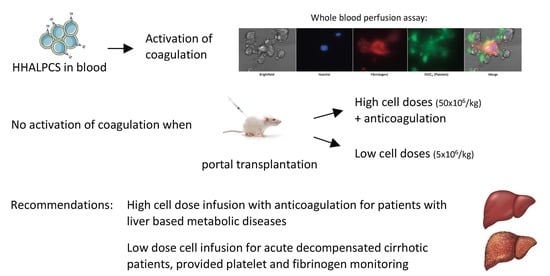
Clinical Protocol to Prevent Thrombogenic Effect of Liver-Derived Mesenchymal Cells for Cell-Based Therapies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Stem Cell Preparation
2.2. In Vitro Studies
2.2.1. Ethical Considerations and Blood Collection
2.2.2. Microfluidic Whole-Blood Model
2.2.3. Chandler Tubing Loop Model
2.2.4. Immunohistochemistry
2.3. In Vivo Studies
2.3.1. Ethical Considerations
2.3.2. Cell Transplantation
2.3.3. Immunohistochemistry
2.3.4. Intravital Microscopy
2.3.5. Statistical Analysis
3. Results
3.1. Anticoagulant Drugs Suppress the Coagulation Induced by HHALPCs in Whole Blood In Vitro
3.2. Coagulation Activation of HHALPCs by TF in a Shear Stress Model
3.3. HHALPCs Induce a Less Explosive Activation of Coagulation in an In Vitro Model with Whole Blood of Cirrhotic Patients
3.4. Infusion of a High Dose of HHALPCs Induces Coagulation and Alterations in Liver Blood Flow In Vivo
3.5. Low Dose Infusion of HHALPCs In Vivo in Xenotransplant Rat Model without Thrombogenic Effect
3.6. Thrombogenic Effect Associated with the High Cell Dose Infusion In Vivo is Suppressed by Anticoagulant Drugs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jung, J.W.; Kwon, M.; Choi, J.C.; Shin, J.W.; Park, I.W.; Choi, B.W.; Kim, J.Y. Familial occurrence of pulmonary embolism after intravenous, adipose tissue-derived stem cell therapy. Yonsei Med. J. 2013, 54, 1293–1296. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhang, S.; Zhou, L.; Cai, J.; Tan, J.; Gao, X.; Zeng, Z.; Li, D. Thromboembolism Induced by Umbilical Cord Mesenchymal Stem Cell Infusion: A Report of Two Cases and Literature Review. Transplant. Proc. 2017, 49, 1656–1658. [Google Scholar] [CrossRef] [PubMed]
- Sokal, E.M.; Stephenne, X.; Ottolenghi, C.; Jazouli, N.; Clapuyt, P.; Lacaille, F.; Najimi, M.; de Lonlay, P.; Smets, F. Liver engraftment and repopulation by in vitro expanded adult derived human liver stem cells in a child with ornithine carbamoyltransferase deficiency. Jimd Rep. 2014, 13, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Melmed, G.Y.; Pandak, W.M.; Casey, K.; Abraham, B.; Valentine, J.; Schwartz, D.; Awais, D.; Bassan, I.; Lichtiger, S.; Sands, B.; et al. Human Placenta-derived Cells (PDA-001) for the Treatment of Moderate-to-severe Crohn’s Disease: A Phase 1b/2a Study. Inflamm. Bowel Dis. 2015, 21, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Strange, C.; Nietert, P.J.; Wang, J.; Turnbull, T.L.; Cloud, C.; Owczarski, S.; Shuford, B.; Duke, T.; Gilkeson, G.; et al. Autologous Mesenchymal Stem Cell and Islet Cotransplantation: Safety and Efficacy. Stem Cells Transl. Med. 2018, 7, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Moll, G.; Ankrum, J.A.; Kamhieh-Milz, J.; Bieback, K.; Ringden, O.; Volk, H.D.; Geissler, S.; Reinke, P. Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol. Med. 2019, 25, 149–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moll, G.; Ignatowicz, L.; Catar, R.; Luecht, C.; Sadeghi, B.; Hamad, O.; Jungebluth, P.; Dragun, D.; Schmidtchen, A.; Ringden, O. Different Procoagulant Activity of Therapeutic Mesenchymal Stromal Cells Derived from Bone Marrow and Placental Decidua. Stem Cells Dev. 2015, 24, 2269–2279. [Google Scholar] [CrossRef]
