Prognostic Value of miRNAs in Head and Neck Cancers: A Comprehensive Systematic and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Meta-Analysis and Assessment of Heterogeneity
2.6. Publication Bias
3. Results
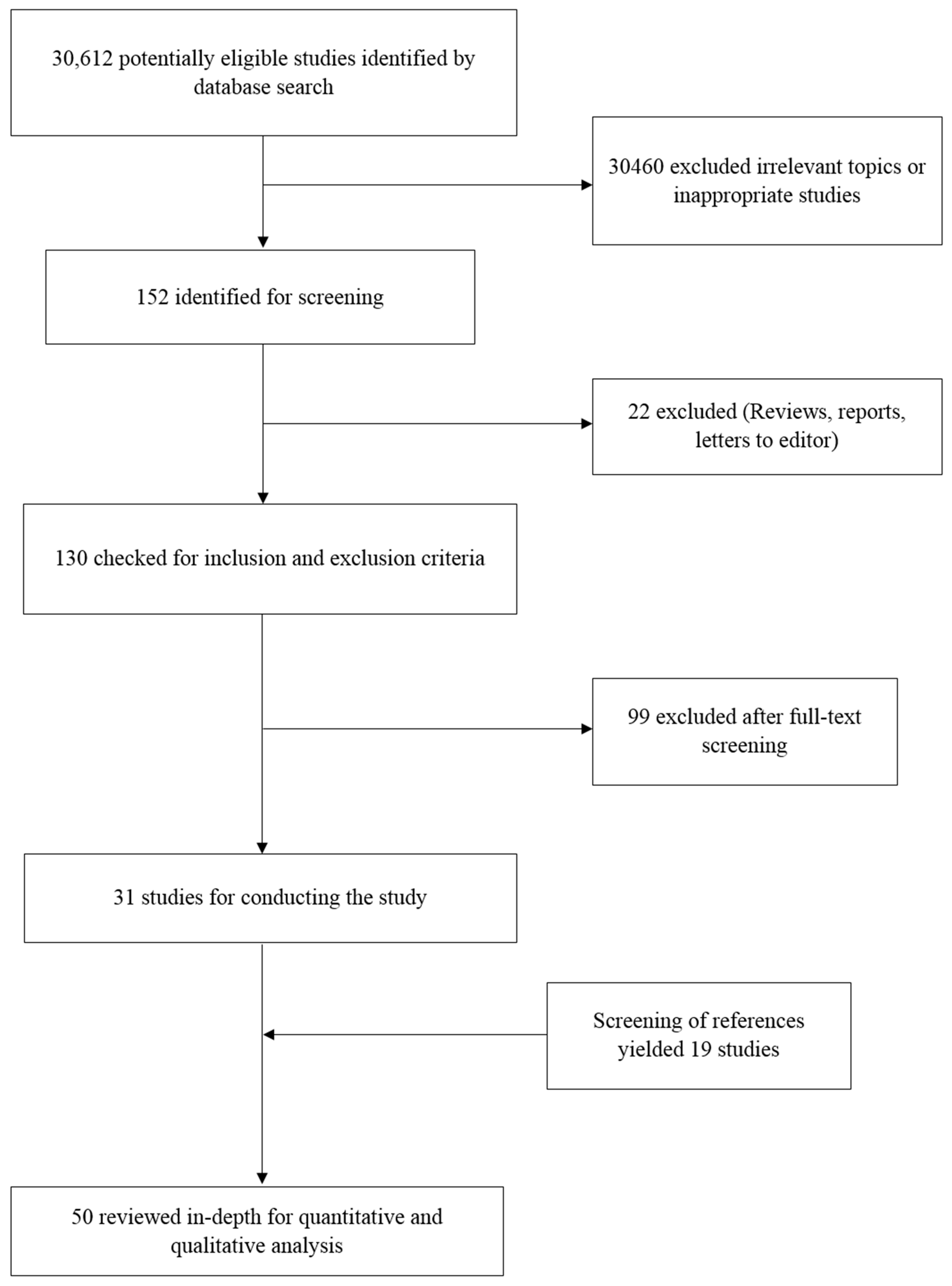
3.1. Study Selection and Data Extraction
3.2. Study Characteristics
3.3. Meta-Analysis
3.4. Subgroup Analysis
3.4.1. Upregulation and Downregulation Subgroups
3.4.2. miRNA Subgroups
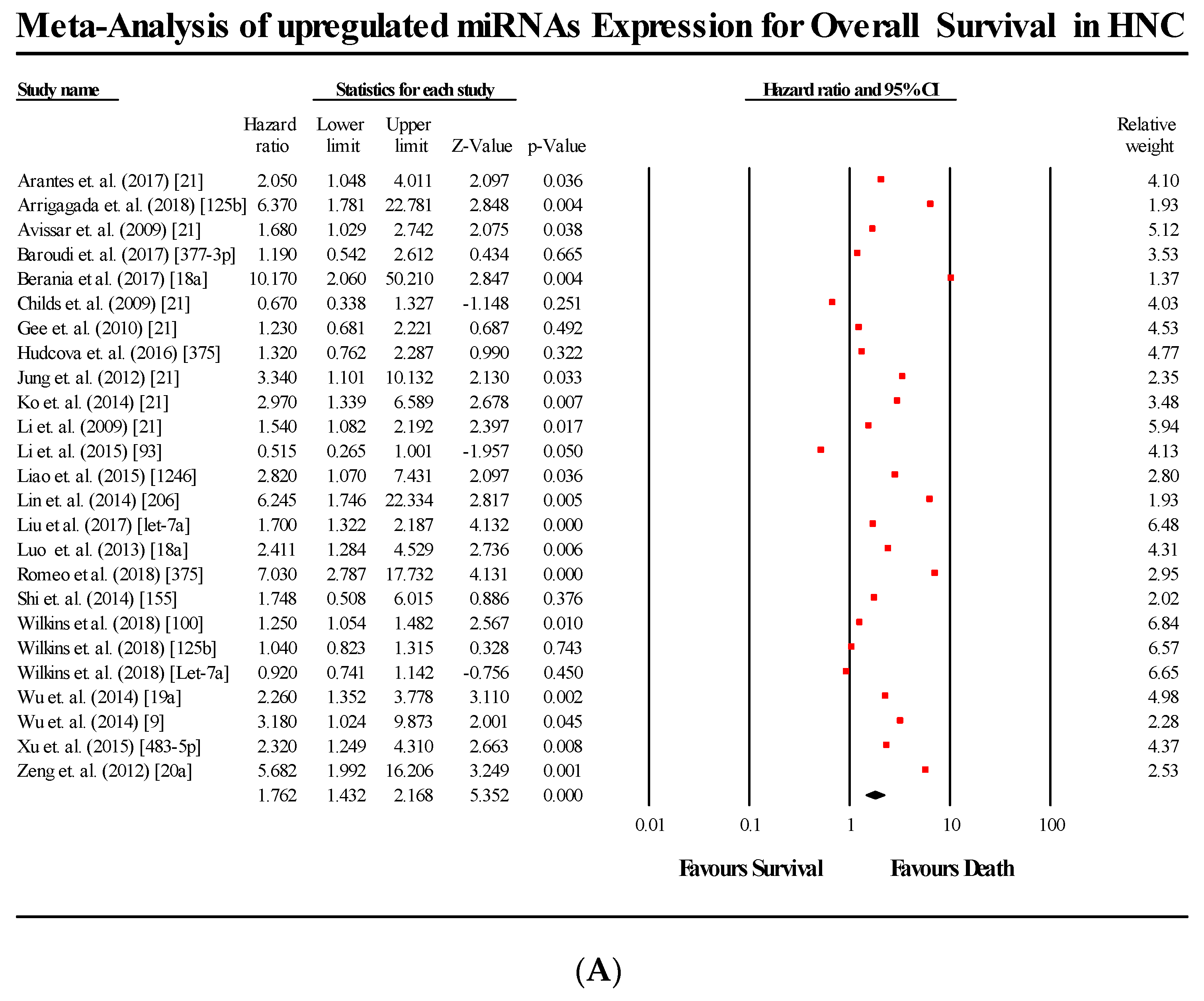
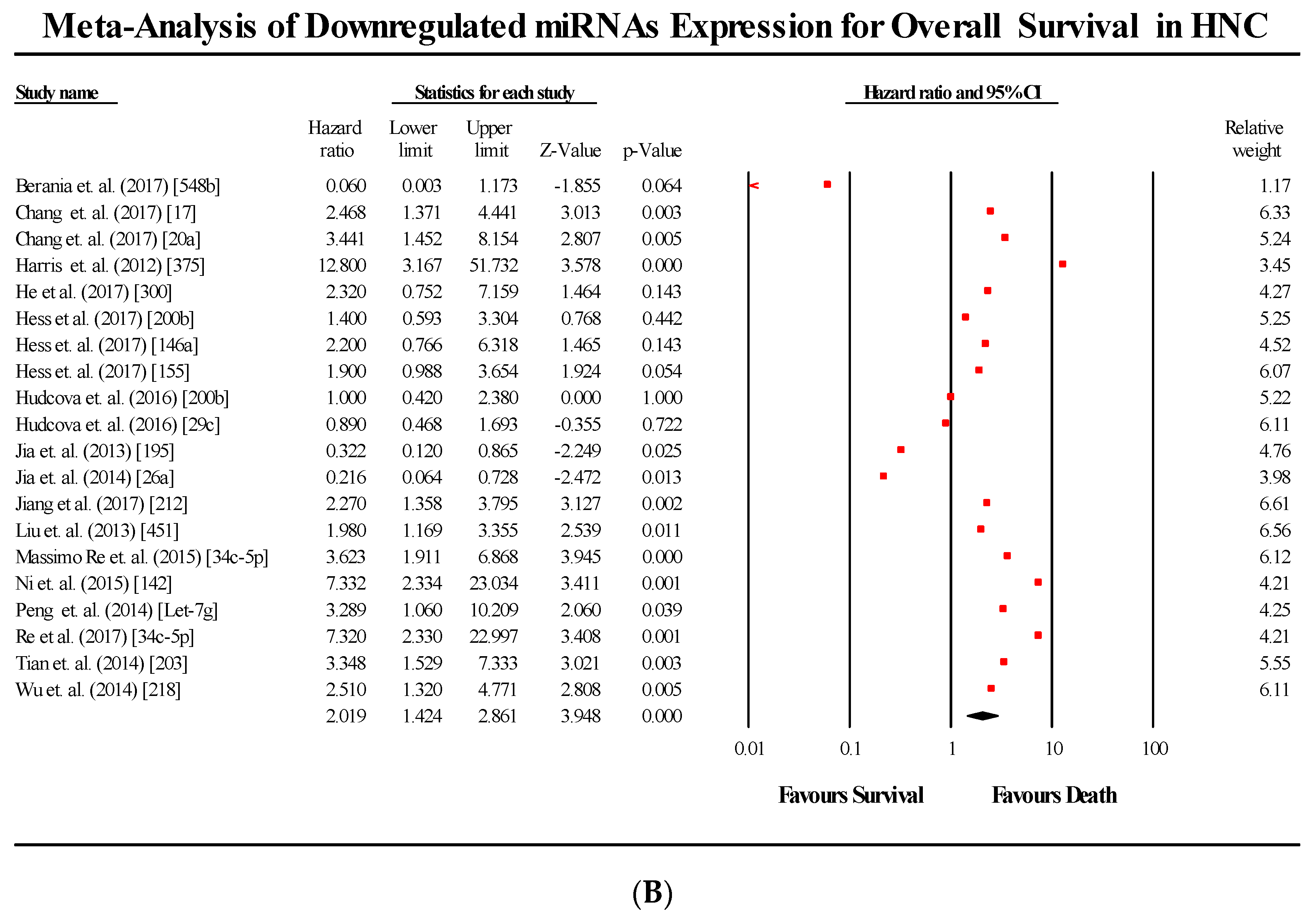
3.5. Overall Survival Group
3.5.1. miR-21
3.5.2. miR-200b
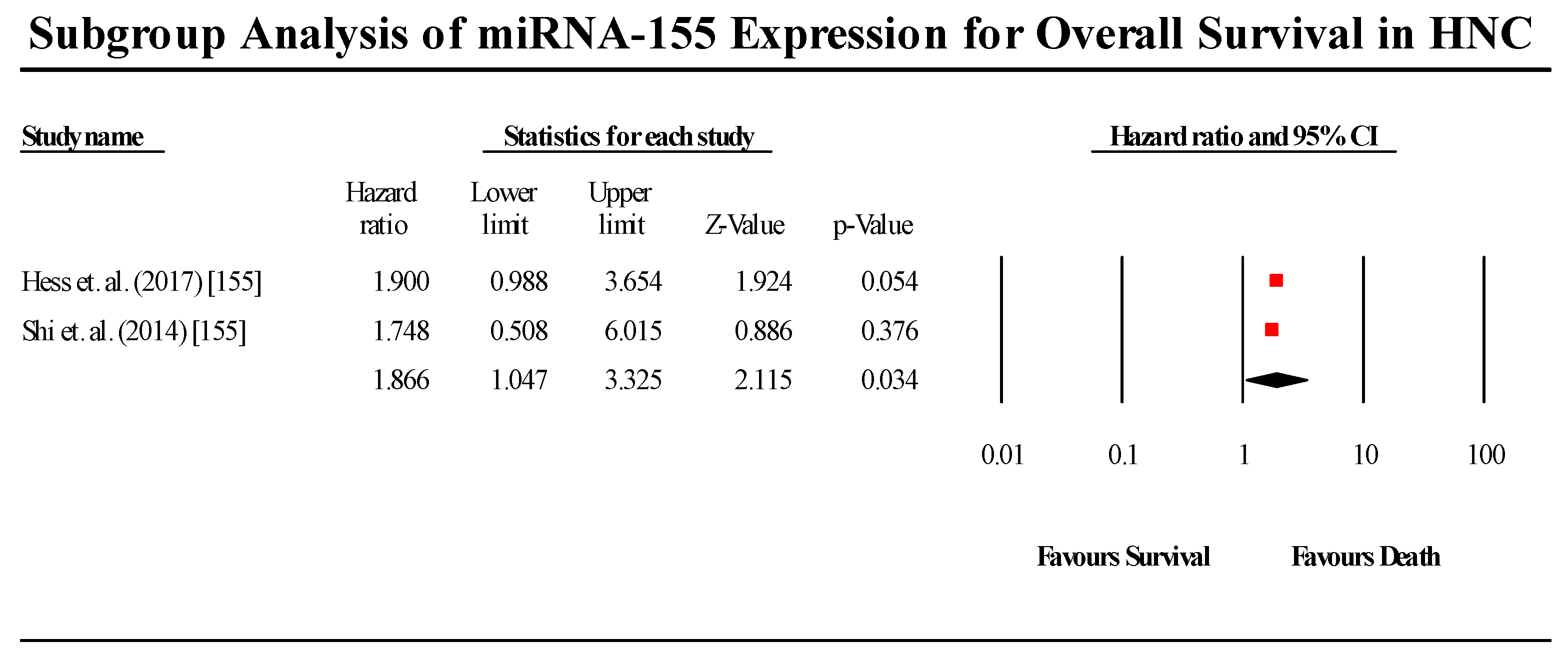
3.5.3. miR-155
3.5.4. miR-18a
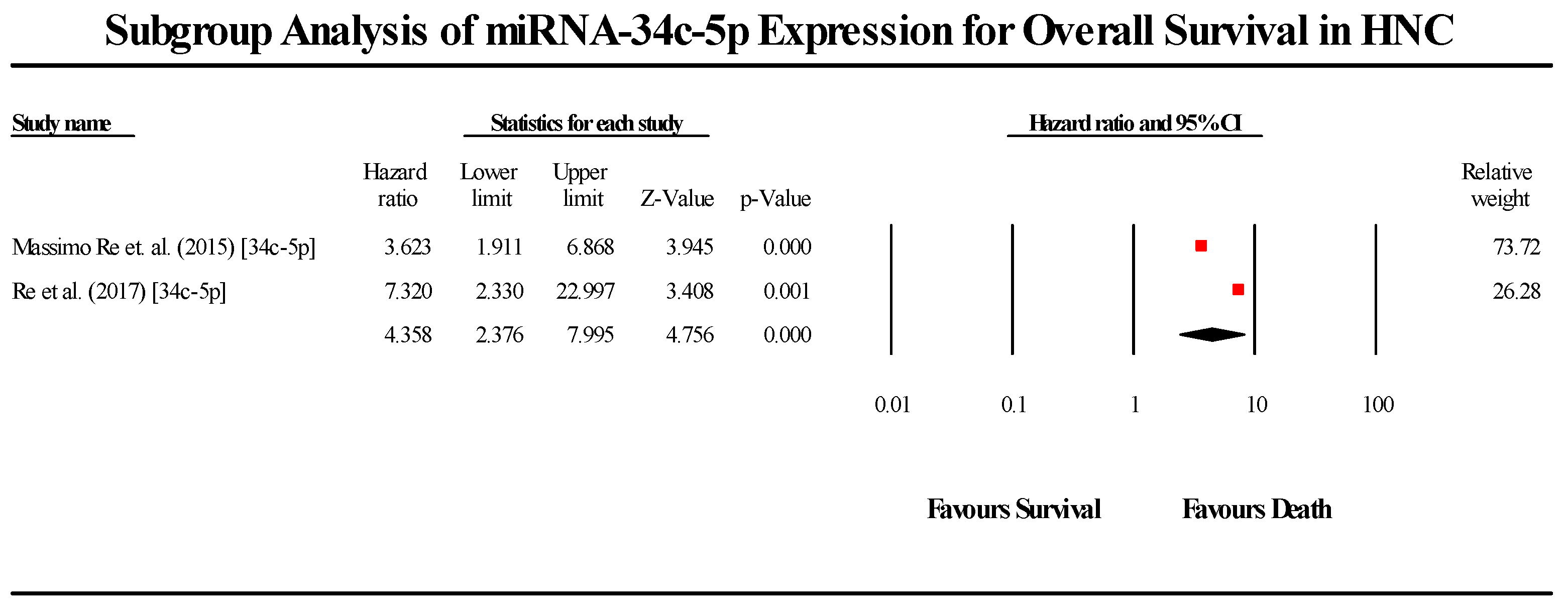
3.5.5. miR-34c-5p
3.5.6. miR-125b
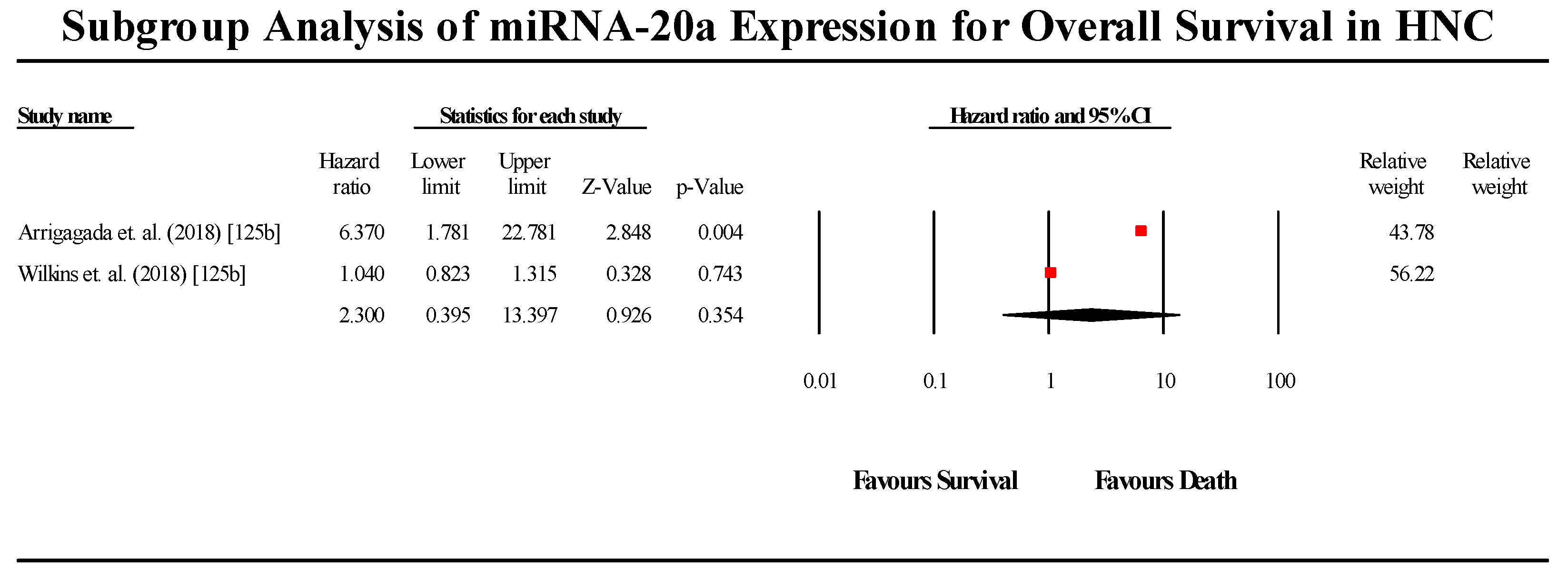
3.5.7. miR-20a
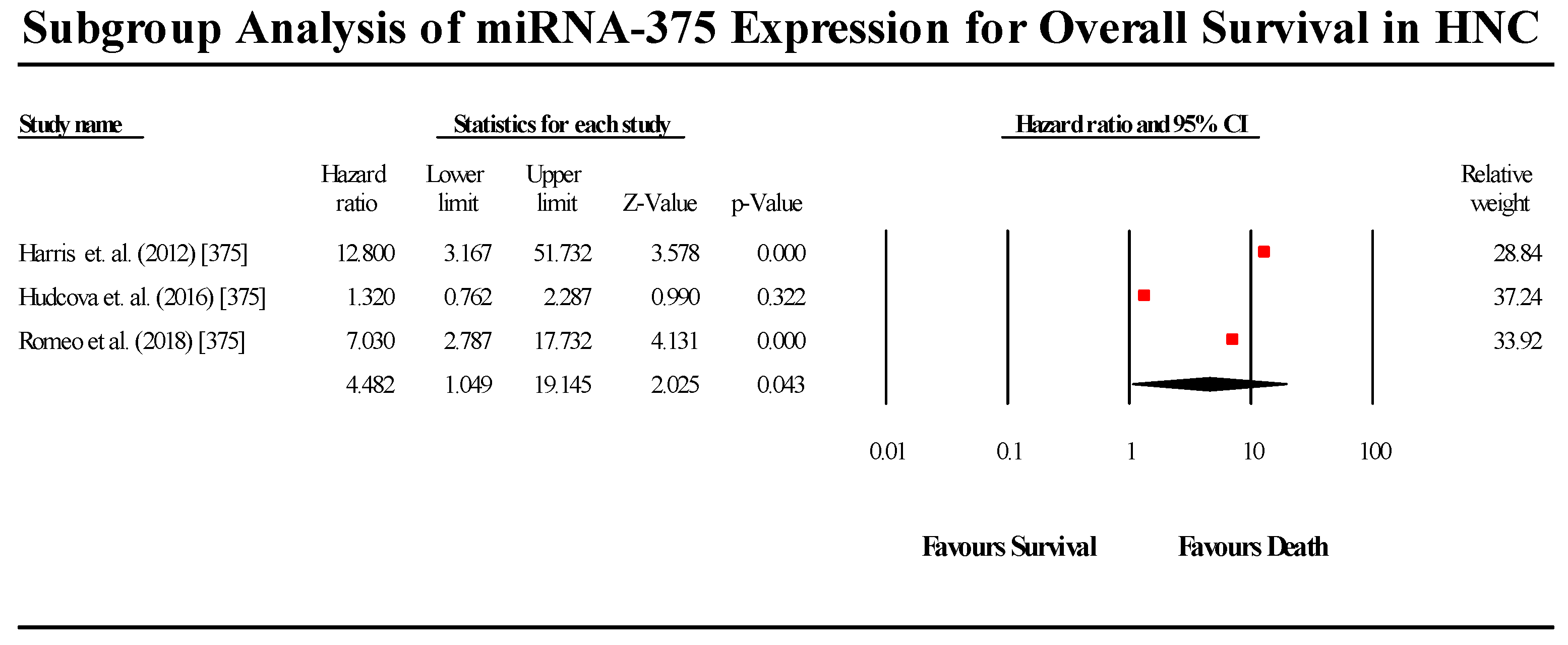
3.5.8. miR-375
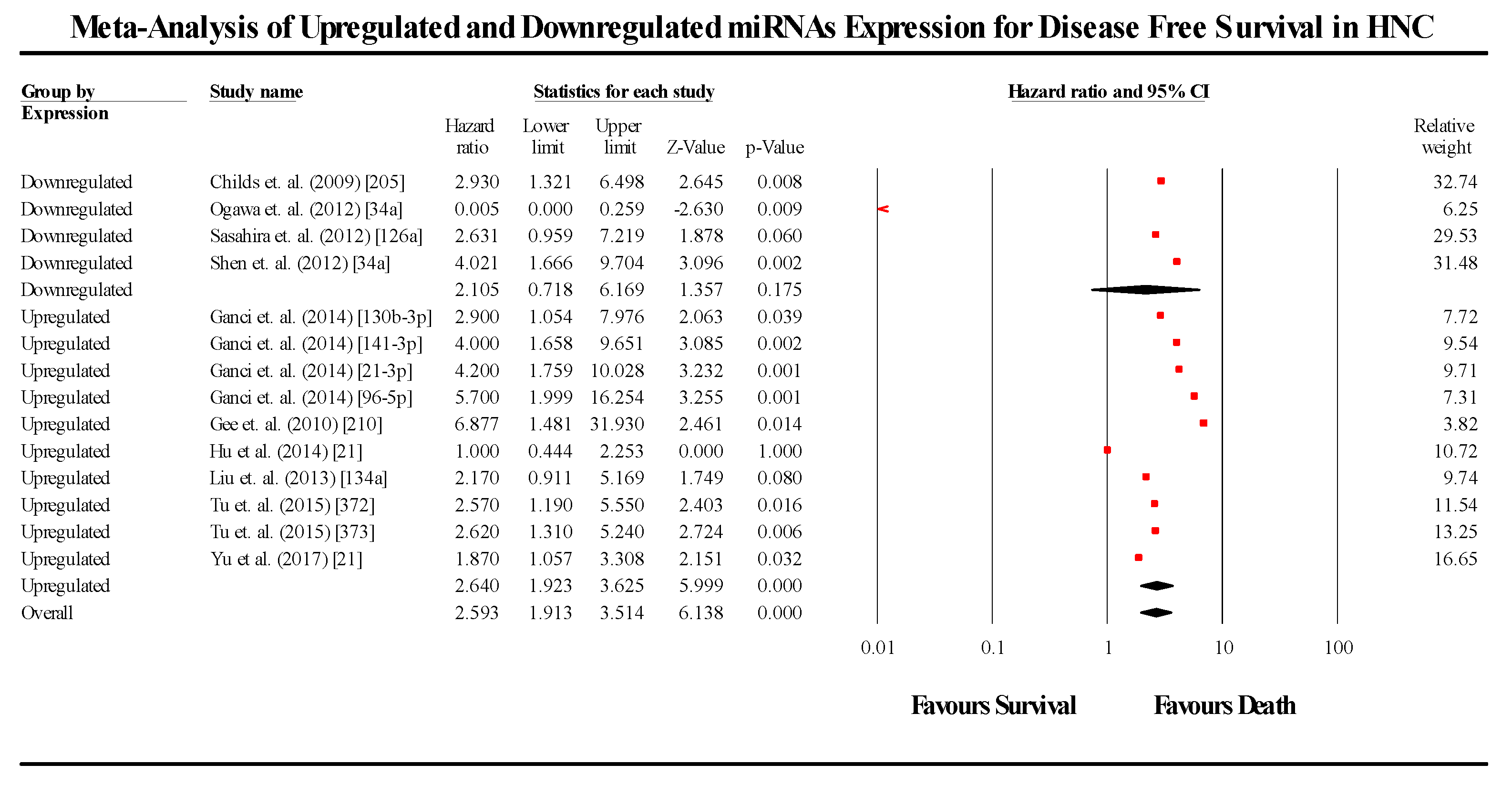
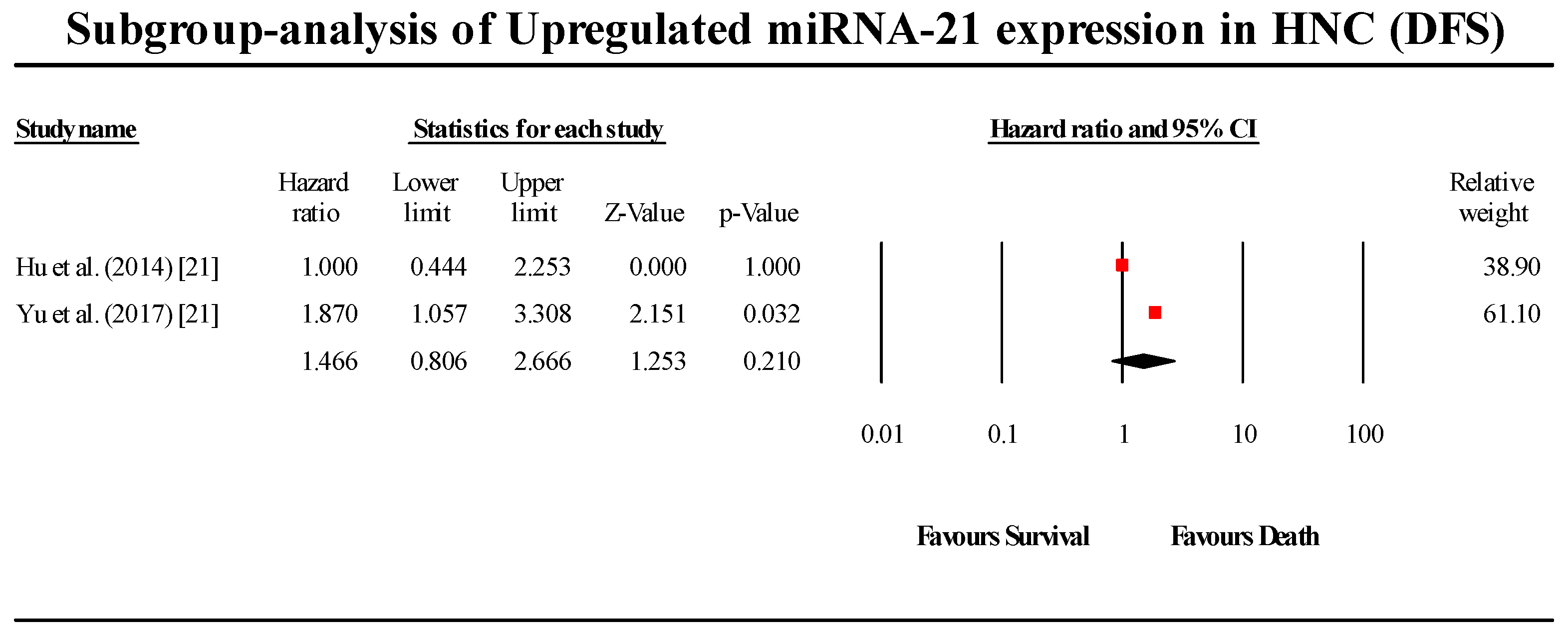
