MicroRNA-21 Mediates the Protective Effect of Cardiomyocyte-Derived Conditioned Medium on Ameliorating Myocardial Infarction in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals and Induction of Acute Myocardial Infarction in Rats
2.3. Culturing of Cardiac Cells
2.4. Collection of Exosomes
2.5. Quantitative Reverse Transcription Polymerase Chain Reaction
2.6. Histopathological and Immunostaining
2.7. Western Blot
2.8. Statistical Analysis
3. Results
3.1. Oxygen–Glucose Deprivation Regulates microRNA Expression Profiles in Cardiac Cells
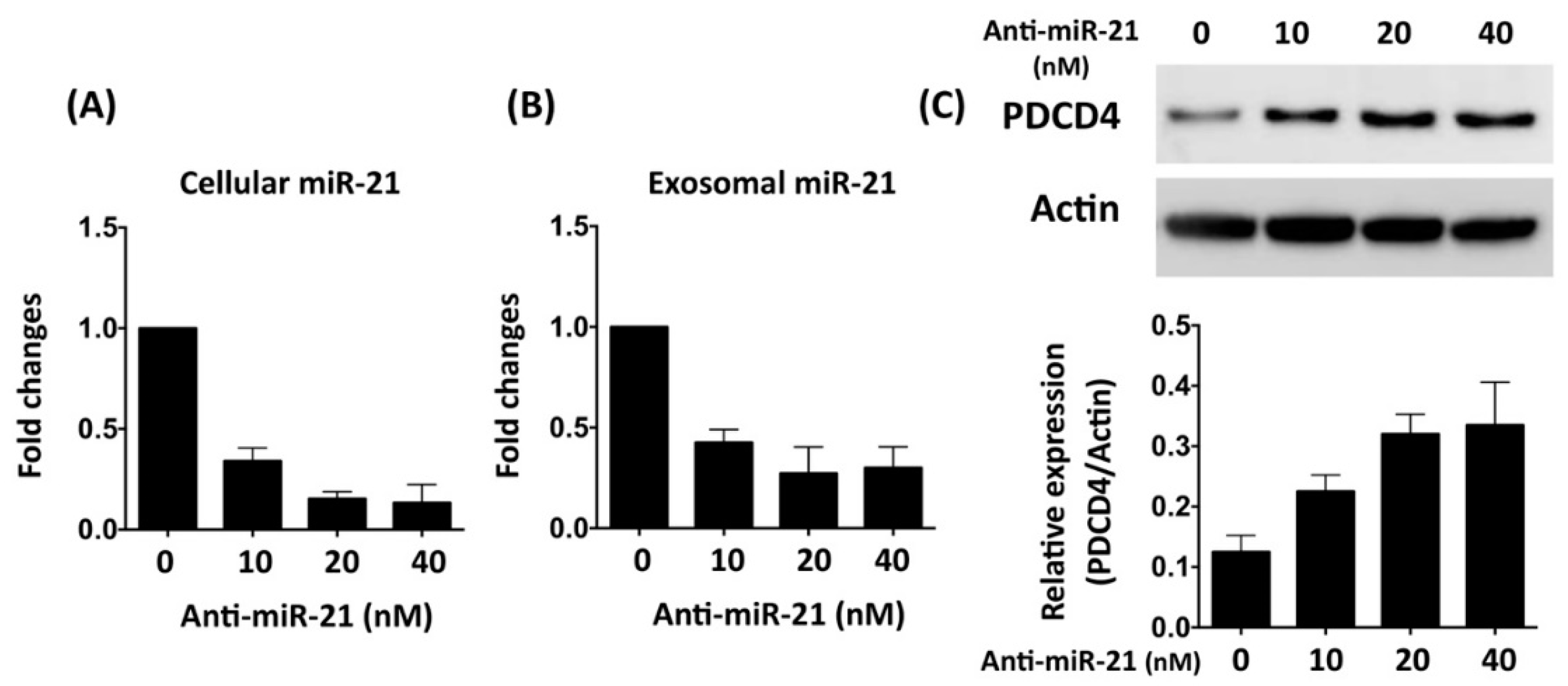
3.2. Inhibition of miR-21 Reduced the Protective Effect of Cardiomyocyte-Derived Conditioned Medium Cardiomyocytes Against Oxidative Stress
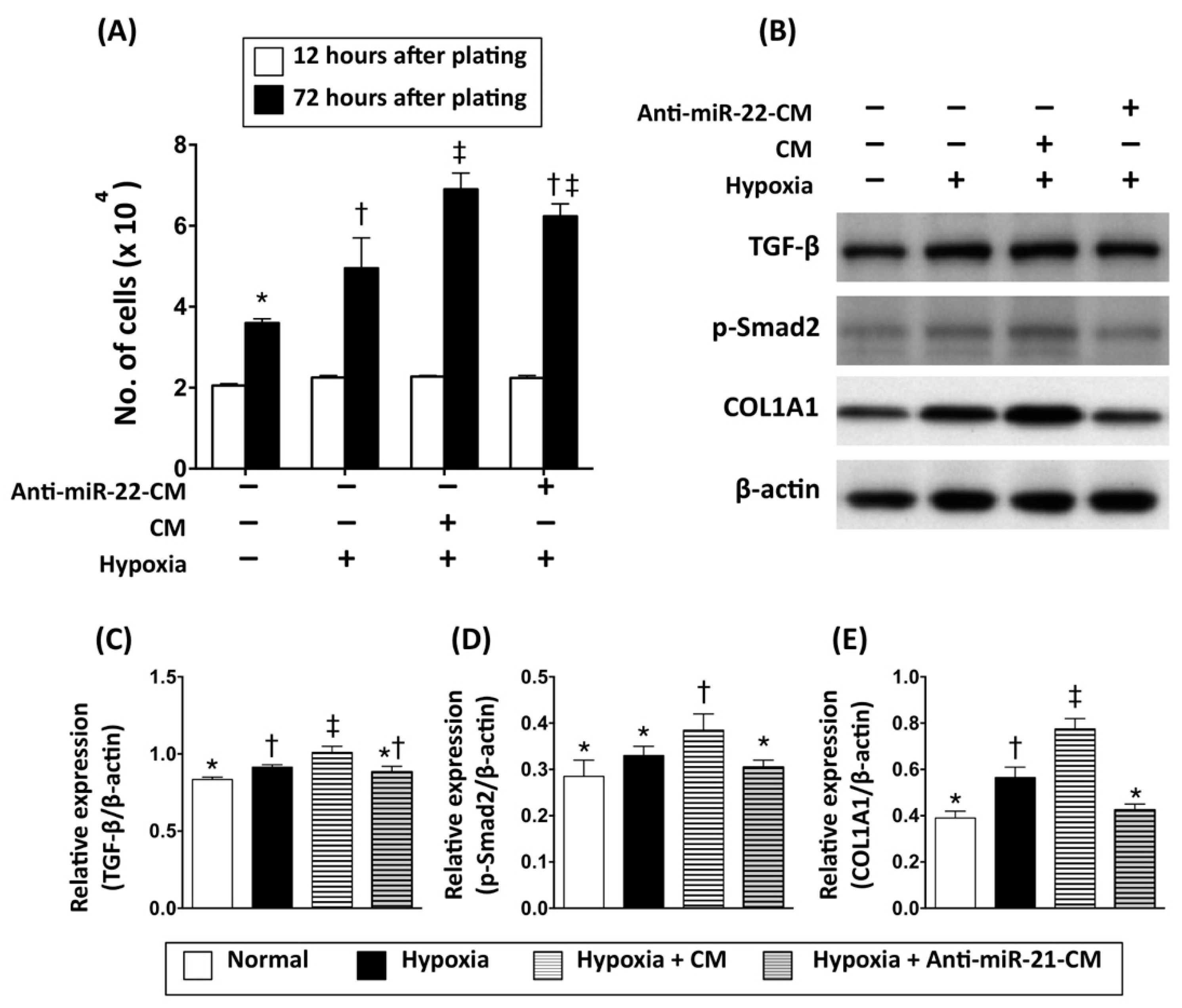
3.3. Inhibition of miR-21 Reduced the Effect of Conditioned Medium on Activation of Cardiac Fibroblasts
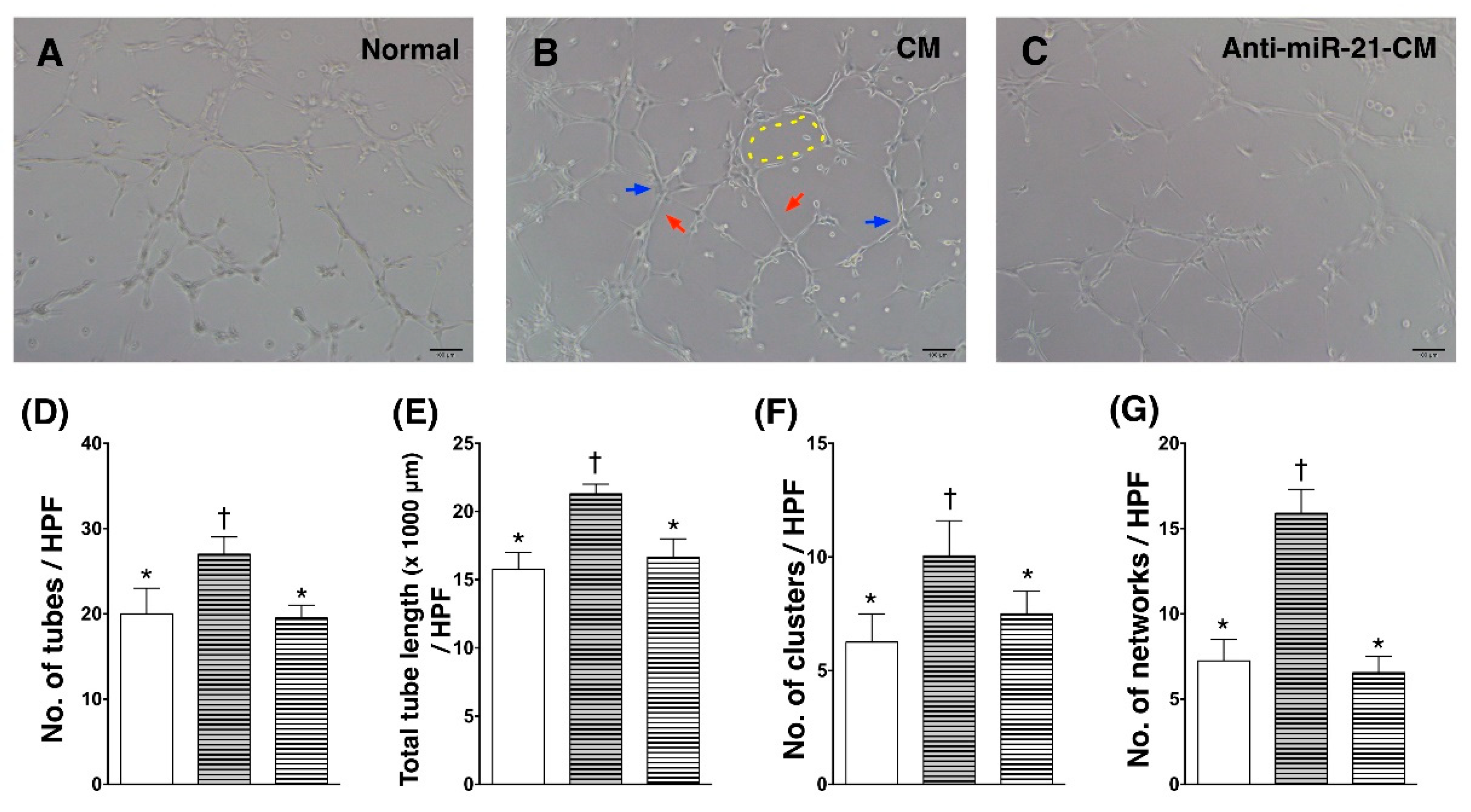
3.4. Inhibition of miR-21 Reduced the Effect of Conditioned Medium on Angiogenic Enhancement in Endothelial Cells
3.5. Inhibition of miR-21 Reduced the Effect of Conditioned Medium on Ameliorating Myocardial Injury in Rats with Acute Myocardial Infarction
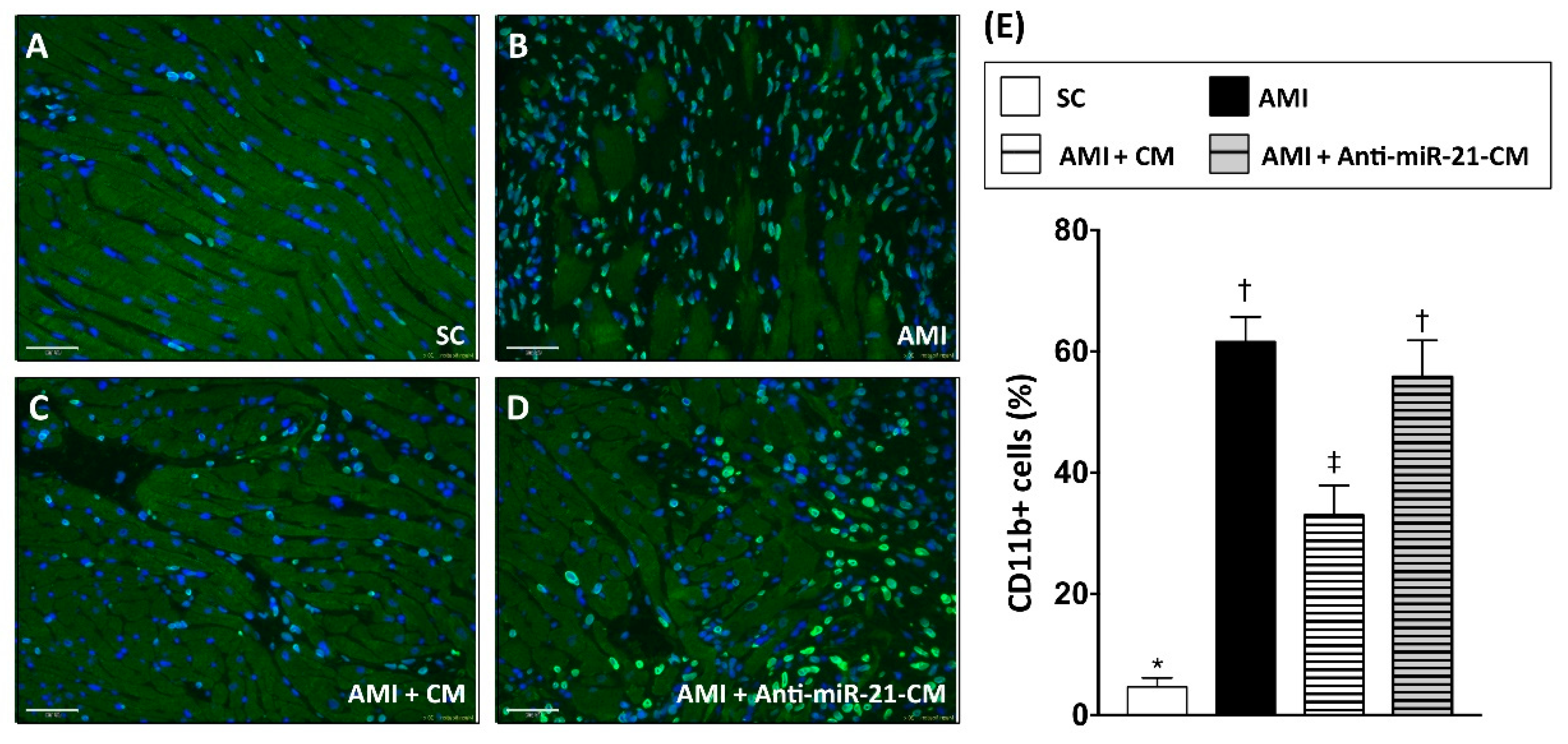
3.6. Inhibition of miR-21 Reduced the Effect of Conditioned Medium on Reducing Immune Cell Infiltration in Myocardium with Acute Myocardial Infarction in Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goldberg, R.J.; Gore, J.M.; Alpert, J.S.; Osganian, V.; de Groot, J.; Bade, J.; Chen, Z.; Frid, D.; Dalen, J.E. Cardiogenic shock after acute myocardial infarction. Incidence and mortality from a community-wide perspective, 1975 to 1988. New Engl. J. Med. 1991, 325, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Lopez, A.D. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997, 349, 1269–1276. [Google Scholar] [CrossRef]
