y.A.; Cho, H.J.; Min, B.-I.; Bae, H. Treatment with Pyranopyran-1, 8-Dione Attenuates Airway Responses in Cockroach Allergen Sensitized Asthma in Mice. PLoS ONE 2014, 9, e87558. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Patil, S.; Zhuge, J.; Wen, M.-C.; Bolleddula, J.; Doddaga, S.; Goldfarb, J.; Sampson, H.A.; Li, X.-M. Glycyrrhiza uralensis flavonoids present in anti-asthma formula, ASHMI™, inhibit memory Th2 responses in vitro and in vivo. Phytother. Res. PTR 2013, 27, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Chu, X.; Guan, M.; Yang, X.; Xie, X.; Liu, F.; Chen, C.; Deng, X. Protocatechuic acid suppresses ovalbumin-induced airway inflammation in a mouse allergic asthma model. Int. Immunopharmacol. 2013, 15, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Wei, M.; Yang, X.; Cao, Q.; Xie, X.; Guan, M.; Wang, D.; Deng, X. Effects of an anthraquinone derivative from Rheum officinale Baill, emodin, on airway responses in a murine model of asthma. Food Chem. Toxicol. 2012, 50, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Wang, C.-N.; Lai, Y.-T.; Kang, J.-J.; Liao, J.-W.; Chiang, B.-L.; Chen, H.-C.; Cheng, Y.-W. Shikonin inhibits maturation of bone marrow-derived dendritic cells and suppresses allergic airway inflammation in a murine model of asthma. Br. J. Pharmacol. 2010, 161, 1496–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.L.; Lin, B.F. Effects of triterpenoid-rich extracts of Ganoderma tsugae on airway hyperreactivity and Th2 responses in vivo. Int. Arch. Allergy Immunol. 2007, 143, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.F.; Xie, Y.C.; Zhang, M.S.; Zhao, X.; Cheng, H.; Wang, H.; Yin, K.S.; Huang, M. Ligustrazine corrects Th1/Th2 and Treg/Th17 imbalance in a mouse asthma model. Int. Immunopharmacol. 2014, 21, 76–81. [Google Scholar] [CrossRef]
- Do, J.S.; Hwang, J.K.; Seo, H.J.; Woo, W.H.; Nam, S.Y. Antiasthmatic activity and selective inhibition of type 2 helper T cell response by aqueous extract of semen armeniacae amarum. Immunopharmacol. Immunotoxicol. 2006, 28, 213–225. [Google Scholar] [CrossRef]
- Jung, H.W.; Kang, S.Y.; Kang, J.S.; Kim, A.R.; Woo, E.R.; Park, Y.K. Effect of Kuwanon G isolated from the root bark of Morus alba on ovalbumin-induced allergic response in a mouse model of asthma. Phytother. Res. 2014, 28, 1713–1719. [Google Scholar] [CrossRef]
- Ok, I.S.; Kim, S.H.; Kim, B.K.; Lee, J.C.; Lee, Y.C. Pinellia ternata, Citrus reticulata, and their combinational prescription inhibit eosinophil infiltration and airway hyperresponsiveness by suppressing CCR3+ and Th2 cytokines production in the ovalbumin-induced asthma model. Mediat. Inflamm. 2009, 2009, 413270. [Google Scholar] [CrossRef]
- Lee, M.Y.; Shin, I.S.; Jeon, W.Y.; Lim, H.S.; Kim, J.H.; Ha, H. Pinellia ternata Breitenbach attenuates ovalbumin-induced allergic airway inflammation and mucus secretion in a murine model of asthma. Immunopharmacol. Immunotoxicol. 2013, 35, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Lee, J.A.; Seo, C.S.; Ha, H.; Lee, N.H.; Shin, H.K. Protective effects of Mentha haplocalyx ethanol extract (MH) in a mouse model of allergic asthma. Phytother. Res. 2011, 25, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Hwang, Y.P.; Lee, H.S.; Jeong, H.G. Inhibitory effect of Platycodi Radix on ovalbumin-induced airway inflammation in a murine model of asthma. Food Chem. Toxicol. 2009, 47, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Han, E.H.; Park, J.H.; Kim, J.Y.; Chung, Y.C.; Jeong, H.G. Inhibitory mechanism of saponins derived from roots of Platycodon grandiflorum on anaphylactic reaction and IgE-mediated allergic response in mast cells. Food Chem. Toxicol. 