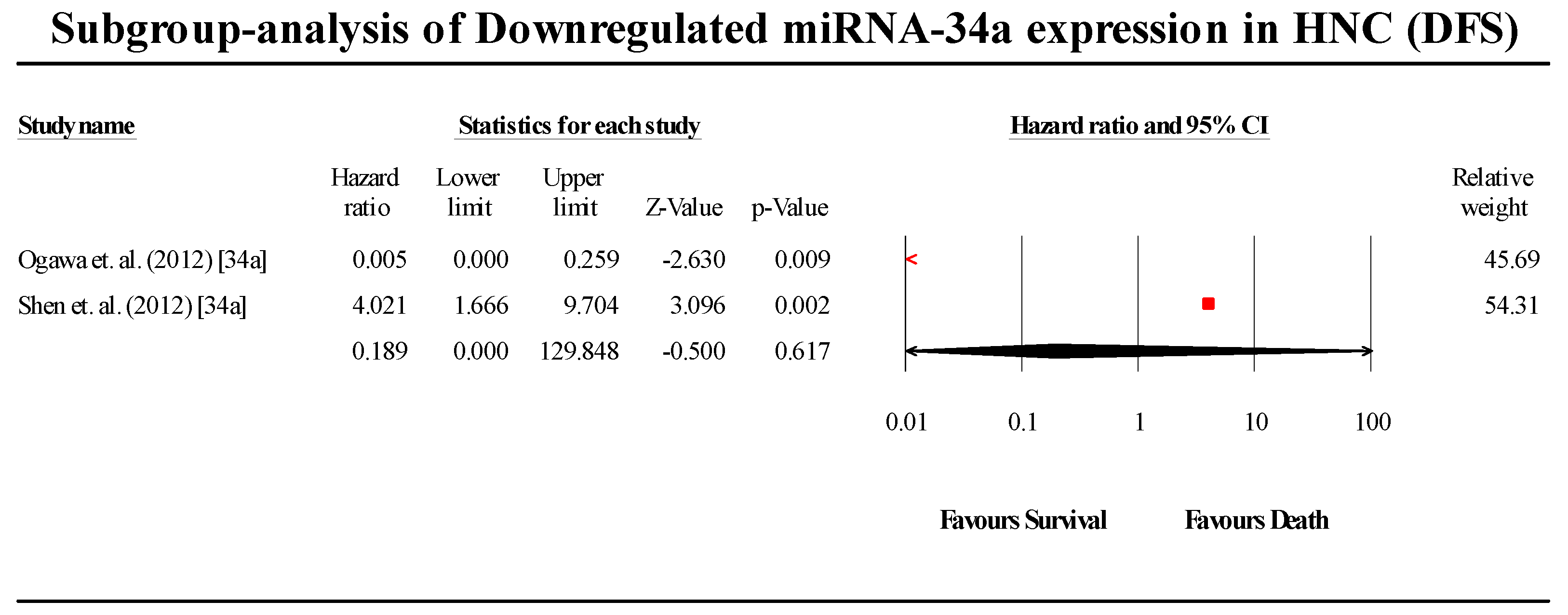
3.6. Disease-Free Survival Group
3.6.1. miR-21
3.6.2. miR-34a
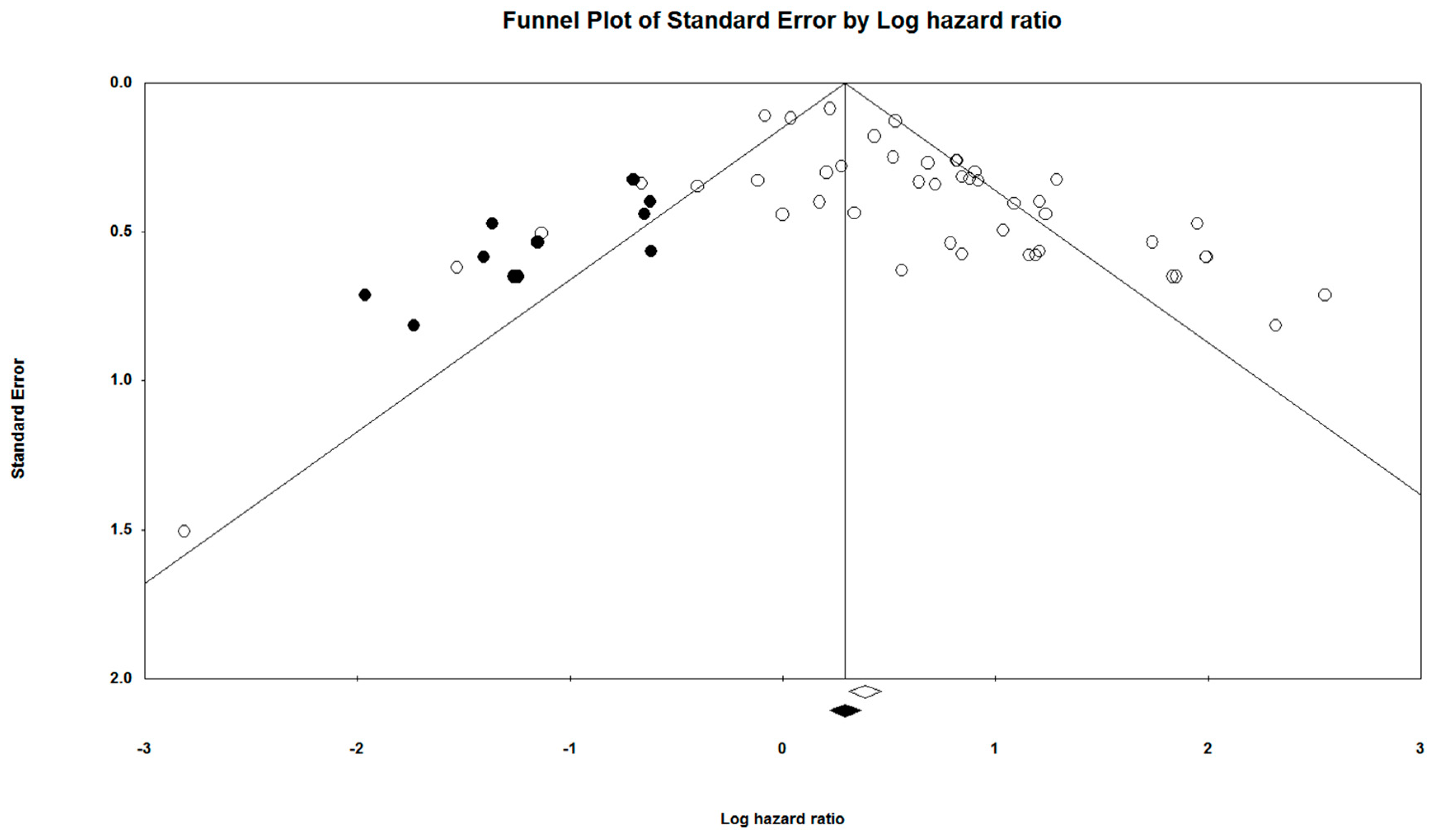
3.7. Publication Bias
3.8. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. Ca A Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Dhull, A.K.; Atri, R.; Dhankhar, R.; Chauhan, A.K.; Kaushal, V. Major Risk Factors in Head and Neck Cancer: A Retrospective Analysis of 12-Year Experiences. World J. Oncol. 2018, 9, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; West, C.M.L.; Nutting, C.; Paleri, V. Head and neck cancer—Part 2: Treatment and prognostic factors. Bmj 2010, 341, c4690. [Google Scholar] [CrossRef] [PubMed]
- Denaro, N.; Merlano, M.C.; Russi, E.G. Follow-up in head and neck cancer: Do more does it mean do better? A systematic review and our proposal based on our experience. Clin. Exp. Otorhinolaryngol. 