- Yellon, D.M.; Alkhulaifi, A.M.; Pugsley, W.B. Preconditioning the human myocardium. Lancet 1993, 342, 276–277. [Google Scholar] [CrossRef]
- Verdouw, P.D.; Gho, B.C.; Koning, M.M.; Schoemaker, R.G.; Duncker, D.J. Cardioprotection by ischemic and nonischemic myocardial stress and ischemia in remote organs. Implications for the concept of ischemic preconditioning. Ann. N. Y. Acad. Sci. 1996, 793, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.R.; Fleischhauer, J.; Montino, H.; Chakupurakal, R.; Foresti, M.; Schuetz, T.; Sack, S.; Mohri, M.; Arras, M.; Schaper, W.; et al. Infarct Size Reduction by Ischemic Preconditioning Is a Monophasic, Short-Lived Phenomenon in Anesthetized Pigs. J. Cardiovasc. Pharm. 1998, 3, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, Y.; Cote, G.M.; Guo, X.; Lebrasseur, N.K.; Cui, L.; Liao, R.; Sawyer, D.B. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J. Biol. Chem. 2004, 279, 51141–51147. [Google Scholar] [CrossRef] [PubMed]
- Kassiri, Z.; Defamie, V.; Hariri, M.; Oudit, G.Y.; Anthwal, S.; Dawood, F.; Liu, P.; Khokha, R. Simultaneous transforming growth factor beta-tumor necrosis factor activation and cross-talk cause aberrant remodeling response and myocardial fibrosis in Timp3-deficient heart. J. Biol. Chem. 2009, 284, 29893–29904. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.L.; Blaxall, B.C. Cardiac intercellular communication: Are myocytes and fibroblasts fair-weather friends? J. Cardiovasc. Transl. Res. 2012, 5, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, F.G.; Yee, K.O. Communication signals between cardiac fibroblasts and cardiac myocytes. J. Cardiovasc. Pharmacol. 2011, 57, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.M.; Frazier, O.H.; Buja, L.M. Fibrosis and heart failure. Heart Fail. Rev. 2014, 19, 173–185. [Google Scholar] [CrossRef]