2009, 47, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.Y.; Ahn, K.S.; Park, M.J.; Kwon, O.K.; Lee, H.K.; Oh, S.R. Skullcapflavone II inhibits ovalbumin-induced airway inflammation in a mouse model of asthma. Int. Immunopharmacol. 2012, 12, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.I.G.; Rios-Santos, F.; Balogun, S.O.; de Almeida, D.A.T.; Damazo, A.S.; da Cruz, T.C.D.; Pavan, E.; Barbosa, R.D.S.; Alvim, T.D.C.; Soares, I.M.; et al. Hydroethanolic extract from Echinodorus scaber Rataj leaves inhibits inflammation in ovalbumin-induced allergic asthma. J. Ethnopharmacol. 2017, 203, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Lee, D.Y.; Choi, Y.H.; Yea, S.S.; Choi, I.; Park, S.G.; Seo, S.K.; Lee, S.W.; Lee, C.M.; Kim, S.K.; et al. Caffeic acid phenethyl ester attenuates allergic airway inflammation and hyperresponsiveness in murine model of ovalbumin-induced asthma. Life Sci. 2008, 82, 797–805. [Google Scholar] [CrossRef]
- Chen, C.G.; Wang, H.Y.; Dai, Y.; Wang, J.L.; Xu, W.H. Tripterygium polyglycosid attenuates the established airway inflammation in asthmatic mice. Chin. J. Integr. Med. 2013, 19, 282–288. [Google Scholar] [CrossRef]
- Liu, Q. Triptolide and its expanding multiple pharmacological functions. Int. Immunopharmacol. 2011, 11, 377–383. [Google Scholar] [CrossRef]
- Chen, M.; Lv, Z.; Zhang, W.; Huang, L.; Lin, X.; Shi, J.; Zhang, W.; Liang, R.; Jiang, S. Triptolide suppresses airway goblet cell hyperplasia and Muc5ac expression via NF-kappaB in a murine model of asthma. Mol. Immunol. 2015, 64, 99–105. [Google Scholar] [CrossRef]
- Sy, L.B.; Wu, Y.L.; Chiang, B.L.; Wang, Y.H.; Wu, W.M. Propolis extracts exhibit an immunoregulatory activity in an OVA-sensitized airway inflammatory animal model. Int. Immunopharmacol. 2006, 6, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Hsiao, H.B.; Lin, W.C. A standardized aqueous extract of Anoectochilus formosanus modulated airway hyperresponsiveness in an OVA-inhaled murine model. Phytomedicine 2010, 17, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Kuo, M.L.; Li, M.L.; Yang, R.C.; Liou, C.J.; Shen, J.J. Gynostemma pentaphyllum decreases allergic reactions in a murine asthmatic model. Am. J. Chin. Med. 2008, 36, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Gu, X.Y.; Feng, G.Z.; Shen, L.; Cui, J.; Cai, J.K.; Huang, M.; Yin, K.S. Effects of astragaloside IV on the expressions of transforming growth factor-beta1 and thymic stromal lymphopoietin in a murine model of asthma. Zhonghua Yi Xue Za Zhi 2011, 91, 3139–3142. [Google Scholar] [PubMed]
- Tanaka, T.; Takahashi, R. Flavonoids and asthma. Nutrients 2013, 5, 2128–2143. [Google Scholar] [CrossRef]
- Karunaweera, N.; Raju, R.; Gyengesi, E.; Munch, G. Plant polyphenols as inhibitors of NF-kappaB induced cytokine production-a potential anti-inflammatory treatment for Alzheimer’s disease? Front. Mol. Neurosci. 2015, 8, 24. [Google Scholar] [CrossRef]
- Marzulli, G.; Magrone, T.; Kawaguchi, K.; Kumazawa, Y.; Jirillo, E. Fermented grape marc (FGM): Immunomodulating properties and its potential exploitation in the treatment of neurodegenerative diseases. Curr. Pharm. Des. 2012, 18, 43–50. [Google Scholar] [CrossRef]
- Gong, J.H.; Shin, D.; Han, S.Y.; Kim, J.L.; Kang, Y.H. Kaempferol suppresses eosionphil infiltration and airway inflammation in airway epithelial cells and in mice with allergic asthma. J. Nutr. 2012, 142, 47–56. [Google Scholar] [CrossRef]
- Heras, B.; Hortelano, S. Molecular Basis of the Anti-Inflammatory Effects of Terpenoids. Inflamm. Allergy-Drug Targets 2009, 8, 28–39. [Google Scholar] [CrossRef]
- Bae, Y.; Lee, S.; Kim, S.H. Chrysin suppresses mast cell-mediated allergic inflammation: Involvement of calcium, caspase-1 and nuclear factor-kappaB. Toxicol. Appl. Pharmacol. 2011, 254, 56–64. [Google Scholar] [CrossRef]
