The G119S Acetylcholinesterase (Ace-1) Target Site Mutation Confers Carbamate Resistance in the Major Malaria Vector Anopheles gambiae from Cameroon: A Challenge for the Coming IRS Implementation
Abstract
:1. Introduction
2. Methods
2.1. Mosquito Sampling
2.2. Insecticide Bioassays
2.3. Species Identification and Ace-1 G119S Mutation Genotyping
2.4. Ace-1 Gene Amplification, Sequencing, and Cloning
3. Results
3.1. Mosquito Collection and Species Molecular Identification
3.2. Insecticide Bioassay
3.3. Ace-1 Mutation Genotyping and Association with Insecticide Resistance Profile
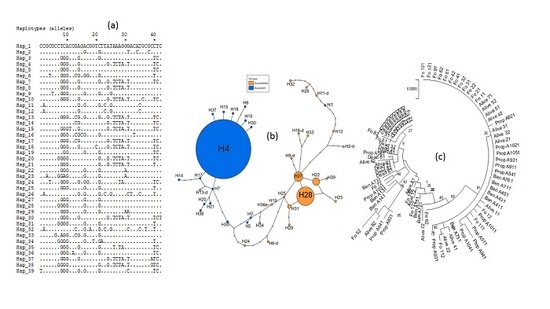
3.4. Genetic Diversity of Ace-1 in Bankeng
3.5. Investigation of Duplication of Ace-1 in Bankeng
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhatt, S.; Weiss, D.; Cameron, E.; Bisanzio, D.; Mappin, B.; Dalrymple, U.; Battle, K.; Moyes, C.; Henry, A.; Eckhoff, P. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015, 526, 207. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2018; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Hemingway, J. The role of vector control in stopping the transmission of malaria: Threats and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130431. [Google Scholar] [CrossRef]
- Riveron, J.M.; Tchouakui, M.; Mugenzi, L.; Menze, B.D.; Chiang, M.-C.; Wondji, C.S. Insecticide resistance in malaria vectors: An update at a global scale. In Towards Malaria Elimination—A Leap Forward; IntechOpen: London, UK, 2018. [Google Scholar]
- Churcher, T.S.; Lissenden, N.; Griffin, J.T.; Worrall, E.; Ranson, H. The impact of pyrethroid resistance on the efficacy and effectiveness of bednets for malaria control in Africa. eLife 2016, 5, e16090. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Plan for Insecticide Resistance Management in Malaria Vectors; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Akogbéto, M.C.; Padonou, G.G.; Gbénou, D.; Irish, S.; Yadouleton, A. Bendiocarb, a potential alternative against pyrethroid resistant Anopheles gambiae in Benin, West Africa. Malar. J. 2010, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Agossa, F.R.; Aïkpon, R.; Azondékon, R.; Govoetchan, R.; Padonou, G.G.; Oussou, O.; Oké-Agbo, F.; Akogbéto, M.C. Efficacy of various insecticides recommended for indoor residual spraying: Pirimiphos methyl, potential alternative to bendiocarb for pyrethroid resistance management in Benin, West Africa. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Asidi, A.N.; N’Guessan, R.; Koffi, A.A.; Curtis, C.F.; Hougard, J.-M.; Chandre, F.; Corbel, V.; Darriet, F.; Zaim, M.; Rowland, M.W. Experimental hut evaluation of bednets treated with an organophosphate (chlorpyrifos-methyl) or a pyrethroid (lambdacyhalothrin) alone and in combination against insecticide-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes. Malar. J. 2005, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Guillet, P.; N’guessan, R.; Darriet, F.; Traore-Lamizana, M.; Chandre, F.; Carnevale, P. Combined pyrethroid and carbamate ‘two-in-one’ treated mosquito nets: Field efficacy against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus. Med. Vet. Entomol. 