2016, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, H.; Kong, A.; Ahmed, S.K. Recurrent head and neck cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S181–S190. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z.; Asl Aminabadi, N.; Attaran, R.; Pournagiazar, F.; Ghertasi Oskouei, S.; Ahmadpour, F. MicroRNAs as prognostic molecular signatures in human head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2015, 51, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, L.-A.; Murphy, P.R. MicroRNA: Biogenesis, Function and Role in Cancer. Curr. Genom. 2010, 11, 537–561. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ma, X.; Chen, L.; Li, H.; Gu, L.; Gao, Y.; Zhang, Y.; Li, X.; Fan, Y.; Chen, J. MicroRNAs with prognostic significance in bladder cancer: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 5619. [Google Scholar] [CrossRef]
- Gu, W.; Xu, Y.; Xie, X.; Wang, T.; Ko, J.H.; Zhou, T. The role of RNA structure at 5’ untranslated region in microRNA-mediated gene regulation. RNA 2014, 20, 1369–1375. [Google Scholar] [CrossRef]
- Felekkis, K.; Touvana, E.; Stefanou, C.; Deltas, C. microRNAs: A newly described class of encoded molecules that play a role in health and disease. Hippokratia 2010, 14, 236–240. [Google Scholar]
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim. Biophys. Acta (Bba)-Mol. Cell Res. 2010, 1803, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Schoof, C.R.G.; Botelho, E.L.d.S.; Izzotti, A.; Vasques, L.D.R. MicroRNAs in cancer treatment and prognosis. Am. J. Cancer Res. 2012, 2, 414–433. [Google Scholar] [PubMed]
- Khawar, M.B.; Fatima, N.; Abbasi, M.H.; Mehmood, R.; Suqaina, S.K.; Sheikh, N. Head and neck cancer: Epidemiology and role of microRNAs. In Diagnosis and Management of Head and Neck Cancer; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- National Heart, L.a.B.I. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 12 February 2018).