- Medeiros, K.C.P.; Faustino, L.; Borduchi, E.; Nascimento, R.J.; Silva, T.M.S.; Gomes, E.; Piuvezam, M.R.; Russo, M. Preventive and curative glycoside kaempferol treatments attenuate the TH2-driven allergic airway disease. Int. Immunopharmacol. 2009, 9, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Higa, S.; Hirano, T.; Kotani, M.; Matsumoto, M.; Fujita, A.; Suemura, M.; Kawase, I.; Tanaka, T. Fisetin, a flavonol, inhibits TH2-type cytokine production by activated human basophils. J. Allergy Clin. Immunol. 2003, 111, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Li, M.L.; Xia, M.Y.; Shao, J.Y. Fisetin-treatment alleviates airway inflammation through inhbition of MyD88/NF-kappaB signaling pathway. Int. J. Mol. Med. 2018, 42, 208–218. [Google Scholar] [PubMed]
- Leyva-Lopez, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as Cytokine Modulators: A Possible Therapy for Inflammation-Related Diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef] [PubMed]
- Kempuraj, D.; Madhappan, B.; Christodoulou, S.; Boucher, W.; Cao, J.; Papadopoulou, N.; Cetrulo, C.L.; Theoharides, T.C. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br. J. Pharmacol. 2005, 145, 934–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segawa, S.; Yasui, K.; Takata, Y.; Kurihara, T.; Kaneda, H.; Watari, J. Flavonoid glycosides extracted from hop (Humulus lupulus L.) as inhibitors of chemical mediator release from human basophilic KU812 cells. Biosci. Biotechnol. Biochem. 2006, 70, 2990–2997. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, A.D.; Liu, Z.; Mukherjee, A.A.; Bodhankar, S.L. Therapeutic potential of Morin in Ovalbumin-induced allergic asthma via modulation of SUMF2/IL-13 and BLT2/NF-kB signaling pathway. Curr. Mol. Pharmacol. 2019, 12, 122–138. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, L.; Fukuda, K.; Ouyang, S.; Chen, X.; Wang, C.; Zhang, C.J.; Martin, B.; Gu, C.; Qin, L.; et al. The flavonoid cyanidin blocks binding of the cytokine interleukin-17A to the IL-17RA subunit to alleviate inflammation in vivo. Sci Signal 2017, 10, eaaf8823. [Google Scholar] [CrossRef]
- Liu, L.L.; Li, F.H.; Zhang, Y.; Zhang, X.F.; Yang, J. Tangeretin has anti-asthmatic effects via regulating PI3K and Notch signaling and modulating Th1/Th2/Th17 cytokine balance in neonatal asthmatic mice. Braz. J. Med. Biol. Res. 2017, 50, e5991. [Google Scholar] [CrossRef]
- Liu, L.L.; Zhang, Y.; Zhang, X.F.; Li, F.H. Influence of rutin on the effects of neonatal cigarette smoke exposure-induced exacerbated MMP-9 expression, Th17 cytokines and NF-kappaB/iNOS-mediated inflammatory responses in asthmatic mice model. Korean J. Physiol. Pharmacol. 2018, 22, 481–491. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Zhang, Y.; Zhao, P.; Zhang, X. Regulatory effect of baicalin on the imbalance of Th17/Treg responses in mice with allergic asthma. J. Ethnopharmacol. 2017, 208, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Dai, J.; Liu, H.; Li, R.R.; Sun, P.L.; Du, Q.; Pang, L.L.; Chen, Z.; Yin, K.S. Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-kappaB activity in a murine model of asthma. Can. J. Physiol. Pharmacol. 2009, 87, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Ci, X.; Zhong, W.; Ren, H.; Wen, Z.; Li, D.; Peng, L. Esculentoside A Attenuates Allergic Airway Inflammation via Activation of the Nrf-2 Pathway. Int. Arch. Allergy Immunol. 2015, 167, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Hamalainen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-inflammatory effects of flavonoids: Genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediat. Inflamm. 2007, 2007, 45673. [Google Scholar]