2001, 15, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Tchicaya, E.S.; Nsanzabana, C.; Smith, T.A.; Donzé, J.; de Hipsl, M.L.; Tano, Y.; Müller, P.; Briët, O.J.; Utzinger, J.; Koudou, B.G. Micro-encapsulated pirimiphos-methyl shows high insecticidal efficacy and long residual activity against pyrethroid-resistant malaria vectors in central Côte d’Ivoire. Malar. J. 2014, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- N’Guessan, R.; Boko, P.; Odjo, A.; Chabi, J.; Akogbeto, M.; Rowland, M. Control of pyrethroid and DDT-resistant Anopheles gambiae by application of indoor residual spraying or mosquito nets treated with a long-lasting organophosphate insecticide, chlorpyrifos-methyl. Malar. J. 2010, 9, 44. [Google Scholar] [CrossRef]
- Akogbeto, M.; Padonou, G.G.; Bankole, H.S.; Gazard, D.K.; Gbedjissi, G.L. Dramatic decrease in malaria transmission after large-scale indoor residual spraying with bendiocarb in Benin, an area of high resistance of Anopheles gambiae to pyrethroids. Am. J. Trop. Med. Hyg. 2011, 85, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Charlwood, J.; Qassim, M.; Elnsur, E.; Donnelly, M.; Petrarca, V.; Billingsley, P.F.; Pinto, J.; Smith, T. The impact of indoor residual spraying with malathion on malaria in refugee camps in eastern Sudan. Acta Trop. 2001, 80, 1–8. [Google Scholar] [CrossRef]
- Hamel, M.J.; Otieno, P.; Bayoh, N.; Kariuki, S.; Were, V.; Marwanga, D.; Laserson, K.F.; Williamson, J.; Slutsker, L.; Gimnig, J. The combination of indoor residual spraying and insecticide-treated nets provides added protection against malaria compared with insecticide-treated nets alone. Am. J. Trop. Med. Hyg. 2011, 85, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Sharp, B.L.; Ridl, F.C.; Govender, D.; Kuklinski, J.; Kleinschmidt, I. Malaria vector control by indoor residual insecticide spraying on the tropical island of Bioko, Equatorial Guinea. Malar. J. 2007, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Aregawi, M.W.; Ali, A.S.; Al-Mafazy, A.-W.; Molteni, F.; Katikiti, S.; Warsame, M.; Njau, R.J.; Komatsu, R.; Korenromp, E.; Hosseini, M. Reductions in malaria and anaemia case and death burden at hospitals following scale-up of malaria control in Zanzibar, 1999–2008. Malar. J. 2011, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Bradley, D. The epidemiology of ricefield-associated diseases. In Vector-Borne Disease Control in Humans through Rice Agroecosystems Management; Experts on Environmental Management for Vector Control, Ed.; International Rice Research Institute/WHO/FAO/UNEP: Los Baños, Philippines, 1988; pp. 29–39. [Google Scholar]
- Dengela, D.; Seyoum, A.; Lucas, B.; Johns, B.; George, K.; Belemvire, A.; Caranci, A.; Norris, L.C.; Fornadel, C.M. Multi-country assessment of residual bio-efficacy of insecticides used for indoor residual spraying in malaria control on different surface types: Results from program monitoring in 17 PMI/USAID-supported IRS countries. Parasites Vectors 2018, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Kigozi, R.; Baxi, S.M.; Gasasira, A.; Sserwanga, A.; Kakeeto, S.; Nasr, S.; Rubahika, D.; Dissanayake, G.; Kamya, M.R.; Filler, S. Indoor residual spraying of insecticide and malaria morbidity in a high transmission intensity area of Uganda. PLoS ONE 2012, 7, e42857. [Google Scholar] [CrossRef] [PubMed]
