The Lipopeptide MALP-2 Promotes Collateral Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Mice and Cells
2.3. Experimental Femoral Artery Ligation (FAL)
2.4. Laser Speckle Imaging
2.5. Histology and Immunohistochemistry
2.6. Organ Chamber Experiments (Wire Myography)
2.7. Real-Time PCR
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Western Blot
2.10. Griess Assay
2.11. Adhesion Assay
2.12. Statistical Analysis
3. Results
3.1. MALP-2 Improved Perfusion Recovery and Collateral Growth in the Hind Limb Following FAL in Hypercholesterolemic Apoe-Deficient Mice
3.2. MALP-2 Increased Pericollateral Macrophage Accumulation, Endothelial Cell Proliferation and Downstream Angiogenesis Following FAL
3.3. MALP-2 Improved NO-Dependent Vascular Relaxation and Enhanced Endothelial Cell-Derived NO Release
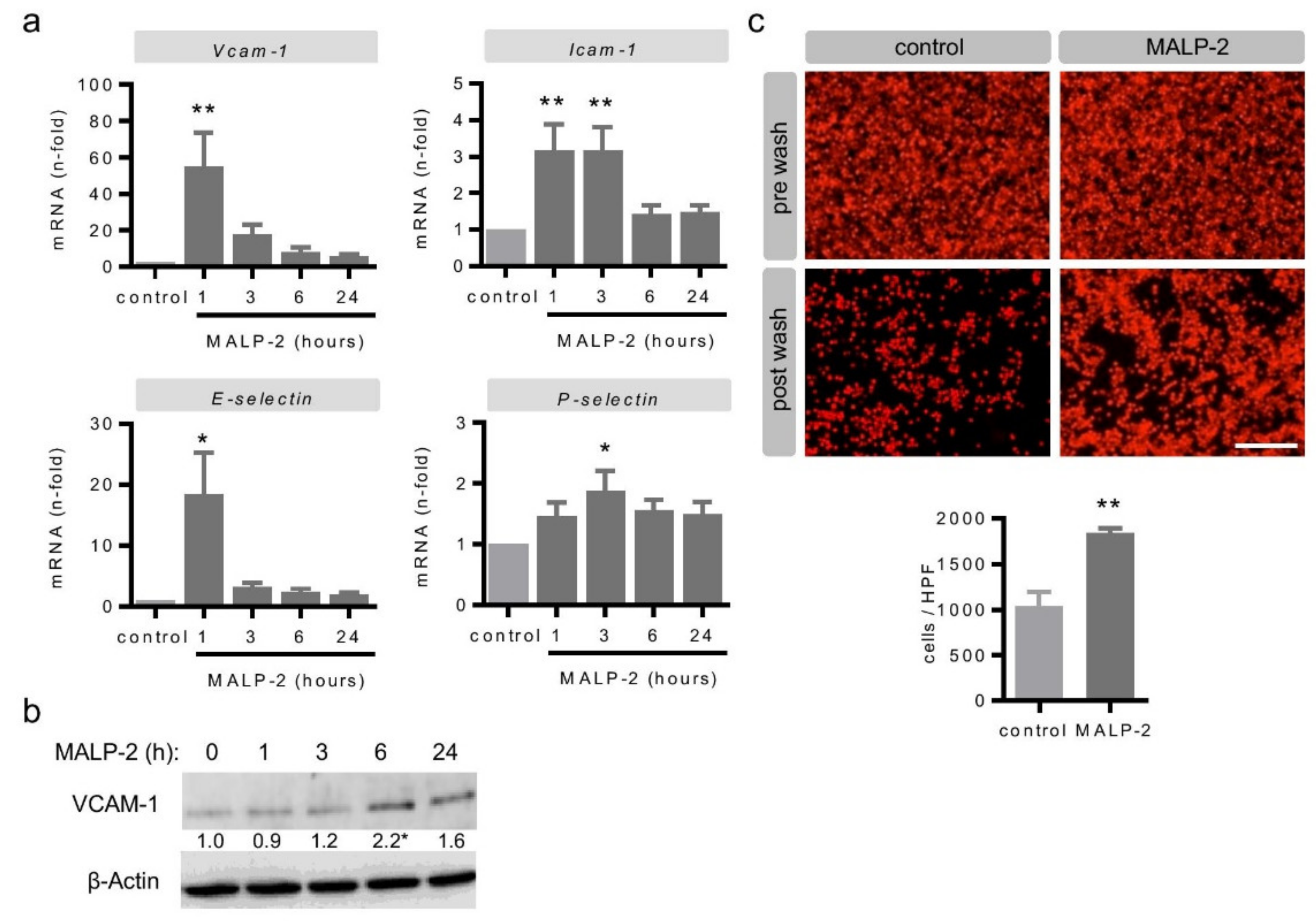
3.4. MALP-2 Up-Regulated Endothelial Adhesion Molecules and Enhanced the Endothelial Adhesion of Monocytic Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowkes, F.G.; Aboyans, V.; Fowkes, F.J.; McDermott, M.M.; Sampson, U.K.; Criqui, M.H. Peripheral artery disease: Epidemiology and global perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K.; Libby, P.; Tabas, I. Inflammation and plaque vulnerability. J. Intern. Med. 2015, 278, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Seiler, C.; Stoller, M.; Pitt, B.; Meier, P. The human coronary collateral circulation: Development and clinical importance. Eur. Heart J. 2013, 34, 2674–2682. [Google Scholar] [CrossRef] [Green Version]
- Pipp, F.; Boehm, S.; Cai, W.J.; Adili, F.; Ziegler, B.; Karanovic, G.; Ritter, R.; Balzer, J.; Scheler, C.; Schaper, W.; et al. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1664–1668. [Google Scholar] [CrossRef] [Green Version]
- Eitenmuller, I.; Volger, O.; Kluge, A.; Troidl, K.; Barancik, M.; Cai, W.J.; Heil, M.; Pipp, F.; Fischer, S.; Horrevoets, A.J.; et al. The range of adaptation by collateral vessels after femoral artery occlusion. Circ. Res. 2006, 99, 656–662. [Google Scholar] [CrossRef]
- Troidl, K.; Tribulova, S.; Cai, W.-J.; Rüding, I.; Apfelbeck, H.; Schierling, W.; Troidl, C.; Schmitz-Rixen, T.; Schaper, W. Effects of endogenous nitric oxide and of DETA NONOate in arteriogenesis. J. Cardiovasc. Pharmacol. 2010, 55, 153–160. [Google Scholar] [CrossRef]
- Shireman, P. The chemokine system in arteriogenesis and hind limb ischemia. J. Vasc. Surg. 2007, 45, A48–A56. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, L.A.; Golenbock, D.; Bowie, A.G. The history of Toll-like receptors—redefining innate immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef]
- Lundberg, A.M.; Hansson, G.K. Innate immune signals in atherosclerosis. Clin. Immunol. 2010, 134, 5–24. [Google Scholar] [CrossRef]