- Lee, J.S.; Lee, C.M.; Jeong, Y.I.; Jung, I.D.; Kim, B.H.; Seong, E.Y.; Kim, J.I.; Choi, I.W.; Chung, H.Y.; Park, Y.M. D-pinitol regulates Th1/Th2 balance via suppressing Th2 immune response in ovalbumin-induced asthma. FEBS Lett. 2007, 581, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, R.R.; Elmahdy, M.K.; Suddek, G.M. Flavocoxid attenuates airway inflammation in ovalbumin-induced mouse asthma model. Chem. Biol. Interact. 2018, 292, 15–23. [Google Scholar]
- Li, J.; Zhang, B. Apigenin protects ovalbumin-induced asthma through the regulation of Th17 cells. Fitoterapia 2013, 91, 298–304. [Google Scholar] [CrossRef]
- Jin, M.; Yang, J.H.; Lee, E.; Lu, Y.; Kwon, S.; Son, K.H.; Son, J.K.; Chang, H.W. Antiasthmatic activity of luteolin-7-O-glucoside from Ailanthus altissima through the downregulation of T helper 2 cytokine expression and inhibition of prostaglandin E2 production in an ovalbumin-induced asthma model. Biol. Pharm. Bull. 2009, 32, 1500–1503. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Fallahi, M.; Khamaneh, A.M.; Ebrahimi Saadatlou, M.A.; Saadat, S.; Keyhanmanesh, R. Effect of alpha-Hederin on IL-2 and IL-17 mRNA and miRNA-133a Levels in Lungs of Ovalbumin-Sensitized Male Rats. Drug Dev. Res. 2016, 77, 87–93. [Google Scholar] [CrossRef]
- Fallahi, M.; Keyhanmanesh, R.; Khamaneh, A.M.; Saadatlou, M.A.E.; Saadat, S.; Ebrahimi, H. Effect of Alpha-Hederin, the active constituent of Nigella sativa, on miRNA-126, IL-13 mRNA levels and inflammation of lungs in ovalbumin-sensitized male rats. Avicenna J. Phytomed. 2016, 6, 77. [Google Scholar]
- Junchao, Y.; Zhen, W.; Yuan, W.; Liying, X.; Libin, J.; Yuanhong, Z.; Wei, Z.; Ruilin, C.; Lu, Z. Anti- trachea inflammatory effects of diosgenin from Dioscorea nipponica through interactions with glucocorticoid receptor alpha. J. Int. Med. Res. 2017, 45, 101–113. [Google Scholar] [CrossRef]
- Ninave, P.B.; Patil, S.D. Antiasthmatic potential of Zizyphus jujuba Mill and Jujuboside B. - Possible role in the treatment of asthma. Respir. Physiol. Neurobiol. 2019, 260, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, N.; Song, Y.; Wang, L.; Zi, J.; Zhang, S.; Dunkin, D.; Busse, P.; Weir, D.; Tversky, J.; et al. Ganoderic acid C1 isolated from the anti-asthma formula, ASHMI suppresses TNF-alpha production by mouse macrophages and peripheral blood mononuclear cells from asthma patients. Int. Immunopharmacol. 2015, 27, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.A.; de las Heras, B.; Garcia, M.D.; Saenz, M.T.; Villar, A. New insights into the mechanism of action of the anti-inflammatory triterpene lupeol. J. Pharm. Pharmacol. 2001, 53, 1533–1539. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, J.F.; Teixeira, M.M.; Barbosa-Filho, J.M.; Lucio, A.S.; Almeida, J.R.; de Queiroz, L.P.; Ribeiro-Dos-Santos, R.; Soares, M.B. The triterpenoid lupeol attenuates allergic airway inflammation in a murine model. Int. Immunopharmacol. 2008, 8, 1216–1221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, X.; Sang, L.; Liu, H.; Xu, Q.; Liu, Z. Boswellic acid attenuates asthma phenotypes by downregulation of GATA3 via pSTAT6 inhibition in a murine model of asthma. Int. J. Clin. Exp. Pathol. 2015, 8, 236–243. [Google Scholar]
- Jung, H.W.; Chung, Y.S.; Kim, Y.S.; Park, Y.K. Celastrol inhibits production of nitric oxide and proinflammatory cytokines through MAPK signal transduction and NF-kappaB in LPS-stimulated BV-2 microglial cells. Exp.Mol. Med. 2007, 39, 715–721. [Google Scholar] [CrossRef]
- Lindner, I.; Meier, C.; Url, A.; Unger, H.; Grassauer, A.; Prieschl-Grassauer, E.; Doerfler, P. Beta-escin has potent anti-allergic efficacy and reduces allergic airway inflammation. BMC Immunol. 2010, 11, 24. [Google Scholar] [CrossRef]