- Borenstein, M.; Rothstein, H. Comprehensive Meta-Analysis; Biostat: Englewood, NJ, USA, 1999. [Google Scholar]
- Hedges, L.V.; Vevea, J.L. Fixed-and random-effects models in meta-analysis. Psychol. Methods 1998, 3, 486. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Cochran, W.G. The comparison of percentages in matched samples. Biometrika 1950, 37, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Book Series; Cochrane Collaboration: London, UK, 2008; pp. 243–296. [Google Scholar]
- Von Hippel, P.T. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Kumarasamy, C.; Madhav, M.R.; Pandey, V.; Sabarimurugan, S.; Ramesh, N.; Gothandam, K.M.; Baxi, S. Comment on “Systematic Review and Meta-Analysis of Diagnostic Accuracy of miRNAs in Patients with Pancreatic Cancer”. Dis. Mark. 2018, 2018, 6904569. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Kumarasamy, C. Comment on ‘Prognostic biomarkers for oral tongue squamous cell carcinoma: A systematic review and meta-analysis’. Br. J. Cancer 2018, 118, e11. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Kumarasamy, C.; Sabarimurugan, S.; Baxi, S. Letter to the Editor in response to the article. “The epidemiology of oral human papillomavirus infection in healthy populations: A systematic review and meta-analysis”. Oral Oncol. 2018, 84, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Kumarasamy, C.; Sabarimurugan, S.; Baxi, S. Commentary: Blood-Derived microRNAs for Pancreatic Cancer Diagnosis: A Narrative Review and Meta-Analysis. Front. Physiol. 2018, 9, 1896. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Kumarasamy, C. Conceptual interpretation of analysing and reporting of results on systematic review and meta-analysis of optimal extent of lateral neck dissection for well-differentiated thyroid carcinoma with metastatic lateral neck lymph nodes. Oral Oncol. 2019, 89, 153. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Kumarasamy, C.J.A.R. Conceptual, statistical and clinical interpretation of results from a systematic review and meta-analysis of prevalence of cervical HPV infection in women with SLE. Autoimmun. Rev. 2019, 18, 433–434. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Kumarasamy, C.; Gothandam, K.M. Letter to the editor “Prognostic value of microRNAs in colorectal cancer: A meta-analysis”. Cancer Manag. Res 2018, 10, 3501–3503. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Kumarasamy, C. Letter to the Editor about the Article: “Performance of different imaging techniques in the diagnosis of head and neck cancer mandibular invasion: A systematic review and meta-analysis”. J. Oral Oncol. 2018, 89, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, C.; Devi, A.; Jayaraj, R. Prognostic value of microRNAs in head and neck cancers: A systematic review and meta-analysis protocol. Syst. Rev. 2018, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Kumarasamy, C. Comment on, “Survival for HPV-positive oropharyngeal squamous cell carcinoma with surgical versus non-surgical treatment approach: A systematic review and meta-analysis”. J. Oral Oncol. 2018, 90, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.; Kumarasamy, C. Systematic review and meta-analysis of cancer studies evaluating diagnostic test accuracy and prognostic values: Approaches to improve clinical interpretation of results. Cancer Manag. Res. 2018, 10, 4669–4670. [Google Scholar] [CrossRef]
- Jayaraj, R.; Kumarasamy, C.; Ramalingam, S.; Devi, A. Systematic review and meta-analysis of risk-reductive dental strategies for medication related osteonecrosis of the jaw among cancer patients: Approaches and strategies. Oral Oncol. 2018, 86, 312–313. [Google Scholar] [CrossRef]
- Sabarimurugan, S.; Kumarasamy, C.; Baxi, S.; Devi, A.; Jayaraj, R. Systematic review and meta-analysis of prognostic microRNA biomarkers for survival outcome in nasopharyngeal carcinoma. PLoS ONE 2019, 14, e0209760. [Google Scholar] [CrossRef]
- Sabarimurugan, S.; Madurantakam Royam, M.; Das, A.; Das, S.; Gothandam, K.M.; Jayaraj, R. Systematic Review and Meta-analysis of the Prognostic Significance of miRNAs in Melanoma Patients. Mol. Diagn. 2018, 22, 653–669. [Google Scholar] [CrossRef]
- Jayaraj, R.; Kumarasamy, C.; Piedrafita, D. Systematic review and meta-analysis protocol for Fasciola DNA vaccines. Online J. Vet. Res. 2018, 22, 517. [Google Scholar]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Orwin, R.G. A fail-safe N for effect size in meta-analysis. J. Educ. Stat. 1983, 8, 157–159. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- De Carvalho, A.C.; Scapulatempo-Neto, C.; Maia, D.C.C.; Evangelista, A.F.; Morini, M.A.; Carvalho, A.L.; Vettore, A.L. Accuracy of microRNAs as markers for the detection of neck lymph node metastases in patients with head and neck squamous cell carcinoma. BMC Med. 2015, 13, 108. [Google Scholar]
- Hou, B.; Ishinaga, H.; Midorikawa, K.; Shah, S.A.; Nakamura, S.; Hiraku, Y.; Oikawa, S.; Murata, M.; Takeuchi, K. Circulating microRNAs as novel prognosis biomarkers for head and neck squamous cell carcinoma. Cancer Biol. Ther. 2015, 16, 1042–1046. [Google Scholar] [CrossRef]
- Maia, D.; de Carvalho, A.C.; Horst, M.A.; Carvalho, A.L.; Scapulatempo-Neto, C.; Vettore, A.L. Expression of miR-296-5p as predictive marker for radiotherapy resistance in early-stage laryngeal carcinoma. J. Transl. Med. 2015, 13, 262. [Google Scholar] [CrossRef]
- Hudcova, K.; Raudenska, M.; Gumulec, J.; Binkova, H.; Horakova, Z.; Kostrica, R.; Babula, P.; Adam, V.; Masarik, M. Expression profiles of miR-29c, miR-200b and miR-375 in tumour and tumour-adjacent tissues of head and neck cancers. Tumor Biol. 2016, 37, 12627–12633. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, G.; Wu, Z.; Lu, B.; Yuan, D.; Li, X.; Lu, Z. MicoRNA-451 is a novel tumor suppressor via targeting c-myc in head and neck squamous cell carcinomas. J. Cancer Res. Ther. 2015, 11, 216. [Google Scholar]
- Arantes, L.M.R.B.; Laus, A.C.; Melendez, M.E.; de Carvalho, A.C.; Sorroche, B.P.; De Marchi, P.R.M.; Evangelista, A.F.; Scapulatempo-Neto, C.; de Souza Viana, L.; Carvalho, A.L. MiR-21 as prognostic biomarker in head and neck squamous cell carcinoma patients undergoing an organ preservation protocol. Oncotarget 2017, 8, 9911. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, Y.; Zhao, H.; Yang, X.; Luo, Y.; Ren, Y.; Liu, W.; Li, N. Serum miR-483-5p: A novel diagnostic and prognostic biomarker for patients with oral squamous cell carcinoma. Tumor Biol. 2016, 37, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Ren, S.; Su, Z.; Liu, C.; Deng, T.; Huang, D.; Tian, Y.; Qiu, Y.; Liu, Y. Increased expression of miR-93 is associated with poor prognosis in head and neck squamous cell carcinoma. Tumor Biol. 2015, 36, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Huang, J.-J.; Xu, W.-H.; Jin, X.-J.; Li, J.-P.; Tang, Y.-J.; Huang, X.-F.; Cui, H.-J.; Sun, G.-B. miR-21 and miR-375 microRNAs as candidate diagnostic biomarkers in squamous cell carcinoma of the larynx: Association with patient survival. Am. J. Transl. Res. 2014, 6, 604. [Google Scholar] [PubMed]
- Hedbäck, N.; Jensen, D.H.; Specht, L.; Fiehn, A.-M.K.; Therkildsen, M.H.; Friis-Hansen, L.; Dabelsteen, E.; von Buchwald, C. MiR-21 expression in the tumor stroma of oral squamous cell carcinoma: An independent biomarker of disease free survival. PLoS ONE 2014, 9, e95193. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, B.; Lin, Z.; Yao, Y.; Chen, Y.; Li, Y.; Chen, J.; Yu, D.; Tang, Z.; Wang, B. MiR-320a acts as a prognostic factor and Inhibits metastasis of salivary adenoid cystic carcinoma by targeting ITGB3. Mol. Cancer 2015, 14, 96. [Google Scholar] [CrossRef]