- Gleeson, B.M.; Martin, K.; Ali, M.T.; Kumar, A.H.; Pillai, M.G.; Kumar, S.P.; O’Sullivan, J.F.; Whelan, D.; Stocca, A.; Khider, W.; et al. Bone Marrow-Derived Mesenchymal Stem Cells Have Innate Procoagulant Activity and Cause Microvascular Obstruction Following Intracoronary Delivery: Amelioration by Antithrombin Therapy. Stem Cells (Dayt. Ohio) 2015, 33, 2726–2737. [Google Scholar] [CrossRef]
- Tatsumi, K.; Ohashi, K.; Matsubara, Y.; Kohori, A.; Ohno, T.; Kakidachi, H.; Horii, A.; Kanegae, K.; Utoh, R.; Iwata, T.; et al. Tissue factor triggers procoagulation in transplanted mesenchymal stem cells leading to thromboembolism. Biochem. Biophys. Res. Commun. 2013, 431, 203–209. [Google Scholar] [CrossRef]
- Christy, B.A.; Herzig, M.C.; Montgomery, R.K.; Delavan, C.; Bynum, J.A.; Reddoch, K.M.; Cap, A.P. Procoagulant activity of human mesenchymal stem cells. J. Trauma Acute Care Surg. 2017, 83, S164–s169. [Google Scholar] [CrossRef]
- Liao, L.; Shi, B.; Chang, H.; Su, X.; Zhang, L.; Bi, C.; Shuai, Y.; Du, X.; Deng, Z.; Jin, Y. Heparin improves BMSC cell therapy: Anticoagulant treatment by heparin improves the safety and therapeutic effect of bone marrow-derived mesenchymal stem cell cytotherapy. Theranostics 2017, 7, 106–116. [Google Scholar] [CrossRef]
- George, M.J.; Prabhakara, K.; Toledano-Furman, N.E.; Wang, Y.-W.; Gill, B.S.; Wade, C.E.; Olson, S.D.; Cox, C.S. Clinical Cellular Therapeutics Accelerate Clot Formation. Stem Cells Transl. Med. 2018, 7, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Moberg, L.; Johansson, H.; Lukinius, A.; Berne, C.; Foss, A.; Kallen, R.; Ostraat, O.; Salmela, K.; Tibell, A.; Tufveson, G.; et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet (Lond. Engl. ) 2002, 360, 2039–2045. [Google Scholar] [CrossRef]
- Stephenne, X.; Vosters, O.; Najimi, M.; Beuneu, C.; Dung, K.N.; Wijns, W.; Goldman, M.; Sokal, E.M. Tissue factor-dependent procoagulant activity of isolated human hepatocytes: relevance to liver cell transplantation. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2007, 13, 599–606. [Google Scholar] [CrossRef]
- Gustafson, E.K.; Elgue, G.; Hughes, R.D.; Mitry, R.R.; Sanchez, J.; Haglund, U.; Meurling, S.; Dhawan, A.; Korsgren, O.; Nilsson, B. The instant blood-mediated inflammatory reaction characterized in hepatocyte transplantation. Transplantation 2011, 91, 632–638. [Google Scholar] [CrossRef]
- Moll, G.; Rasmusson-Duprez, I.; von Bahr, L.; Connolly-Andersen, A.M.; Elgue, G.; Funke, L.; Hamad, O.A.; Lonnies, H.; Magnusson, P.U.; Sanchez, J.; et al. Are therapeutic human mesenchymal stromal cells compatible with human blood? Stem Cells (Dayt. Ohio) 2012, 30, 1565–1574. [Google Scholar] [CrossRef]