- Isik, S.; Karaman, M.; Micili, S.C.; Caglayan-Sozmen, S.; Bagriyanik, H.A.; Arikan-Ayyildiz, Z.; Uzuner, N.; Karaman, O. Sinomenine ameliorates the airway remodelling, apoptosis of airway epithelial cells, and Th2 immune response in a murine model of chronic asthma. Allergol. Immunopathol. 2018, 46, 67–75. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, J.H.; Lee, Y.C. Chelidonine, a principal isoquinoline alkaloid of Chelidonium majus, attenuates eosinophilic airway inflammation by suppressing IL-4 and eotaxin-2 expression in asthmatic mice. Pharmacol. Rep. 2015, 67, 1168–1177. [Google Scholar] [CrossRef]
- Song, Y.; Wu, Y.; Li, X.; Shen, Y.; Ding, Y.; Zhu, H.; Liu, F.; Yu, K.; Sun, L.; Qian, F. Protostemonine attenuates alternatively activated macrophage and DRA-induced asthmatic inflammation. Biochem. Pharmacol. 2018, 155, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Fang, Z.Y.; Tao, X.N.; Bai, M.; Feng, G. Effect and mechanism of ligustrazine on Th1/Th2 cytokines in a rat asthma model. Am. J. Chin. Med. 2007, 35, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, B.F. Differential modulation of IgE-dependent activation of human basophils by ambroxol and related secretolytic analogues. Int. J. Immunopathol. Pharmacol. 2009, 22, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, J.; Zhang, N.; Li, C. Berberine improves airway inflammation and inhibits NF-kappaB signaling pathway in an ovalbumin-induced rat model of asthma. J. Asthma 2016, 53, 999–1005. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, H.J.; Lee, C.M.; Choi, I.W.; Moon, D.O.; Roh, H.J.; Lee, H.K.; Park, Y.M. Epigallocatechin-3-gallate protects toluene diisocyanate-induced airway inflammation in a murine model of asthma. FEBS Lett. 2006, 580, 1883–1890. [Google Scholar] [CrossRef] [Green Version]
- Rogerio, A.P.; Fontanari, C.; Borducchi, E.; Keller, A.C.; Russo, M.; Soares, E.G.; Albuquerque, D.A.; Faccioli, L.H. Anti-inflammatory effects of Lafoensia pacari and ellagic acid in a murine model of asthma. Eur. J. Pharmacol. 2008, 580, 262–270. [Google Scholar] [CrossRef]
- Zhou, E.; Fu, Y.; Wei, Z.; Yang, Z. Inhibition of allergic airway inflammation through the blockage of NF-kappaB activation by ellagic acid in an ovalbumin-induced mouse asthma model. Food Funct. 2014, 5, 2106–2112. [Google Scholar] [CrossRef]
- Lee, M.; Kim, S.; Kwon, O.K.; Oh, S.R.; Lee, H.K.; Ahn, K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int. Immunopharmacol. 2009, 9, 418–424. [Google Scholar] [CrossRef]
- Kim, S.Y.; Moon, K.A.; Jo, H.Y.; Jeong, S.; Seon, S.H.; Jung, E.; Cho, Y.S.; Chun, E.; Lee, K.Y. Anti-inflammatory effects of apocynin, an inhibitor of NADPH oxidase, in airway inflammation. Immunol. Cell Biol. 2012, 90, 441–448. [Google Scholar] [CrossRef]
- Chen, M.; Lv, Z.; Jiang, S. The effects of triptolide on airway remodelling and transforming growth factor-beta(1)/Smad signalling pathway in ovalbumin-sensitized mice. Immunology 2011, 132, 376–384. [Google Scholar] [CrossRef]
- Bao, Z.; Guan, S.; Cheng, C.; Wu, S.; Wong, S.H.; Kemeny, D.M.; Leung, B.P.; Wong, W.S. A novel antiinflammatory role for andrographolide in asthma via inhibition of the nuclear factor-kappaB pathway. Am. J. Respir. Crit. Care Med. 2009, 179, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Munroe, M.E.; Businga, T.R.; Kline, J.N.; Bishop, G.A. Anti-inflammatory effects of the neurotransmitter agonist Honokiol in a mouse model of allergic asthma. J. Immunol. 2010, 185, 5586–5597. [Google Scholar] [CrossRef] [PubMed]
- Keyhanmanesh, R.; Boskabady, M.H.; Khamneh, S.; Doostar, Y. Effect of thymoquinone on the lung pathology and cytokine levels of ovalbumin-sensitized guinea pigs. Pharmacol. Rep. 2010, 62, 910–916. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Li, J. The Immunoregulatory Effects of Traditional Chinese Medicine on Treatment of Asthma or Asthmatic Inflammation. Am. J. Chin. Med. 2015, 43, 1059–1081. [Google Scholar] [CrossRef] [PubMed]