- Saito, K.; Inagaki, K.; Kamimoto, T.; Ito, Y.; Sugita, T.; Nakajo, S.; Hirasawa, A.; Iwamaru, A.; Ishikura, T.; Hanaoka, H. MicroRNA-196a is a putative diagnostic biomarker and therapeutic target for laryngeal cancer. PLoS ONE 2013, 8, e71480. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Sun, L.; Yang, M.; Pan, C.; Chen, W.; Wu, D.; Lin, Z.; Zeng, C.; Yao, Y. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin. Cancer Res. 2009, 15, 3998–4008. [Google Scholar] [CrossRef]
- Liu, N.; Chen, N.-Y.; Cui, R.-X.; Li, W.-F.; Li, Y.; Wei, R.-R.; Zhang, M.-Y.; Sun, Y.; Huang, B.-J.; Chen, M. Prognostic value of a microRNA signature in nasopharyngeal carcinoma: A microRNA expression analysis. Lancet Oncol. 2012, 13, 633–641. [Google Scholar] [CrossRef]
- Summerer, I.; Niyazi, M.; Unger, K.; Pitea, A.; Zangen, V.; Hess, J.; Atkinson, M.J.; Belka, C.; Moertl, S.; Zitzelsberger, H. Changes in circulating microRNAs after radiochemotherapy in head and neck cancer patients. Radiat. Oncol. 2013, 8, 296. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.E.; Raulf, N.; Gäken, J.; Lawler, K.; Urbano, T.G.; Bullenkamp, J.; Gobeil, S.; Huot, J.; Odell, E.; Tavassoli, M. MicroRNA-196a promotes an oncogenic effect in head and neck cancer cells by suppressing annexin A1 and enhancing radioresistance. Int. J. Cancer 2015, 137, 1021–1034. [Google Scholar] [CrossRef]
- Ogawa, T.; Saiki, Y.; Shiga, K.; Chen, N.; Fukushige, S.; Sunamura, M.; Nagase, H.; Hashimoto, S.; Matsuura, K.; Saijo, S. mi R-34a is downregulated in cis-diamminedichloroplatinum treated sinonasal squamous cell carcinoma patients with poor prognosis. Cancer Sci. 2012, 103, 1737–1743. [Google Scholar] [CrossRef]
- Avissar, M.; McClean, M.D.; Kelsey, K.T.; Marsit, C.J. MicroRNA expression in head and neck cancer associates with alcohol consumption and survival. Carcinogenesis 2009, 30, 2059–2063. [Google Scholar] [CrossRef] [PubMed]
- Re, M.; Çeka, A.; Rubini, C.; Ferrante, L.; Zizzi, A.; Gioacchini, F.M.; Tulli, M.; Spazzafumo, L.; Sellari-Franceschini, S.; Procopio, A.D. Micro RNA-34c-5p is related to recurrence in laryngeal squamous cell carcinoma. Laryngoscope 2015, 125, E306–E312. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, J.; Cao, W.; Wang, X.; Xu, Q.; Yan, M.; Wu, X.; Chen, W. Dysregulated miR-363 affects head and neck cancer invasion and metastasis by targeting podoplanin. Int. J. Biochem. Cell Biol. 2013, 45, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Li, M.; Ge, J.; Guo, Y.; Sun, Y.; Liu, M.; Xiao, H. MiR-203 is downregulated in laryngeal squamous cell carcinoma and can suppress proliferation and induce apoptosis of tumours. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 5953–5963. [Google Scholar] [CrossRef]
- Chang, C.-C.; Yang, Y.-J.; Li, Y.-J.; Chen, S.-T.; Lin, B.-R.; Wu, T.-S.; Lin, S.-K.; Kuo, M.Y.-P.; Tan, C.-T. MicroRNA-17/20a functions to inhibit cell migration and can be used a prognostic marker in oral squamous cell carcinoma. Oral Oncol. 2013, 49, 923–931. [Google Scholar] [CrossRef]
- Gee, H.E.; Camps, C.; Buffa, F.M.; Patiar, S.; Winter, S.C.; Betts, G.; Homer, J.; Corbridge, R.; Cox, G.; West, C.M.; et al. hsa-mir-210 is a marker of tumor hypoxia and a prognostic factor in head and neck cancer. Cancer 2010, 116, 2148–2158. [Google Scholar] [CrossRef] [PubMed]
- Lenarduzzi, M.; Hui, A.B.; Alajez, N.M.; Shi, W.; Williams, J.; Yue, S.; O’Sullivan, B.; Liu, F.F. MicroRNA-193b enhances tumor progression via down regulation of neurofibromin 1. PLoS ONE 2013, 8, e53765. [Google Scholar] [CrossRef]
- Childs, G.; Fazzari, M.; Kung, G.; Kawachi, N.; Brandwein-Gensler, M.; McLemore, M.; Chen, Q.; Burk, R.D.; Smith, R.V.; Prystowsky, M.B.; et al. Low-level expression of microRNAs let-7d and miR-205 are prognostic markers of head and neck squamous cell carcinoma. Am. J. Pathol. 2009, 174, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Zhan, G.; Ye, D.; Ren, Y.; Cheng, L.; Wu, Z.; Guo, J. MicroRNA-34a affects the occurrence of laryngeal squamous cell carcinoma by targeting the antiapoptotic gene survivin. Med. Oncol. 2012, 29, 2473–2480. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Dai, Y.; Zhang, L.; Jiang, C.; Li, Z.; Yang, J.; McCarthy, J.B.; She, X.; Zhang, W.; Ma, J.; et al. miR-18a promotes malignant progression by impairing microRNA biogenesis in nasopharyngeal carcinoma. Carcinogenesis 2013, 34, 415–425. [Google Scholar] [CrossRef]
- Jung, H.M.; Phillips, B.L.; Patel, R.S.; Cohen, D.M.; Jakymiw, A.; Kong, W.W.; Cheng, J.Q.; Chan, E.K. Keratinization-associated miR-7 and miR-21 regulate tumor suppressor reversion-inducing cysteine-rich protein with kazal motifs (RECK) in oral cancer. J. Biol. Chem. 2012, 287, 29261–29272. [Google Scholar] [CrossRef]
- Sasahira, T.; Kurihara, M.; Bhawal, U.K.; Ueda, N.; Shimomoto, T.; Yamamoto, K.; Kirita, T.; Kuniyasu, H. Downregulation of miR-126 induces angiogenesis and lymphangiogenesis by activation of VEGF-A in oral cancer. Br. J. Cancer 2012, 107, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Shen, W.G.; Peng, S.Y.; Cheng, H.W.; Kao, S.Y.; Lin, S.C.; Chang, K.W. miR-134 induces oncogenicity and metastasis in head and neck carcinoma through targeting WWOX gene. Int. J. Cancer 2014, 134, 811–821. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.J.; Zhang, C.Y.; Zhou, Z.T.; Ma, J.Y.; Liu, Y.; Bao, Z.X.; Jiang, W.W. MicroRNA-155 in oral squamous cell carcinoma: Overexpression, localization, and prognostic potential. Head Neck 2015, 37, 970–976. [Google Scholar] [CrossRef]
- Harris, T.; Jimenez, L.; Kawachi, N.; Fan, J.-B.; Chen, J.; Belbin, T.; Ramnauth, A.; Loudig, O.; Keller, C.E.; Smith, R.; et al. Low-level expression of miR-375 correlates with poor outcome and metastasis while altering the invasive properties of head and neck squamous cell carcinomas. Am. J. Pathol. 2012, 180, 917–928. [Google Scholar] [CrossRef]
- Huang, W.C.; Chan, S.H.; Jang, T.H.; Chang, J.W.; Ko, Y.C.; Yen, T.C.; Chiang, S.L.; Chiang, W.F.; Shieh, T.Y.; Liao, C.T.; et al. miRNA-491-5p and GIT1 serve as modulators and biomarkers for oral squamous cell carcinoma invasion and metastasis. Cancer Res. 2014, 74, 751–764. [Google Scholar] [CrossRef]
- Shiiba, M.; Shinozuka, K.; Saito, K.; Fushimi, K.; Kasamatsu, A.; Ogawara, K.; Uzawa, K.; Ito, H.; Takiguchi, Y.; Tanzawa, H. MicroRNA-125b regulates proliferation and radioresistance of oral squamous cell carcinoma. Br. J. Cancer 2013, 108, 1817–1821. [Google Scholar] [CrossRef]
- Zeng, X.; Xiang, J.; Wu, M.; Xiong, W.; Tang, H.; Deng, M.; Li, X.; Liao, Q.; Su, B.; Luo, Z.; et al. Circulating miR-17, miR-20a, miR-29c, and miR-223 combined as non-invasive biomarkers in nasopharyngeal carcinoma. PLoS ONE 2012, 7, e46367. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Jiang, N.; Guo, R.; Jiang, W.; He, Q.M.; Xu, Y.F.; Li, Y.Q.; Tang, L.L.; Mao, Y.P.; Sun, Y.; et al. MiR-451 inhibits cell growth and invasion by targeting MIF and is associated with survival in nasopharyngeal carcinoma. Mol. Cancer 2013, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Hung, P.S.; Wang, P.W.; Liu, C.J.; Chu, T.H.; Cheng, H.W.; Lin, S.C. miR-181 as a putative biomarker for lymph-node metastasis of oral squamous cell carcinoma. J. Oral Pathol. Med. 2011, 40, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-Y.; Zhang, T.-H.; Qu, L.-M.; Feng, J.-P.; Tian, L.-L.; Zhang, B.-H.; Li, D.-D.; Sun, Y.-N.; Liu, M. MiR-19a is correlated with prognosis and apoptosis of laryngeal squamous cell carcinoma by regulating TIMP-2 expression. Int. J. Clin. Exp. Pathol. 2013, 7, 56–63. [Google Scholar] [PubMed]