- Padonou, G.G.; Gbedjissi, G.; Yadouleton, A.; Azondekon, R.; Razack, O.; Oussou, O.; Gnanguenon, V.; Rock, A.; Sezonlin, M.; Akogbeto, M. Decreased proportions of indoor feeding and endophily in Anopheles gambiae sl populations following the indoor residual spraying and insecticide-treated net interventions in Benin (West Africa). Parasites Vectors 2012, 5, 262. [Google Scholar] [CrossRef] [PubMed]
- Aïkpon, R.; Agossa, F.; Ossè, R.; Oussou, O.; Aïzoun, N.; Oké-Agbo, F.; Akogbéto, M. Bendiocarb resistance in Anopheles gambiae sl. populations from Atacora department in Benin, West Africa: A threat for malaria vector control. Parasites Vectors 2013, 6, 192. [Google Scholar] [CrossRef]
- Alou, L.P.A.; Koffi, A.A.; Adja, M.A.; Tia, E.; Kouassi, P.K.; Koné, M.; Chandre, F. Distribution of ace-1 R and resistance to carbamates and organophosphates in Anopheles gambiae ss populations from Côte d’Ivoire. Malar. J. 2010, 9, 167. [Google Scholar] [CrossRef]
- Dabiré, K.; Diabaté, A.; Namontougou, M.; Djogbenou, L.; Kengne, P.; Simard, F.; Bass, C.; Baldet, T. Distribution of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae s.l. populations from Burkina Faso (West Africa). Trop. Med. Int. Health 2009, 14, 396–403. [Google Scholar] [CrossRef]
- Essandoh, J.; Yawson, A.E.; Weetman, D. Acetylcholinesterase (Ace-1) target site mutation 119S is strongly diagnostic of carbamate and organophosphate resistance in Anopheles gambiae ss and Anopheles coluzzii across southern Ghana. Malar. J. 2013, 12, 404. [Google Scholar] [CrossRef] [PubMed]
- N’guessan, R.; Darriet, F.; Guillet, P.; Carnevale, P.; Traore-Lamizana, M.; Corbel, V.; Koffi, A.; Chandre, F. Resistance to carbosulfan in Anopheles gambiae from Ivory Coast, based on reduced sensitivity of acetylcholinesterase. Med. Vet. Entomol. 2003, 17, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Edi, C.V.; Koudou, B.G.; Jones, C.M.; Weetman, D.; Ranson, H. Multiple-insecticide resistance in Anopheles gambiae mosquitoes, Southern Côte d’Ivoire. Emerg. Infect. Dis. 2012, 18, 1508. [Google Scholar] [CrossRef] [PubMed]
- Aïkpon, R.; Sèzonlin, M.; Ossè, R.; Akogbéto, M. Evidence of multiple mechanisms providing carbamate and organophosphate resistance in field An. gambiae population from Atacora in Benin. Parasites Vectors 2014, 7, 568. [Google Scholar] [CrossRef]
- Dabiré, R.K.; Namountougou, M.; Diabaté, A.; Soma, D.D.; Bado, J.; Toé, H.K.; Bass, C.; Combary, P. Distribution and frequency of kdr mutations within Anopheles gambiae s.l. populations and first report of the Ace.1G119S mutation in Anopheles arabiensis from Burkina Faso (West Africa). PLoS ONE 2014, 9, e101484. [Google Scholar] [CrossRef]
- Weill, M.; Lutfalla, G.; Mogensen, K.; Chandre, F.; Berthomieu, A.; Berticat, C.; Pasteur, N.; Philips, A.; Fort, P.; Raymond, M. Comparative genomics: Insecticide resistance in mosquito vectors. Nature 2003, 423, 136. [Google Scholar] [CrossRef] [PubMed]
- Weill, M.; Malcolm, C.; Chandre, F.; Mogensen, K.; Berthomieu, A.; Marquine, M.; Raymond, M. The unique mutation in ace-1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect Mol. Biol. 2004, 13. [Google Scholar] [CrossRef]
- Antonio-Nkondjio, C.; Poupardin, R.; Tene, B.F.; Kopya, E.; Costantini, C.; Awono-Ambene, P.; Wondji, C.S. Investigation of mechanisms of bendiocarb resistance in Anopheles gambiae populations from the city of Yaoundé, Cameroon. Malar. J. 2016, 15, 424. [Google Scholar] [CrossRef]