- van den Borne, S.W.; Diez, J.; Blankesteijn, W.M.; Verjans, J.; Hofstra, L.; Narula, J. Myocardial remodeling after infarction: The role of myofibroblasts. Nat. Reviews. Cardiol. 2010, 7, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.; Kao, Y.H.; Sun, C.K.; Lin, Y.C.; Tsai, T.H.; Chang, L.T.; Chua, S.; Yeh, K.H.; Wu, C.J.; Fu, M.; et al. Myocardium-derived conditioned medium improves left ventricular function in rodent acute myocardial infarction. J. Transl. Med. 2011, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Courts, C.; Madea, B. Micro-RNA—A potential for forensic science? Forensic Sci. Int. 2010, 203, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Dykxhoorn, D.M. MicroRNAs and metastasis: Little RNAs go a long way. Cancer Res. 2010, 70, 6401–6406. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, W.; Xu, R.; Nie, Y.; Cao, X.; Meng, J.; Xu, X.; Hu, S.; Zheng, Z. MicroRNA-24 regulates cardiac fibrosis after myocardial infarction. J. Cell. Mol. Med. 2012, 16, 2150–2160. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.J.; Ray, A. MicroRNAs in the search for understanding human diseases. Biodrugs Clin. Immunother. Biopharm. Gene Ther. 2007, 21, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, X. The function of miRNA in cardiac hypertrophy. Cell. Mol. Life Sci. Cmls 2012, 69, 3561–3570. [Google Scholar] [CrossRef] [Green Version]
- Thum, T.; Galuppo, P.; Wolf, C.; Fiedler, J.; Kneitz, S.; van Laake, L.W.; Doevendans, P.A.; Mummery, C.L.; Borlak, J.; Haverich, A.; et al. MicroRNAs in the human heart: A clue to fetal gene reprogramming in heart failure. Circulation 2007, 116, 258–267. [Google Scholar] [CrossRef]