- Stephenne, X.; Nicastro, E.; Eeckhoudt, S.; Hermans, C.; Nyabi, O.; Lombard, C.; Najimi, M.; Sokal, E. Bivalirudin in combination with heparin to control mesenchymal cell procoagulant activity. PLoS ONE 2012, 7, e42819. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Heemskerk, J.W.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef]
- Thomassen, S.; Mastenbroek, T.G.; Swieringa, F.; Winckers, K.; Feijge, M.A.H.; Schrijver, R.; Cosemans, J.; Maroney, S.A.; Mast, A.E.; Hackeng, T.M.; et al. Suppressive Role of Tissue Factor Pathway Inhibitor-alpha in Platelet-Dependent Fibrin Formation under Flow Is Restricted to Low Procoagulant Strength. Thromb. Haemost. 2018, 118, 502–513. [Google Scholar] [CrossRef]
- Baaten, C.; Ten Cate, H.; van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet populations and priming in hematological diseases. Blood Rev. 2017, 31, 389–399. [Google Scholar] [CrossRef]
- Smets, F.; Dobbelaere, D.; McKiernan, P.; Dionisi-Vici, C.; Broue, P.; Jacquemin, E.; Lopes, A.I.; Goncalves, I.; Mandel, H.; Pawlowska, J.; et al. Phase I/II Trial of Liver Derived Mesenchymal Stem Cells in Pediatric Liver Based Metabolic Disorders: A Prospective, Open Label, Multicenter, Partially Randomized, Safety Study of One Cycle of Heterologous Human Adult Liver-Derived Progenitor Cells (HepaStem(R)) in Urea Cycle Disorders and Crigler-Najjar Syndrome patients. Transplantation 2019. [Google Scholar] [CrossRef]
- Sokal, E.M. Treating inborn errors of liver metabolism with stem cells: current clinical development. J. Inherit. Metab. Dis. 2014, 37, 535–539. [Google Scholar] [CrossRef]
- El-Kehdy, H.; Sargiacomo, C.; Fayyad-Kazan, M.; Fayyad-Kazan, H.; Lombard, C.; Lagneaux, L.; Sokal, E.; Najar, M.; Najimi, M. Immunoprofiling of Adult-Derived Human Liver Stem/Progenitor Cells: Impact of Hepatogenic Differentiation and Inflammation. Stem Cells Int. 2017, 2017, 15. [Google Scholar] [CrossRef]
- Najimi, M.; Berardis, S.; El-Kehdy, H.; Rosseels, V.; Evraerts, J.; Lombard, C.; El Taghdouini, A.; Henriet, P.; van Grunsven, L.; Sokal, E.M. Human liver mesenchymal stem/progenitor cells inhibit hepatic stellate cell activation: in vitro and in vivo evaluation. Stem Cell Res. Ther. 2017, 8, 131. [Google Scholar] [CrossRef]
- Frederik, N.; Thierry, G.; Pierre-François, L.; Luc, L.; Enev, H.L.; Victor, V.; Virginie, B.; Clerget-Chossat, N.; Sokal, E. GS-16-Safety and tolerability of liver-derived stem cells (HepaStem) infused in patients with acute-on-chronic liver failure or acute decompensation: a European phase I/IIa open-labelled study. J. Hepatol. 2019, 70, e83. [Google Scholar] [CrossRef]
- Lisman, T.; Porte, R.J. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood 2010, 116, 878–885. [Google Scholar] [CrossRef] [Green Version]
- Tripodi, A.; Primignani, M.; Mannucci, P.M.; Caldwell, S.H. Changing Concepts of Cirrhotic Coagulopathy. Am. J. Gastroenterol. 2017, 112, 274–281. [Google Scholar] [CrossRef]