- Mühlradt, P.F.; Kiess, M.; Meyer, H.; Sussmuth, R.; Jung, G. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J. Exp. Med. 1997, 185, 1951–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, O.; Kaufmann, A.; Grote, K.; Kawai, T.; Hoshino, K.; Morr, M.; Muhlradt, P.F.; Akira, S. Cutting edge: Preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 2000, 164, 554–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, O.; Kawai, T.; Muhlradt, P.F.; Morr, M.; Radolf, J.D.; Zychlinsky, A.; Takeda, K.; Akira, S. Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int. Immunol. 2001, 13, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Grote, K.; Schuett, H.; Salguero, G.; Grothusen, C.; Jagielska, J.; Drexler, H.; Muhlradt, P.F.; Schieffer, B. Toll-like receptor 2/6 stimulation promotes angiogenesis via GM-CSF as a potential strategy for immune defense and tissue regeneration. Blood 2010, 115, 2543–2552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grote, K.; Sonnenschein, K.; Kapopara, P.R.; Hillmer, A.; Grothusen, C.; Salguero, G.; Kotlarz, D.; Schuett, H.; Bavendiek, U.; Schieffer, B. Toll-like receptor 2/6 agonist macrophage-activating lipopeptide-2 promotes reendothelialization and inhibits neointima formation after vascular injury. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 2097–2104. [Google Scholar] [CrossRef] [Green Version]
- Grote, K.; Petri, M.; Liu, C.; Jehn, P.; Spalthoff, S.; Kokemuller, H.; Luchtefeld, M.; Tschernig, T.; Krettek, C.; Haasper, C.; et al. Toll-like receptor 2/6-dependent stimulation of mesenchymal stem cells promotes angiogenesis by paracrine factors. Eur. Cell. Mater. 2013, 26, 66–79. [Google Scholar] [CrossRef]
- Limbourg, A.; Korff, T.; Napp, L.C.; Schaper, W.; Drexler, H.; Limbourg, F.P. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat. Protoc. 2009, 4, 1737–1746. [Google Scholar] [CrossRef]
- Scholz, D.; Ito, W.; Fleming, I.; Deindl, E.; Sauer, A.; Wiesnet, M.; Busse, R.; Schaper, J.; Schaper, W. Ultrastructure and molecular histology of rabbit hind-limb collateral artery growth (arteriogenesis). Virchows Arch. 2000, 436, 257–270. [Google Scholar] [CrossRef]
- Ito, W.D.; Arras, M.; Winkler, B.; Scholz, D.; Schaper, J.; Schaper, W. Monocyte chemotactic protein-1 increases collateral and peripheral conductance after femoral artery occlusion. Circ. Res. 1997, 80, 829–837. [Google Scholar] [CrossRef]
- Buschmann, I.R.; Hoefer, I.E.; van Royen, N.; Katzer, E.; Braun-Dulleaus, R.; Heil, M.; Kostin, S.; Bode, C.; Schaper, W. GM-CSF: A strong arteriogenic factor acting by amplification of monocyte function. Atherosclerosis 2001, 159, 343–356. [Google Scholar] [CrossRef]
- Das, S.; Goldstone, A.B.; Wang, H.; Farry, J.; D’Amato, G.; Paulsen, M.J.; Eskandari, A.; Hironaka, C.E.; Phansalkar, R.; Sharma, B.; et al. A Unique Collateral Artery Development Program Promotes Neonatal Heart Regeneration. Cell 2019, 176, 1128–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chennupati, R.; Lamers, W.H.; Koehler, S.E.; De Mey, J.G. Endothelium-dependent hyperpolarization-related relaxations diminish with age in murine saphenous arteries of both sexes. Br. J. Pharmacol. 2013, 169, 1486–1499. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Qadura, M.; Terenzi, D.C.; Verma, S.; Al-Omran, M.; Hess, D.A. Concise Review: Cell Therapy for Critical Limb Ischemia: An Integrated Review of Preclinical and Clinical Studies. Stem Cells 2018, 36, 161–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighat, L.; Ionescu, C.N.; Regan, C.J.; Altin, S.E.; Attaran, R.R.; Mena-Hurtado, C.I. Review of the Current Basic Science Strategies to Treat Critical Limb Ischemia. Vasc. Endovascular Surg. 2019, 53, 316–324. [Google Scholar] [CrossRef]