- Peng, S.C.; Liao, C.T.; Peng, C.H.; Cheng, A.J.; Chen, S.J.; Huang, C.G.; Hsieh, W.P.; Yen, T.C. MicroRNAs MiR-218, MiR-125b, and Let-7g predict prognosis in patients with oral cavity squamous cell carcinoma. PLoS ONE 2014, 9, e102403. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Arriagada, W.A.; Olivero, P.; Rodriguez, B.; Lozano-Burgos, C.; de Oliveira, C.E.; Coletta, R.D. Clinicopathological significance of miR-26, miR-107, miR-125b, and miR-203 in head and neck carcinomas. Oral Dis. 2018, 24, 930–939. [Google Scholar] [CrossRef] [PubMed]
- El Baroudi, M.; Machiels, J.P.; Schmitz, S. Expression of SESN1, UHRF1BP1, and miR-377-3p as prognostic markers in mutated TP53 squamous cell carcinoma of the head and neck. Cancer Biol. 2017, 18, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Berania, I.; Cardin, G.B.; Clement, I.; Guertin, L.; Ayad, T.; Bissada, E.; Nguyen-Tan, P.F.; Filion, E.; Guilmette, J.; Gologan, O.; et al. Four PTEN-targeting co-expressed miRNAs and ACTN4-targeting miR-548b are independent prognostic biomarkers in human squamous cell carcinoma of the oral tongue. Int. J. Cancer 2017, 141, 2318–2328. [Google Scholar] [CrossRef] [PubMed]
- He, F.Y.; Liu, H.J.; Guo, Q.; Sheng, J.L. Reduced miR-300 expression predicts poor prognosis in patients with laryngeal squamous cell carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 760–764. [Google Scholar]
- Hess, A.K.; Muer, A.; Mairinger, F.D.; Weichert, W.; Stenzinger, A.; Hummel, M.; Budach, V.; Tinhofer, I. MiR-200b and miR-155 as predictive biomarkers for the efficacy of chemoradiation in locally advanced head and neck squamous cell carcinoma. Eur. J. Cancer 2017, 77, 3–12. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, H.; Zhou, L.; Jiang, T.; Xu, Y.; Xia, L. MicroRNA-212 inhibits the metastasis of nasopharyngeal carcinoma by targeting SOX4. Oncol. Rep. 2017, 38, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Zhou, Q.; Qi, Y.H.; Wang, H.; Yang, L.; Fan, Q.Y. Effects of long non-coding RNA H19 and microRNA let7a expression on thyroid cancer prognosis. Exp. Mol. Pathol. 2017, 103, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Re, M.; Magliulo, G.; Gioacchini, F.M.; Bajraktari, A.; Bertini, A.; Ceka, A.; Rubini, C.; Ferrante, L.; Procopio, A.D.; Olivieri, F. Expression Levels and Clinical Significance of miR-21-5p, miR-let-7a, and miR-34c-5p in Laryngeal Squamous Cell Carcinoma. Biomed Res. Int. 2017, 2017, 3921258. [Google Scholar] [CrossRef] [PubMed]
- Romeo, P.; Colombo, C.; Granata, R.; Calareso, G.; Gualeni, A.V.; Dugo, M.; De Cecco, L.; Rizzetti, M.G.; Zanframundo, A.; Aiello, A.; et al. Circulating miR-375 as a novel prognostic marker for metastatic medullary thyroid cancer patients. Endocr. Relat. Cancer 2018, 25, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, O.M.; Titus, A.J.; Salas, L.A.; Gui, J.; Eliot, M.; Butler, R.A.; Sturgis, E.M.; Li, G.; Kelsey, K.T.; Christensen, B.C. MicroRNA-Related Genetic Variants Associated with Survival of Head and Neck Squamous Cell Carcinoma. Cancer Epidemiol. Biomark. Prev. 2019, 28, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.H.; Tu, H.F.; Wu, C.H.; Yang, C.C.; Chang, K.W. MicroRNA-21 promotes perineural invasion and impacts survival in patients with oral carcinoma. J. Chin. Med. Assoc. JCMA 2017, 80, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Madurantakam, R.M.; Kumarasamy, C.; Baxi, S.; Gupta, A.; Ramesh, N.; Kodiveri, M.G.; Jayaraj, R. Current Evidence on miRNAs as Potential Theranostic Markers for Detecting Chemoresistance in Colorectal Cancer: A Systematic Review and Meta-Analysis of Preclinical and Clinical Studies. Mol. Diagn. Ther. 2019, 23, 65–82. [Google Scholar] [CrossRef]
- Madhav, M.R.; Nayagam, S.G.; Biyani, K.; Pandey, V.; Kamal, D.G.; Sabarimurugan, S.; Ramesh, N.; Gothandam, K.M.; Jayaraj, R. Epidemiologic analysis of breast cancer incidence, prevalence, and mortality in India: Protocol for a systematic review and meta-analyses. Medicine 2018, 97, e13680. [Google Scholar] [CrossRef]
- Poddar, A.; Aranha, R.R.; Muthukaliannan, G.K.; Nachimuthu, R.; Jayaraj, R. Head and neck cancer risk factors in India: Protocol for systematic review and meta-analysis. BMJ Open 2018, 8, e020014. [Google Scholar] [CrossRef]





| Article | Year | miR | Sample Size | Anatomic Location | Assay Method | Study Population | Gender | Stage | Metastasis | Risk Factors | Age |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carvalho et al. [41] | 2015 | miR-203 miR-205 | 127 | Tongue 58.3% Floor of mouth 31.3% Alveolar ridge 8.3% Lower gum 2.1% | qRT-PCR | Brazil | Male (79.2%) | T2 (62.5%) | Metastases + ve (52.083%) Metastases − ve (47.916%) | Smoking (47.9%) | 43–84 |
| Hou et.al. [42] | 2015 | miR-223 miR-99a miR-21 | 16 | Head and Neck | qRT-PCR | Japan | Male (93.75%) | T2 (18.75%) T3 (12.5%) T4 (68.75%) | NA | NA | 48–80 |
| Maia et al. [43] | 2015 | miR-296-5p | 34 | Supraglottic 20.6% Glottic 79.4% | qRT-PCR | Brazil | Male (88.2%) | T1 (47.1%) T2 (52.9%) | NA | Tobacco (91.2%) | ≤60 years (47%) >60 years (53%) |
| Hudcova et al. [44] | 2016 | miR-29c miR-200b miR-375 | 42 | Head and Neck | qRT-PCR | Czech Republic | Male (100%) | T1 + T2 (45%) T3 + T4 (55%) | Metastases + ve (11.42%) Metastases − ve (88.57%) | NA | NA |
| Wang et al. [45] | 2015 | miR-451 | 50 | Head and Neck | qRT-PCR | China | NA | NA | NA | NA | NA |
| Arantes et al. [46] | 2017 | miR-21 | 71 | Oropharynx 49.3% Larynx 39.4% Hypopharynx 11.3% | qRT-PCR | Brazil | Male (95.8%) | T2 + T3 (64.8%) T4 (35.2%) | NA | HPV (8.45%) Tobacco (80.3%) Alcohol (38.0%) | 40–76 |
| Xu et al. [47] | 2015 | miR-483-5p | 101 | Oral Cavity | qRT-PCR | China | Male (76.2%) | T1 + T2 (50.5%) T3 + T4 (49.5%) | NA | Smoking (72.3%) Alcohol (68.3%) | 53.2 ± 10.3 |
| Li et al. [48] | 2015 | miR-93 | 103 | Supraglottic 25.24% Glottic 55.33% Hypopharynx 9.7% Oral Cavity 9.7% | ISH, qRT-PCR | China | Male (96.1%) | T1 (15.5%) T2 (35%) T3 (40.8%) T4 (8.7%) | Metastases + ve (38.83%) | NA | <58 (46%) ≥58 (54%) |
| Hu et al. [49] | 2014 | miR-21 miR-375 | 46 | Glottic 71.7% Supraglottic 23.9% Subglottic 4.4% | qRT-PCR | China | Male (91.3%) | T0 + T1 + T2 (45.7%) T3 + T4 (54.3%) | NA | Smoking (72.1%) Alcohol (46.3%) | 59.2 ± 7.84 |
| Hedback et al. [50] | 2014 | miR-21 | 86 | Oral Cavity | ISH, Immunohistochemistry | Denmark | NA | NA | NA | NA | NA |
| Sun et al. [51] | 2015 | miR-320a | 450 | Salivary Gland | ISH, Immunohistochemistry | China | Male (47.56%) | T1 + T2 (65.33%) T3 + T4 (34.67%) | Metastases + ve (43.56%) | NA | <50 (49%) ≥50 (51%) |
| Saito et al. [52] | 2013 | miR-196a | 84 | Larynx | qRT-PCR | Japan | NA | NA | NA | NA | NA |
| Li et al. [53] | 2009 | miR-21 | 103 | Tongue | qRT-PCR | China | Male (54.36%) | T1 + T2 (58.25%) T3 + T4 (41.75%) | Metastases + ve (27.18%) | NA | <50 (46%) ≥50 (54%) |
| Liu et al. [54] | 2012 | miR-93 miR-142-3p miR-29c miR-26a miR-30e | 465 | Nasopharyngeal | qRT-PCR | China | Male (74.19%) | T1 (21.94%) T2 (27.31%) T3 (23.66%) T4 (27.10%) | Metastases + ve (19.78%)) | NA | 47.09 ± 11 |
| Summerer et al. [55] | 2013 | miR-425-5p miR-21-5p miR-106b-5p miR-93-5p | 18 | Larynx 27.77% Oropharynx 16.66% Mouth floor 11.11% Tongue 11.11% Esophagus 5.55% Hypopharynx 5.55% Maxilla 5.55% Nasopharyngeal 5.55% Sinuses 5.55% Soft palate 5.55% | qRT-PCR | Germany | Male (77.78%) | T1 (22.22%) T2 (11.11%) T3 (33.33%) T4 (33.33%) | Metastases + ve (11.11%) | NA | 45.1–80.6 |
| Suh et al. [56] | 2015 | miR-196a | 16 | Oral Cavity | qRT-PCR | UK | NA | NA | NA | NA | NA |
| Ogawa et al. [57] | 2012 | miR-34a | 24 | Sinonasal | miRNA-Microarray | Japan | Male (66.67%) | T2 (4%) T3 (41.67%) T4 (54.17%) | Metastases + ve (8.33%) | NA | >60 (59%) <60 (41%) |