- Najimi, M.; Khuu, D.N.; Lysy, P.A.; Jazouli, N.; Abarca, J.; Sempoux, C.; Sokal, E.M. Adult-derived human liver mesenchymal-like cells as a potential progenitor reservoir of hepatocytes? Cell Transplant. 2007, 16, 717–728. [Google Scholar] [CrossRef]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013, 144, 1426–1437. [Google Scholar] [CrossRef]
- Swieringa, F.; Kuijpers, M.J.; Lamers, M.M.; van der Meijden, P.E.; Heemskerk, J.W. Rate-limiting roles of the tenase complex of factors VIII and IX in platelet procoagulant activity and formation of platelet-fibrin thrombi under flow. Haematologica 2015, 100, 748–756. [Google Scholar] [CrossRef] [Green Version]
- Swieringa, F.; Baaten, C.C.; Verdoold, R.; Mastenbroek, T.G.; Rijnveld, N.; van der Laan, K.O.; Breel, E.J.; Collins, P.W.; Lance, M.D.; Henskens, Y.M.; et al. Platelet Control of Fibrin Distribution and Microelasticity in Thrombus Formation Under Flow. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Geffen, J.V.; Brouns, S.; Batista, J. High-throughput elucidation of thrombus formation reveals sources of platelet function variability. Haematologica 2019, in press. [Google Scholar] [CrossRef]
- Smets, F.; Najimi, M.; Sokal, E.M. Cell transplantation in the treatment of liver diseases. Pediatric Transplant. 2008, 12, 6–13. [Google Scholar] [CrossRef]
- Lysy, P.A.; Najimi, M.; Stephenne, X.; Bourgois, A.; Smets, F.; Sokal, E.M. Liver cell transplantation for Crigler-Najjar syndrome type I: update and perspectives. World J. Gastroenterol. Wjg 2008, 14, 3464–3470. [Google Scholar] [CrossRef]
- Muraca, M.; Gerunda, G.; Neri, D.; Vilei, M.T.; Granato, A.; Feltracco, P.; Meroni, M.; Giron, G.; Burlina, A.B. Hepatocyte transplantation as a treatment for glycogen storage disease type 1a. Lancet (Lond. Engl.) 2002, 359, 317–318. [Google Scholar] [CrossRef]
- Varma, S.; Stephenne, X.; Komuta, M.; Bouzin, C.; Ambroise, J.; Smets, F.; Reding, R.; Sokal, E.M. The histological quantification of alpha-smooth muscle actin predicts future graft fibrosis in pediatric liver transplant recipients. Pediatric Transplant. 2017, 21. [Google Scholar] [CrossRef]
- Bouzin, C.; Saini, M.L.; Khaing, K.K.; Ambroise, J.; Marbaix, E.; Gregoire, V.; Bol, V. Digital pathology: elementary, rapid and reliable automated image analysis. Histopathology 2016, 68, 888–896. [Google Scholar] [CrossRef]
- Kuijpers, M.J.; van der Meijden, P.E.; Feijge, M.A.; Mattheij, N.J.; May, F.; Govers-Riemslag, J.; Meijers, J.C.; Heemskerk, J.W.; Renne, T.; Cosemans, J.M. Factor XII regulates the pathological process of thrombus formation on ruptured plaques. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1674–1680. [Google Scholar] [CrossRef]
- Furlani, D.; Ugurlucan, M.; Ong, L.; Bieback, K.; Pittermann, E.; Westien, I.; Wang, W.; Yerebakan, C.; Li, W.; Gaebel, R.; et al. Is the intravascular administration of mesenchymal stem cells safe? Mesenchymal stem cells and intravital microscopy. Microvasc. Res. 2009, 77, 370–376. [Google Scholar] [CrossRef]