- Seiler, C.; Pohl, T.; Wustmann, K.; Hutter, D.; Nicolet, P.A.; Windecker, S.; Eberli, F.R.; Meier, B. Promotion of collateral growth by granulocyte-macrophage colony-stimulating factor in patients with coronary artery disease: A randomized, double-blind, placebo-controlled study. Circulation 2001, 104, 2012–2017. [Google Scholar] [CrossRef] [Green Version]
- Van Royen, N.; Schirmer, S.H.; Atasever, B.; Behrens, C.Y.; Ubbink, D.; Buschmann, E.E.; Voskuil, M.; Bot, P.; Hoefer, I.; Schlingemann, R.O.; et al. START Trial: A pilot study on STimulation of ARTeriogenesis using subcutaneous application of granulocyte-macrophage colony-stimulating factor as a new treatment for peripheral vascular disease. Circulation 2005, 112, 1040–1046. [Google Scholar] [CrossRef]
- Van den Borne, S.W.; van de Schans, V.A.; Strzelecka, A.E.; Vervoort-Peters, H.T.; Lijnen, P.M.; Cleutjens, J.P.; Smits, J.F.; Daemen, M.J.; Janssen, B.J.; Blankesteijn, W.M. Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc. Res. 2009, 84, 273–282. [Google Scholar] [CrossRef] [Green Version]
- Cho, M.L.; Ju, J.H.; Kim, H.R.; Oh, H.J.; Kang, C.M.; Jhun, J.Y.; Lee, S.Y.; Park, M.K.; Min, J.K.; Park, S.H.; et al. Toll-like receptor 2 ligand mediates the upregulation of angiogenic factor, vascular endothelial growth factor and interleukin-8/CXCL8 in human rheumatoid synovial fibroblasts. Immunol. Lett. 2007, 108, 121–128. [Google Scholar] [CrossRef]
- Wang, W.; Xu, G.L.; Jia, W.D.; Ma, J.L.; Li, J.S.; Ge, Y.S.; Ren, W.H.; Yu, J.H.; Liu, W.B. Ligation of TLR2 by versican: A link between inflammation and metastasis. Arch. Med. Res. 2009, 40, 321–323. [Google Scholar] [CrossRef]
- West, X.Z.; Malinin, N.L.; Merkulova, A.A.; Tischenko, M.; Kerr, B.A.; Borden, E.C.; Podrez, E.A.; Salomon, R.G.; Byzova, T.V. Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 2010, 467, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Curtiss, L.K.; Black, A.S.; Bonnet, D.J.; Tobias, P.S. Atherosclerosis induced by endogenous and exogenous toll-like receptor (TLR)1 or TLR6 agonists. J. Lipid Res. 2012, 53, 2126–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troidl, K.; Schubert, C.; Vlacil, A.-K.; Chennupati, R.; Koch, S.; Schütt, J.; Oberoi, R.; Schaper, W.; Schmitz-Rixen, T.; Schieffer, B.; et al. The Lipopeptide MALP-2 Promotes Collateral Growth. Cells 2020, 9, 997. https://doi.org/10.3390/cells9040997
Troidl K, Schubert C, Vlacil A-K, Chennupati R, Koch S, Schütt J, Oberoi R, Schaper W, Schmitz-Rixen T, Schieffer B, et al. The Lipopeptide MALP-2 Promotes Collateral Growth. Cells. 2020; 9(4):997. https://doi.org/10.3390/cells9040997
Chicago/Turabian StyleTroidl, Kerstin, Christian Schubert, Ann-Kathrin Vlacil, Ramesh Chennupati, Sören Koch, Jutta Schütt, Raghav Oberoi, Wolfgang Schaper, Thomas Schmitz-Rixen, Bernhard Schieffer, and et al. 2020. "The Lipopeptide MALP-2 Promotes Collateral Growth" Cells 9, no. 4: 997. https://doi.org/10.3390/cells9040997