- Adam, O.; Lohfelm, B.; Thum, T.; Gupta, S.K.; Puhl, S.L.; Schafers, H.J.; Bohm, M.; Laufs, U. Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res. Cardiol 2012, 107, 278. [Google Scholar] [CrossRef]
- Williams, A.E. Functional aspects of animal microRNAs. Cell. Mol. Life Sci. Cmls 2008, 65, 545–562. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, X.; Lu, Y.; Yang, B. miRNAs at the heart of the matter. J. Mol. Med. (Berl) 2008, 86, 771–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishi, H.; Sakaguchi, T.; Miyagawa, S.; Yoshikawa, Y.; Fukushima, S.; Saito, S.; Ueno, T.; Kuratani, T.; Sawa, Y. Impact of microRNA expression in human atrial tissue in patients with atrial fibrillation undergoing cardiac surgery. PLoS ONE 2013, 8, e73397. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, Y.; Chen, X.; Cheng, X.; Liao, Y.; Yu, X. Exosomal transfer of miR-30a between cardiomyocytes regulates autophagy after hypoxia. J. Mol. Med. (Berl) 2016, 94, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Felicetti, F.; De Feo, A.; Coscia, C.; Puglisi, R.; Pedini, F.; Pasquini, L.; Bellenghi, M.; Errico, M.C.; Pagani, E.; Care, A. Exosome-mediated transfer of miR-222 is sufficient to increase tumor malignancy in melanoma. J. Transl. Med. 2016, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, W.; Liu, G.; Cai, W.; Millard, R.W.; Wang, Y.; Chang, J.; Peng, T.; Fan, G.C. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J. Mol. Cell Cardiol. 2014, 74, 139–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hergenreider, E.; Heydt, S.; Treguer, K.; Boettger, T.; Horrevoets, A.J.; Zeiher, A.M.; Scheffer, M.P.; Frangakis, A.S.; Yin, X.; Mayr, M.; et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 2012, 14, 249–256. [Google Scholar] [CrossRef]