- Demlova, R.; Valik, D.; Obermannova, R.; ZdraZilova-Dubska, L. The safety of therapeutic monoclonal antibodies: Implications for cancer therapy including immuno-checkpoint inhibitors. Physiol. Res. 2016, 65 (Suppl. 4), S455–S462. [Google Scholar]
- Byard, R.W.; Musgrave, I.; Maker, G.; Bunce, M. What risks do herbal products pose to the Australian community? Med. J. Aust. 2017, 206, 86–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Group | Anti-Cytokine Ab | Drug |
|---|---|---|
| Group 1 | Anti-TSLP Ab | Tezepelumab (Phase 3 clinical trial) |
| Anti-IL-6 Ab | N/A | |
| Anti-IL-25 Ab | N/A | |
| Anti-IL-33 Ab | AMG 282 (Phase 1 clinical trial) ANB020 (Phase 2 clinical trial) | |
| Anti-IL-33R Ab | CNTO 7160 (Phase 1 clinical trial) | |
| Group 2 | Anti-IL-4Rα Ab | Dupilumab (Phase 3 clinical trial) |
| Anti-IL-5 Ab | Mepolizumab (US FDA approved) Reslizumab (US FDA approved) | |
| Anti-IL-5Rα Ab | Benralizumab (US FDA approved) | |
| Anti-IL-9 Ab | MEDI-528 (Phase 2 clinical trial) | |
| Anti-IL-13Ab | Tralokinumab (Phase 3 clinical trial) Lebrikizumab (Phase 3 clinical trial) | |
| Anti-IL-17 Ab | Brodalumab (Phase 2 clinical trial) | |
| Group 3 | Anti-TGF-β Ab | N/A |
| Group | Components | Cytokines * | Ref. |
|---|---|---|---|
| Group 1 | |||
| Cordyceps sinensis | CS-19-22 fraction | IL-1β, TNF- α, IL-6, IL-10 (–) (LPS-activated BALF cells) | [98] |
| Astragalus membranaceus | Astragaloside IV | IL-1β, TNF- α, GM-CSF (–) (Der p 1 activated human blood eosinophils) | [99] |
| Curcuma longa | Curcumin | IL-1β, TNF- α, IL-6, IL-2 (–) (DRA-challenged mice/ LPS-stimulated macrophages) | [100,101] |
| Thuja occidentalis | Extract | IL-6, TNF-α (–) (LPS-stimulated macrophages) | [102] |
| Fritillaria thunbergii | Extract | IL-6, TNF-α (–) (Human mast cell line-1 for childhood asthma) | [89] |
| Scutellaria baicalensis | Extract | TNF-α (–) (compound 48/80-induced HMC-1 cells) | [103] |
| Alstonia scholaris | Total alkaloid | TNF-α, (–) (LPS-induced airway inflammation in rats) | [104] |
| Group 2 | |||
| Astragalus membranaceus | Extract | IL-4, IL-5, IL-13 (–), IFN-γ (+) | [105,106] |
| Astragaloside IV | IFN-γ (+), IL-4, IL-5, IL-13 (–) | [107,108] | |
| Asparagus cochinchinensis | Saponin-enriched extract | IL-4, IL-13 (–) | [109] |
| Peucedanum praeruptorum | Coumarins | IL-4, IL-5, IL-13 (–), IL-10, IFN-γ (+) | [110] |
| (±)-praeruptorin A | IL-4, IL-5, IL-12, IL-13 (–) | [111,112] | |
| Victis fructus | Pyranopyran-1, 8-dione | IL-4, IL-5, IL-13 (–) (Cockroach allergen-induced mice) | [113] |
| Glycyrrhiza uralensis | Isoliquiritigenin 7, 4’-DHF, liquiritigenin | IL-4, IL-5, IL-13, GATA-3 (–), IFN-γ (+) (effector memory Th2 cells D10 and OVAsensitized/challenged mice) | [114] |
| Ilex chineses | Protocatechuic acid | IL-4, IL-5, IL-13 (–) | [115] |
| Rheum officinale | Emodin | IL-4, IL-5, IL-13 (–) | [116] |
| Lithonspermum erythrorthizon | Shikonin | IL-4, IL-5, IL-13, TNF-α (–) (OVA/TSLP-induced BM-DCs maturation) | [117] |
| Ganoderma tsugae | Triterpenoid-rich extracts | IL-4, IL-5 (–) | [118] |
| Thuja orientalis | Extract | IL-4, IL-5, IL-13 (–) (LPS-stimulated macrophages) | [102] |
| Ligusticum wallichi | Ligustrazine | IL-4, IL-5, IL-13, IL-17, TNF-α (–) | [119] |
| Armeniacae amarum | Water extract | IL-4 (–) | [120] |
| Morus alba | Kuwanon G | IL-4, IL-5, IL-13 (–) | [121] |
| Pinellia ternate | Water extract | IL-4, IL-5, IL-13, TNF-α (–) | [122,123] |
| Mentha haplocalyx | Ethanol extract | IL-5 (–) | [124] |
| Platycodon grandiflorum | Water extract | IL-4, IFN-γ, IL-5, IL-13, TNF-α (–) | [125] |
| Saponins | IL-4, TNF-α (–) (IgE antibody-induced RBL-2H3 cell) | [126] | |
| Scutellaria baicalensis | Skullcapflavone II | IL-4, TNF-β1 (–) | [127] |
| Echinodarus scaber | Hydroethanolic extract | IL-4, IL-5, IL-13 (–) | [128] |