- USAID/President’s Malaria Initiative. Malaria Operational Plan FY 2018 and FY 2019; USAID/PMI: Cameroon, 2018. [Google Scholar]
- Antonio-Nkondjio, C.; Sonhafouo-Chiana, N.; Ngadjeu, C.S.; Doumbe-Belisse, P.; Talipouo, A.; Djamouko-Djonkam, L.; Kopya, E.; Bamou, R.; Awono-Ambene, P.; Wondji, C.S. Review of the evolution of insecticide resistance in main malaria vectors in Cameroon from 1990 to 2017. Parasites Vectors 2017, 10, 472. [Google Scholar] [CrossRef]
- Antonio-Nkondjio, C.; Fossog, B.T.; Ndo, C.; Djantio, B.M.; Togouet, S.Z.; Awono-Ambene, P.; Costantini, C.; Wondji, C.S.; Ranson, H. Anopheles gambiae distribution and insecticide resistance in the cities of Douala and Yaoundé (Cameroon): Influence of urban agriculture and pollution. Malar. J. 2011, 10, 154. [Google Scholar] [CrossRef]
- Bigoga, J.D.; Nanfack, F.M.; Awono-Ambene, P.H.; Patchoké, S.; Atangana, J.; Otia, V.S.; Fondjo, E.; Moyou, R.S.; Leke, R.G. Seasonal prevalence of malaria vectors and entomological inoculation rates in the rubber cultivated area of Niete, South Region of Cameroon. Parasites Vectors 2012, 5, 197. [Google Scholar] [CrossRef]
- Nwane, P.; Etang, J.; Chouaїbou, M.; Toto, J.C.; Koffi, A.; Mimpfoundi, R.; Simard, F. Multiple insecticide resistance mechanisms in Anopheles gambiae s.l. populations from Cameroon, Central Africa. Parasites Vectors 2013, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Gillies, M.T.; de Meillon, B. The Anophelinae of Africa South of the Sahara (Ethiopian Zoogeographical Region); South African Institute for Medical Research: Johannesburg, South Africa, 1968. [Google Scholar]
- Gillies, M.; Coetzee, M. A Supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region); South African Institute for Medical Research: Johannesburg, South Africa, 1987. [Google Scholar]
- World Health Organization. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes, 2nd ed.; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Livak, K.J. Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 1984, 107, 611–634. [Google Scholar]
- Santolamazza, F.; Mancini, E.; Simard, F.; Qi, Y.; Tu, Z.; della Torre, A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malar. J. 2008, 7, 163. [Google Scholar] [CrossRef]
- Bass, C.; Nikou, D.; Vontas, J.; Williamson, M.S.; Field, L.M. Development of high-throughput real-time PCR assays for the identification of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae. Pestic. Biochem. Physiol. 2010, 96, 80–85. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Information Retrieval Ltd.: London, UK, 1979; pp. c1979–c2000. [Google Scholar]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nei, M.; Dudley, J.; Tamura, K. MEGA: A biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinforma. 2008, 9, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Múrias dos Santos, A.; Cabezas, M.P.; Tavares, A.I.; Xavier, R.; Branco, M. tcsBU: A tool to extend TCS network layout and visualization. Bioinformatics 2015, 32, 627–628. [Google Scholar] [CrossRef] [PubMed]