- Fiedler, J.; Thum, T. MicroRNAs in myocardial infarction. Arter. Thromb. Vasc. Biol. 2013, 33, 201–205. [Google Scholar] [CrossRef]
- Shan, Z.X.; Lin, Q.X.; Fu, Y.H.; Deng, C.Y.; Zhou, Z.L.; Zhu, J.N.; Liu, X.Y.; Zhang, Y.Y.; Li, Y.; Lin, S.G.; et al. Upregulated expression of miR-1/miR-206 in a rat model of myocardial infarction. Biochem. Biophys. Res. Commun. 2009, 381, 597–601. [Google Scholar] [CrossRef]
- Freedman, J.E.; Ercan, B.; Morin, K.M.; Liu, C.T.; Tamer, L.; Ayaz, L.; Kanadasi, M.; Cicek, D.; Seyhan, A.I.; Akilli, R.E.; et al. The distribution of circulating microRNA and their relation to coronary disease. F1000Res 2012, 1, 50. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-miRPath v3.0: Deciphering microRNA function with experimental support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, X.; Zhang, S.; Lin, Y.; Yang, J.; Zhang, C. MicroRNA-21 protects against the H(2)O(2)-induced injury on cardiac myocytes via its target gene PDCD4. J. Mol. Cell Cardiol. 2009, 47, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Pan, Y.; Li, X.H.; Yang, X.Y.; Feng, Y.L.; Tan, H.H.; Jiang, L.; Feng, J.; Yu, X.Y. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016, 7, e2277. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Tang, C.Y.; Chiang, S.C.; Tseng, K.W.; Huang, C.H. Ischemic preconditioning activates prosurvival kinases and reduces myocardial apoptosis. J. Chin. Med. Assoc. 2015, 78, 460–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iliodromitis, E.K.; Lazou, A.; Kremastinos, D.T. Ischemic preconditioning: Protection against myocardial necrosis and apoptosis. Vasc. Health Risk Manag. 2007, 3, 629–637. [Google Scholar] [PubMed]