- Silachev, D.N.; Goryunov, K.V.; Shpilyuk, M.A.; Beznoschenko, O.S.; Morozova, N.Y.; Kraevaya, E.E.; Popkov, V.A.; Pevzner, I.B.; Zorova, L.D.; Evtushenko, E.A.; et al. Effect of MSCs and MSC-Derived Extracellular Vesicles on Human Blood Coagulation. Cells 2019, 8. [Google Scholar] [CrossRef]
- Lisman, T.; Bos, S.; Intagliata, N.M. Mechanisms of enhanced thrombin-generating capacity in patients with cirrhosis. J. Thromb. Haemost. Jth 2018, 16, 1128–1131. [Google Scholar] [CrossRef]
- Ettelaie, C.; Fountain, D.; Collier, M.E.; Elkeeb, A.M.; Xiao, Y.P.; Maraveyas, A. Low molecular weight heparin downregulates tissue factor expression and activity by modulating growth factor receptor-mediated induction of nuclear factor-kappaB. Biochim. Biophys. Acta 2011, 1812, 1591–1600. [Google Scholar] [CrossRef]
- Seeger, F.H.; Rasper, T.; Fischer, A.; Muhly-Reinholz, M.; Hergenreider, E.; Leistner, D.M.; Sommer, K.; Manavski, Y.; Henschler, R.; Chavakis, E.; et al. Heparin disrupts the CXCR4/SDF-1 axis and impairs the functional capacity of bone marrow-derived mononuclear cells used for cardiovascular repair. Circ. Res. 2012, 111, 854–862. [Google Scholar] [CrossRef]
- Groeneveld, D.; Pereyra, D.; Veldhuis, Z.; Adelmeijer, J.; Ottens, P.; Kopec, A.K.; Starlinger, P.; Lisman, T.; Luyendyk, J.P. Intrahepatic fibrin(ogen) deposition drives liver regeneration after partial hepatectomy in mice and humans. Blood 2019. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppin, L.; Najimi, M.; Bodart, J.; Rouchon, M.-S.; van der Smissen, P.; Eeckhoudt, S.; Dahlqvist, G.; Castanares-Zapatero, D.; Komuta, M.; Brouns, S.L.; et al. Clinical Protocol to Prevent Thrombogenic Effect of Liver-Derived Mesenchymal Cells for Cell-Based Therapies. Cells 2019, 8, 846. https://doi.org/10.3390/cells8080846
Coppin L, Najimi M, Bodart J, Rouchon M-S, van der Smissen P, Eeckhoudt S, Dahlqvist G, Castanares-Zapatero D, Komuta M, Brouns SL, et al. Clinical Protocol to Prevent Thrombogenic Effect of Liver-Derived Mesenchymal Cells for Cell-Based Therapies. Cells. 2019; 8(8):846. https://doi.org/10.3390/cells8080846
Chicago/Turabian StyleCoppin, Louise, Mustapha Najimi, Julie Bodart, Marie-Sophie Rouchon, Patrick van der Smissen, Stéphane Eeckhoudt, Géraldine Dahlqvist, Diego Castanares-Zapatero, Mina Komuta, Sanne L. Brouns, and et al. 2019. "Clinical Protocol to Prevent Thrombogenic Effect of Liver-Derived Mesenchymal Cells for Cell-Based Therapies" Cells 8, no. 8: 846. https://doi.org/10.3390/cells8080846
APA StyleCoppin, L., Najimi, M., Bodart, J., Rouchon, M.-S., van der Smissen, P., Eeckhoudt, S., Dahlqvist, G., Castanares-Zapatero, D., Komuta, M., Brouns, S. L., Baaten, C. C., Heemskerk, J. W. M., Horman, S., Belmonte, N., Sokal, E., & Stéphenne, X. (2019). Clinical Protocol to Prevent Thrombogenic Effect of Liver-Derived Mesenchymal Cells for Cell-Based Therapies. Cells, 8(8), 846. https://doi.org/10.3390/cells8080846