| Propolis | Caffeic acid phen-ethyl ester | IL-4, IL-5, TNF-α (–) | [129] |
| Tripterygium polyglycosid | Extract | IL-5 (–) | [130] |
| Triptolide | IL-5, IL-12, TGF-β1 (–) (LPS-stimulated MPM and human MDC) | [131,132] | |
| Propolis | IL-10, IFN-γ, IL-5, IL-6 (–), | [133] | |
| Cordyceps sinensis | CS-19-22 fraction | IFN-γ, IL-12 (+) (LPS-activated BALF cells) | [98] |
| Curcuma longa | FLLL31 (derivative of curcumin) | IL-17 (–) (DRA-challenged mice and LPS-stimulated macrophages | [101] |
| Anoectochilus formosanus | Extract | IL-4, TNF-α (–), IFN-γ, IL-12 (+) | [134] |
| Gynostemma pentaphyllum | Extract | IFN-γ (+) | [135] |
| Group 3 | |||
| Astragalus membranaceus | Astragaloside IV | TGF-β1 (–), IL-10 (+) | [136] |
| Ligusticum wallichii | Ligustrazine | IL-10 (+) | [119] |
| Peucedanum praeruptorum | (±)-Praeruptorin A | TGF-β1 (–) | [111] |
| Tripterygium polyglycosid | Triptolide | TGF-β1 (–) | [132] |
| Type | Compound | Cytokine * | Mechanisms | Ref. |
|---|---|---|---|---|
| Flavonoids | Chrysin | Gr1: IL-1β, IL-6 (–) Gr2: IL-4, TNF-α (–) | Inhibition of the NF-κB signaling pathway and caspase-1 | [142] |
| Kaempferol | Gr2: IL-4, IL-5, IL-13 (–) (A23187-stimulated KU812 cells) | Inhibition of the NF-κB signaling pathway | [143,144] | |
| Fisetin | Gr2: IL-4, IL-5, IL-13, TNF-α (–) (A23187-stimulated KU812 cells) Gr 1: IFN-γ, IL-8, IL-1β (–) | Inhibition of the MyD88 and NF-κB signaling pathways | [144,145,146] | |
| Quercetin | Gr1: IL-1β, IL-6 (–) (A23187-stimulated KU812 cells) Gr2: IL-4, IL-5, TNF-α, IFN-γ (–) Gr3: IL10 (+) (BV-2 LPS-stimulated microglia cells) | Inhibition of protein kinase C θ phosphorylation inhibition of the NF-κB signaling pathway | [144,146,147,148] | |
| Skullcap-flavone II | Gr2: IL-4, IL-5, IL-13 (–) Gr3: TGF-β1 (–) | Acting on TGF-β1/Smad signaling pathways | [127] | |
| Morin | Gr1: IL-1β, IL-6 (–) Gr2: TNF-α, IL-4, IL-13 (–) | up-regulated SUMF2 mRNA expression and down-regulated Leukotriene B4 receptor 2 (BLT2)/NF-kB mRNA expression | [149] | |
| Myricetin | Gr1: IL-6, IL-8, TNF-α (–) (Human umbilical cord blood-derived cultured mast cells) | Inhibition of protein kinase C θ phosphorylation | [147] | |
| Cyanidin | Gr1: IL-17A (–) | Inhibition of the IL-17A/IL-17RA interaction | [150] | |
| Tangeretin | Gr1: IL-6 (–) Gr2: IFN-γ (+), IL-4, IL-5, IL-13, IL-17A (–) | Modulate PI3K/Akt and Notch signaling and Th2/Th1 and Th17 cytokine levels | [151] | |
| Rutin | Gr2: IL-4, IL-5, IL-13, IL-17A (–), IFN-γ (+) Gr3:IL-10 (+) | Inhibition of the NF-κB signaling pathway | [152] | |
| Kaempferol glycosides | Gr2: IL-5, IL-13 (–) | Inhibition of IL-4-induced transcription factor STAT6 activation | [143] | |
| Baicalin | Gr1: IL-6 (–) Gr2: IL-17A (–) Gr3: IL-10 (+) | Suppression of STAT3 expression and promoted FOXP3 expression | [153] | |
| Naringenin | Gr2: IL-4, IL-13 (–) | Inhibition of the NF-κB signaling pathway | [146,154] | |
| Esculento-side A | Gr2: IL-4, IL-5, IL-13 (–) | Nrf-2 activation | [155] | |
| Genistein and Daidzein | Gr1: IL-1β, TNF-α (–) | Inhibition of STAT-1 and NF-κB pathways | [156] | |
| Pinitol | Gr2: IFN-γ (+), IL-4, IL-5 (–) | Blocking the transcription factor GATA binding protein 3 (GATA 3) | [157] | |
| Flavocoxid | Gr2: IL-13 (–) | - | [158] | |
| Apigenin | Gr1: IL-6, TNF-α (–) Gr2: IL-17A (–), IL-4 (–) | Blocking the transcription factor GATA 3 | [146,159] | |
| Luteolin-7-O-glucoside | Gr2: IL-4, IL-5, IL-13 (–) | Downregulation of T helper 2 cytokine transcript | [146,160] | |
| Triterpenoid and glycosides | Astragaloside IV | Gr2: IL-4 (–), IFN-γ (+) Gr3: IL-10 (+) | Inhibition of the synthesis of GATA-3-encoding mRNA and protein in addition to increasing the synthesis of T-bet-encoding mRNA and protein in both lung tissues and CD4+ T cells | [107,108] |