- Xin, H.; Li, Y.; Buller, B.; Katakowski, M.; Zhang, Y.; Wang, X.; Shang, X.; Zhang, Z.G.; Chopp, M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells 2012, 30, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.C.; Veeramachaneni, D.N.; Winger, Q.A.; Carnevale, E.M.; Bouma, G.J. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: A possible new form of cell communication within the ovarian follicle. Biol. Reprod. 2012, 86, 71. [Google Scholar] [CrossRef] [PubMed]
- Izarra, A.; Moscoso, I.; Levent, E.; Canon, S.; Cerrada, I.; Diez-Juan, A.; Blanca, V.; Nunez-Gil, I.J.; Valiente, I.; Ruiz-Sauri, A.; et al. miR-133a enhances the protective capacity of cardiac progenitors cells after myocardial infarction. Stem Cell Rep. 2014, 3, 1029–1042. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Carvalho, V.; da Silva, M.M.; Guimaraes, G.V.; Bacal, F.; Bocchi, E.A. MicroRNAs: New players in heart failure. Mol. Biol. Rep. 2013, 40, 2663–2670. [Google Scholar] [CrossRef]
- Kaudewitz, D.; Zampetaki, A.; Mayr, M. MicroRNA Biomarkers for Coronary Artery Disease? Curr. Atheroscler Rep. 2015, 17, 70. [Google Scholar] [CrossRef]
- Pan, Y.Q.; Li, J.; Li, X.W.; Li, Y.C.; Li, J.; Lin, J.F. Effect of miR-21/TLR4/NF-kappaB pathway on myocardial apoptosis in rats with myocardial ischemia-reperfusion. Eur. Rev. Med. Pharm. Sci 2018, 22, 7928–7937. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, H.; Ge, D.; Xu, Y.; Xu, H.; Yang, Y.; Gu, M.; Zhou, Y.; Zhu, J.; Ge, T.; et al. Mir-21 Promotes Cardiac Fibrosis After Myocardial Infarction Via Targeting Smad7. Cell Physiol. Biochem. 2017, 42, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Wu, S.; Kong, F.; Cai, X.; Ye, B.; Shan, P.; Huang, W. MicroRNA-21 protects against cardiac hypoxia/reoxygenation injury by inhibiting excessive autophagy in H9c2 cells via the Akt/mTOR pathway. J. Cell. Mol. Med. 2017, 21, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yang, K.; Li, A. microRNA-21 