| Avissar et al. [58] | 2009 | miR-375 miR-21 | 169 | Oral 64% Pharynx 21% Larynx 15% | qRT-PCR | USA | Male (68%) | T1 + T2 (28%) T3 + T4 (72%) | NA | HPV (17.4%) Alcohol (88.5%) Smoking (84.5%) | 61.5 ± 11.9 |
| Massimo Re et al. [59] | 2015 | miR-34c-5p | 90 | Supraglottic 21.1% Transglottic 73.3% Subglottic 5.6% | qRT-PCR | Italy | Male (96.6%) | T3 (66.7%) T4 (33.3%) | Metastases + ve (0%) | NA | 66.51 ± 8.02 |
| Sun et al. [60] | 2013 | miR-363 | 62 | Tongue 41.9% Gingival 21% Cheek 11.3% Floor of Mouth 17.7% Oropharynx 8.1% | qRT-PCR | China | Male (69.4%) | T1 + T2 (43.5%) T3 + T4 (36.5%) | Metastases + ve (54.83%) | Smoking (48.4%) Drinking (32.3%) | ≥60 (42%) <60 (58%) |
| Tian et al. [61] | 2014 | miR-203 | 56 | Glottic 53.57% Supraglottic 46.43% | qRT-PCR | China | Male (71.43%) | T1 + T2 (42.85%) T3 + T4 (57.14%) | Metastases + ve (50%) | NA | ≥59 (57%) <59 (43%) |
| Chang et al. [62] | 2013 | miR-17 miR-20a | 98 | Buccal Mucosa 43.88% Tongue 29.59% Gingiva 21.43% Floor of Mouth 5.10% | qRT-PCR | Taiwan | Male (84.7%) | T1 + T2 (44.9%) T3 + T4 (55.1%) | Metastases + ve (37.75%) | Smoking (82.65%) | >50 (35%) <50 (65%) |
| Gee et al. [63] | 2010 | miR-210 | 46 | Oral Cavity 21% Oropharynx 46% Hypopharynx 19% Larynx 11% Paranasal Sinus 2% | qRT-PCR | UK | Male (80.43%) | T1 (10.87%) T2 (30.43%) T3 (15.22%) T4 (43.48%) | NA | Smoking (86.96%) Alcohol (78.26%) | 43–92 |
| Lenarduzzi et al. [64] | 2013 | miR-193b | 51 | Head and Neck | qRT-PCR | Canada | NA | NA | NA | NA | NA |
| Childs et al. [65] | 2009 | miR-205 Let-7d miR-21 | 104 | Oral Cavity 30% Oropharynx 46% Hypopharynx 9% Larynx 31% | qRT-PCR | US | Male (68%) | T1 + T2 (23%) T3 + T4 (77%) | NA | Smoking (82%) HPV (36%) | <60 (40%) >60 (61%) |
| Shen et al. [66] | 2012 | miR-34a | 69 | Larynx | qRT-PCR | China | NA | T1 + T2 (60.87%) T3 + T4 (39.13%) | Metastases + ve (34.78%) | NA | <60 (48%) ≥60 (52%) |
| Luo et al. [67] | 2013 | miR-18a | 168 | Nasopharyngeal | qRT-PCR | China | Male (75.6%) | T1 + T2 (42.86%) T3 + T4 (57.14%) | Metastases + ve (64.88%) | NA | ≥50 (59%) <50 (41.%) |
| Jung et al. [68] | 2012 | miR-21 | 17 | Tongue 94.12% Oropharynx 5.88% | qRT-PCR | USA | NA | NA | NA | HPV (58.82%) | 41–69 |
| Sasahira et al. [69] | 2012 | miR-126a | 118 | Tongue 54.24% Other 45.76% | qRT-PCR | Japan | Male (57.63%) | T1 + T2 (76.27%) T3 + T4 (23.73%) | Metastases + ve (28.81%) | NA | ≤65 (39%) >65 (61%) |
| Liu et al. [70] | 2014 | miR-134a | 96 | Buccal Mucosa 35.41% Tongue 27.08% Oral pharynx 37.5% | qRT-PCR | Taiwan | Male (93.75%) | T1 + T2 + T3 (28.12%) T4 (71.88%) | Metastases + ve (6.25%) | NA | 53.5 (Average) |
| Shi et al. [71] | 2014 | miR-155 | 30 | Oral Cavity | qRT-PCR, FISH | China | Male (63.33%) | T1 (10%) T2 (16.67%) T3 (33.33%) T4 (40%) | NA | Smoking (46.67%) Alcohol (53.33%) | 56.4 ± 8.6 (40-75) |
| Harris et al. [72] | 2012 | miR-375 | 123 | Oral Cavity 35% Oropharynx 30% Larynx 35% | qRT-PCR | US | Male (69.1%) | T1 + T2 (19.5%) T3 + T4 (80.5%) | NA | Smoking (60.9%) Alcohol (27.6%) HPV (25.2%) | ≤58 (37%) 59–66 (31%) ≥67 (33%) |
| Huang et al. [73] | 2014 | miR-491-p5 | 33 | Oral Cavity | qRT-PCR, FISH | Taiwan | Male (96.9%) | T1 (9.1%) T2 (51.5%) T3 (3.0%) T4 (36.4%) | NA | NA | ≤60 (21%) >60 (79%) |
| Shiiba et al. [74] | 2013 | miR-125b | 50 | Oral Cavity | qRT-PCR | Japan | NA | T1 (10%) T2 (12%) T3 (14%) T4 (64%) | NA | NA | NA |
| Zeng et al. [75] | 2012 | miR-20a | 160 | Nasopharyngeal | qRT-PCR | China | Male (61.25%) | T1 (1,25%) T2 (15.63%) T3 (34.38%) T4 (40%) | NA | NA | 46.41 ± 10.74 |
| Liu et al. [76] | 2013 | miR-451 | 280 | Nasopharyngeal | qRT-PCR | Taiwan | Male (73.57%) | T1 + T2 (50.71%) T3 + T4 (49.28%) | NA | NA | ≤45 (49%) >45 (51%) |
| Yang et al. [77] | 2011 | miR-181a | 39 | Oral Cavity | qRT-PCR | Taiwan | Male (44.87%) | T1 + T2 + T3 (33.33%) T4 (66.66%) | NA | NA | NA |
| Wu et al. [78] | 2014 | miR-19a | 83 | Laryngeal | qRT-PCR | China | Male (68.67%) | NA | Metastases + ve (34.93%) | NA | ≥56 (51%) <56 (49%) |
| Peng et al. [79] | 2014 | Let-7g miR-125b miR-218 | 29 | Oral Cavity | qRT-PCR | Taiwan | NA | NA | NA | NA | NA |
| Arriagada et al. [80] | 2018 | miR-215b | 32 | Head and Neck | qRT-PCR | Chile | Male (55.9%) | T1 + T2 (75.2%) T3 + T4 (47.7%) | NA | Smoking (62.5%) Drinking (50.5%) | <64 (86%) ≥64 years (44%) |
| Baroudi et al. [81] | 2017 | miR-377-3p | 199 | Larynx 31% Oral cavity 64% Oropharynx 5% | GSEA | NA | Male (28%) | T1 (9%) T2 (18%) T3 (27%) T4 (53%) | NA | Smoking (52%) Alcohol (66%) | ≤ 70 years (80%) > 70 years (20%) |
| Berania et al. [82] | 2017 | miR-18a miR-548b | 58 | Oral tongue squamous cell carcinoma | qRT-PCR | Canada | Male (71%) | NA | NA | Smoking (72%) Drinking (41%) HPV (22%) | ≤ 50 (28%) > 50 (72%) |
| He et al. [83] | 2017 | miR-300 | 133 | Laryngeal squamous cell carcinoma | qRT-PCR | China | Male (65%) | T1 + T2 (50%) T3 + T4 (50%) | Metastasis + ve (55%) | NA | <50 (35%) ≥50 (65%) |
| Hess et al. [84] | 2017 | miR-200b miR-155 miR-146a | 149 | Oropharynx 52% Hypopharynx 48% | qRT-PCR | Germany | NA | NA | NA | NA | NA |
| Jiang et al. [85] | 2017 | miR-212 | 73 | Nasopharyngeal | qRT-PCR | China | Male (59%) | T1 + T2 (34%) T3 + T4 (66%) | Metastasis + ve (56%) | NA | ≤45 (48%) >45 (52%) |
| Liu et al. [86] | 2017 | let-7a | 131 | Thyroid | qRT-PCR | China | Male (33%) | T1 + T2 (39%) T3 + T4 (61%) | Metastasis + ve (53%) | NA | < 45 (44%) ≥ 45 (56%) |
| Re et al. [87] | 2017 | miR-34c-5p | 43 | Supraglottic (18.60%) Transglottic (76.74%) Subglottic (4.65%) | qRT-PCR | Italy | Male (97.67%) | T3 (72%) T4 (28%) | Metastasis + ve (0%) | NA | 66.51 ± 8.02 |
| Romeo et al. [88] | 2018 | miR-375 | 36 | Medullary thyroid | qRT-PCR | Italy | Male (58.3%) | T1 + T2 (25%) T3 + T4 (63.8%) | Metastasis + ve (72.2%) | NA | Mean 55.5 |
| Wilkins et al. [89] | 2018 | miR-100 miR-125b Let-7a | 2083 | Oral cavity (31.7%) Pharynx (52.7%) Larynx (15.6%) | Axiom miRNA Target Site Genotyping Array | USA | Male (24.5%) | T1 + T2 (25.9%) T3 + T4 (74.1%) | NA | Smoking [current] (25.9%) Smoking [former] (42.9%) | ≤50 (24.8%) >50 to ≤60 (36.2%) >60 to ≤70 (25.9%) >70 (13.1%) |
| Yu et al. [90] | 2017 | miR-21 | 100 | Buccal mucosa (37%) Tongue (35%) Mouth floor (12%) Others (16%) | Immunohistochemistry | China | Male (92%) | T1 + T2 (23%) T3 + T4 (77%) | Metastasis + ve (28%) | NA | ≤55 (56%) >55 (44%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumarasamy, C.; Madhav, M.R.; Sabarimurugan, S.; Krishnan, S.; Baxi, S.; Gupta, A.; Gothandam, K.M.; Jayaraj, R. Prognostic Value of miRNAs in Head and Neck Cancers: A Comprehensive Systematic and Meta-Analysis. Cells 2019, 8, 772. https://doi.org/10.3390/cells8080772
Kumarasamy C, Madhav MR, Sabarimurugan S, Krishnan S, Baxi S, Gupta A, Gothandam KM, Jayaraj R. Prognostic Value of miRNAs in Head and Neck Cancers: A Comprehensive Systematic and Meta-Analysis. Cells. 2019; 8(8):772. https://doi.org/10.3390/cells8080772
Chicago/Turabian StyleKumarasamy, Chellan, Madurantakam Royam Madhav, Shanthi Sabarimurugan, Sunil Krishnan, Siddhartha Baxi, Ajay Gupta, K M Gothandam, and Rama Jayaraj. 2019. "Prognostic Value of miRNAs in Head and Neck Cancers: A Comprehensive Systematic and Meta-Analysis" Cells 8, no. 8: 772. https://doi.org/10.3390/cells8080772
APA StyleKumarasamy, C., Madhav, M. R., Sabarimurugan, S., Krishnan, S., Baxi, S., Gupta, A., Gothandam, K. M., & Jayaraj, R. (2019). Prognostic Value of miRNAs in Head and Neck Cancers: A Comprehensive Systematic and Meta-Analysis. Cells, 8(8), 772. https://doi.org/10.3390/cells8080772