| α-Hederin | Gr2:IL-13, IL-17A (–), IL-2 (+) | Th1 cells (increases the Th1/Th2 ratio) | [161,162] | |
| Diosgenin | Gr1: TNF-α, IL1-β, IL-6 (–) | Enhancing the expression of glucocorticosteroid receptors, SLPI, GILZ, and MKP-1, and inhibiting the expression of HSP70 | [163] | |
| Jujuboside B | Gr2: IL-4, IL-5 (–) | - | [164] | |
| Ganoderic acid C1 | Gr1: TNF-α (–) (RAW264.7 cells and peripheral bloodmononuclear cells (peripheral blood mononuclear cells; PBMCs) from asthma patients) | Downregulation of NF-κB expression, and partial suppression of MAPK and AP-1 signaling pathways | [165] | |
| Lupeol | Gr1: TNF-α, IL-1β (–) Gr2: IL-4, IL-5, IL-13 (–) | A mechanism distinct of glucocorticoids, | [166,167] | |
| Boswellic acid | Gr2: IL-4, IL-5, IL-13 (–) | Decreasing the expression of pSTAT6 and GATA-3 | [168] | |
| Celastrol | Gr1: TNF-α, IL-1β (–) (LPS-stimulated BV-2 cells) | Inhibition of extracellular signal-regulated kinase 1 and 2 (ERK1/2) phosphorylation and NF-κB activation | [169] | |
| B-Escin | Gr2: IL-5, IL-13 (–) | - | [170] | |
| Lupeol | Gr2: IL-4, IL-5, IL-13 (–) (LPS-treated marcophages) | - | [167] | |
| Alkaloids | Sinomenine | Gr2: IL-4, IL-5, IL-13 (–) Gr3: TGF-β (–) | Inhibition of TH2 immune response, apoptosis of airway ECs and airway remodeling | [171] |
| Chelidonine | Gr2:, IL-4, IL-13 (–) | STAT6 and Foxp3 pathways | [172] | |
| Protostemonine | Gr2: IL-4, IL-5, IL-13, IL-33 (–) (dust mites, ragweed and aspergillus-induced asthma) | Inhibition of STAT6, KLF4, and IRF4 | [173] | |
| Ligustrazine | Gr2: IL-4 (–), IFN-γ (+) | Modulating key master switches GATA-3 and T-bet | [174] | |
| Ambroxol | Gr2: IL-4, L-13 (–) | Inhibiting IgE-dependent basophil mediator release and p38 MAPK activity | [175] | |
| Berberine | Gr1: IL-1β, IL-6 (–) Gr2: IL-4, IL-5, IL-13, IL-17 (–) | Inhibition of the NF-κB signaling pathway | [176] | |
| Polyphenols | Epigallocatechin-3-gallate | Gr1: TNF-α (–) Gr2: IL-5 (–) (Toluene diisocyanate-induced asthma model) | Activation of the 5’ AMP-activated protein kinase (AMPK) signaling pathway | [177] |
| Curcumin | Gr1: TNF-α, IL-1, IL-6 (–) Gr2: IL-2, IL-12 (–) | Inhibition of the NF-κB signaling pathway | [101] | |
| Ellagic acid | Gr2: IL-4, IL-5, IL-13 (–) | Inhibition of the NF-κB signaling pathway | [178,179] | |
| Resveratrol | Gr2: IL-4, IL-5 (–) | Inhibition of the NF-κB signaling pathway | [180] | |
| Apocynin | Gr1: TNF-α (–) Gr2: IL-4, IL-5, IL-12, IL-13 (–) | Inhibition of the NF-κB signaling pathway | [181] | |
| Others | Triptolide | Gr2: IL-2 (+) Gr3: TGF-β1 (–) | TGF-β1/Smad pathway | [182] |
| Andrographolide | Gr2: IL-4, IL-5, IL-13 (–) | Inhibition of the NF-κB signaling pathway | [183] | |
| Honokiol | Gr1: TNF-α, IL-6 (–) Gr2: IL-12, IFN-γ (+), IL-13, IL-17(–) Gr3: IL-10, TGF-β (+) | γ-Aminobutyric acid type A-dependent manner | [184] | |
| Thymoquin-one | Gr2: IL-4 (–), IFN-γ (+) | - | [185] | |
| Shikonin | Gr1: TNF-α (–) Gr2: IL-4, IL-5, IL-13 (–) (OVA + TSLP-induced BM-DC maturation, OVA-sensitized/challenged mice) | - | [117] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-C.; Shi, L.-S.; Ye, Y.-L. Advanced Molecular Knowledge of Therapeutic Drugs and Natural Products Focusing on Inflammatory Cytokines in Asthma. Cells 2019, 8, 685. https://doi.org/10.3390/cells8070685
Lin S-C, Shi L-S, Ye Y-L. Advanced Molecular Knowledge of Therapeutic Drugs and Natural Products Focusing on Inflammatory Cytokines in Asthma. Cells. 2019; 8(7):685. https://doi.org/10.3390/cells8070685
Chicago/Turabian StyleLin, Sheng-Chieh, Li-Shian Shi, and Yi-Ling Ye. 2019. "Advanced Molecular Knowledge of Therapeutic Drugs and Natural Products Focusing on Inflammatory Cytokines in Asthma" Cells 8, no. 7: 685. https://doi.org/10.3390/cells8070685