protects against ischemia-reperfusion and hypoxia-reperfusion-induced cardiocyte apoptosis via the phosphatase and tensin homolog/Akt-dependent mechanism. Mol. Med. Rep. 2014, 9, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Gutterman, D.D.; Chabowski, D.S.; Kadlec, A.O.; Durand, M.J.; Freed, J.K.; Ait-Aissa, K.; Beyer, A.M. The Human Microcirculation: Regulation of Flow and Beyond. Circ. Res. 2016, 118, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, W.; Yan, X.; Zhou, H.; Zhang, H.; Liu, J.; Yu, M.; Zhu, X.; Ma, K. Effects of mir-21 on Cardiac Microvascular Endothelial Cells After Acute Myocardial Infarction in Rats: Role of Phosphatase and Tensin Homolog (PTEN)/Vascular Endothelial Growth Factor (VEGF) Signal Pathway. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 3562–3575. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Z.; Li, C.; Chen, Q.; Jing, Y.; Carpenter, R.; Jiang, Y.; Kung, H.F.; Lai, L.; Jiang, B.H. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1alpha expression. PLoS ONE 2011, 6, e19139. [Google Scholar] [CrossRef]
- Reddy, S.; Hu, D.Q.; Zhao, M.; Blay, E., Jr.; Sandeep, N.; Ong, S.G.; Jung, G.; Kooiker, K.B.; Coronado, M.; Fajardo, G.; et al. miR-21 is associated with fibrosis and right ventricular failure. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Cao, W.; Shi, P.; Ge, J.J. miR-21 enhances cardiac fibrotic remodeling and fibroblast proliferation via CADM1/STAT3 pathway. BMC Cardiovasc. Disord. 2017, 17, 88. [Google Scholar] [CrossRef]
- La Sala, L.; Mrakic-Sposta, S.; Micheloni, S.; Prattichizzo, F.; Ceriello, A. Glucose-sensing microRNA-21 disrupts ROS homeostasis and impairs antioxidant responses in cellular glucose variability. Cardiovasc. Diabetol. 2018, 17, 105. [Google Scholar] [CrossRef]
- Canfran-Duque, A.; Rotllan, N.; Zhang, X.; Fernandez-Fuertes, M.; Ramirez-Hidalgo, C.; Araldi, E.; Daimiel, L.; Busto, R.; Fernandez-Hernando, C.; Suarez, Y. Macrophage deficiency of miR-21 promotes apoptosis, plaque necrosis, and vascular inflammation during atherogenesis. EMBO Mol. Med. 2017, 9, 1244–1262. [Google Scholar] [CrossRef]
- Davidson, S.M.; Riquelme, J.A.; Zheng, Y.; Vicencio, J.M.; Lavandero, S.; Yellon, D.M. Endothelial cells release cardioprotective exosomes that may contribute to ischaemic preconditioning. Sci. Rep. 2018, 8, 15885. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boon, R.A.; Vickers, K.C. Intercellular transport of microRNAs. Arter. Thromb. Vasc. Biol. 2013, 33, 186–192. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-H.; Hsu, S.-Y.; Chiu, C.-C.; Leu, S. MicroRNA-21 Mediates the Protective Effect of Cardiomyocyte-Derived Conditioned Medium on Ameliorating Myocardial Infarction in Rats. Cells 2019, 8, 935. https://doi.org/10.3390/cells8080935
Chen C-H, Hsu S-Y, Chiu C-C, Leu S. MicroRNA-21 Mediates the Protective Effect of Cardiomyocyte-Derived Conditioned Medium on Ameliorating Myocardial Infarction in Rats. Cells. 2019; 8(8):935. https://doi.org/10.3390/cells8080935
Chicago/Turabian StyleChen, Chih-Hung, Shu-Yuan Hsu, Chien-Chih Chiu, and Steve Leu. 2019. "MicroRNA-21 Mediates the Protective Effect of Cardiomyocyte-Derived Conditioned Medium on Ameliorating Myocardial Infarction in Rats" Cells 8, no. 8: 935. https://doi.org/10.3390/cells8080935
APA StyleChen, C.-H., Hsu, S.-Y., Chiu, C.-C., & Leu, S. (2019). MicroRNA-21 Mediates the Protective Effect of Cardiomyocyte-Derived Conditioned Medium on Ameliorating Myocardial Infarction in Rats. Cells, 8(8), 935. https://doi.org/10.3390/cells8080935