- Assogba, B.S.; Djogbénou, L.S.; Milesi, P.; Berthomieu, A.; Perez, J.; Ayala, D.; Chandre, F.; Makoutodé, M.; Labbé, P.; Weill, M. An ace-1 gene duplication resorbs the fitness cost associated with resistance in Anopheles gambiae, the main malaria mosquito. Sci. Rep. 2015, 5, 14529. [Google Scholar] [CrossRef]
- Menze, B.D.; Wondji, M.J.; Tchapga, W.; Tchoupo, M.; Riveron, J.M.; Wondji, C.S. Bionomics and insecticides resistance profiling of malaria vectors at a selected site for experimental hut trials in central Cameroon. Malar. J. 2018, 17, 317. [Google Scholar] [CrossRef] [PubMed]
- Djogbénou, L.; Dabiré, R.; Diabaté, A.; Kengne, P.; Akogbéto, M.; Hougard, J.M.; Chandre, F. Identification and geographic distribution of the ACE-1R mutation in the malaria vector Anopheles gambiae in south-western Burkina Faso, West Africa. Am. J. Trop. Med. Hyg. 2008, 78, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Hemingway, J.; Vontas, J.; Poupardin, R.; Raman, J.; Lines, J.; Schwabe, C.; Matias, A.; Kleinschmidt, I. Country-level operational implementation of the Global Plan for Insecticide Resistance Management. Proc. Natl. Acad. Sci. USA 2013, 110, 9397–9402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamgang, B.; Tchapga, W.; Ngoagouni, C.; Sangbakembi-Ngounou, C.; Wondji, M.; Riveron, J.M.; Wondji, C.S. Exploring insecticide resistance mechanisms in three major malaria vectors from Bangui in Central African Republic. Pathog. Glob. Health 2018, 112, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Nardini, L.; Hunt, R.H.; Dahan-Moss, Y.L.; Christie, N.; Christian, R.N.; Coetzee, M.; Koekemoer, L.L. Malaria vectors in the Democratic Republic of the Congo: The mechanisms that confer insecticide resistance in Anopheles gambiae and Anopheles funestus. Malar. J. 2017, 16, 448. [Google Scholar] [CrossRef] [PubMed]
- Sangba, M.L.O.; Sidick, A.; Govoetchan, R.; Dide-Agossou, C.; Ossè, R.A.; Akogbeto, M.; Ndiath, M.O. Evidence of multiple insecticide resistance mechanisms in Anopheles gambiae populations in Bangui, Central African Republic. Parasites Vectors 2017, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Vontas, J.; Grigoraki, L.; Morgan, J.; Tsakireli, D.; Fuseini, G.; Segura, L.; de Carvalho, J.N.; Nguema, R.; Weetman, D.; Slotman, M.A. Rapid selection of a pyrethroid metabolic enzyme CYP9K1 by operational malaria control activities. Proc. Natl. Acad. Sci. USA 2018, 115, 4619–4624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Anopheles gambiae 1000 Genomes Consortium. Genetic diversity of the African malaria vector Anopheles gambiae. Nature 2017, 552, 96. [Google Scholar] [CrossRef] [PubMed]
- Riveron, J.M.; Yunta, C.; Ibrahim, S.S.; Djouaka, R.; Irving, H.; Menze, B.D.; Ismail, H.M.; Hemingway, J.; Ranson, H.; Albert, A. A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol. 2014, 15, R27. [Google Scholar] [CrossRef] [PubMed]
- Weedall, G.D.; Mugenzi, L.M.; Menze, B.D.; Tchouakui, M.; Ibrahim, S.S.; Amvongo-Adjia, N.; Irving, H.; Wondji, M.J.; Tchoupo, M.; Djouaka, R. A cytochrome P450 allele confers pyrethroid resistance on a major African malaria vector, reducing insecticide-treated bednet efficacy. Sci. Trans. Med. 2019, 11, eaat7386. [Google Scholar] [CrossRef] [PubMed]
- Djogbenou, L.; Chandre, F.; Berthomieu, A.; Dabire, R.; Koffi, A.; Alout, H.; Weill, M. Evidence of introgression of the ace-1R mutation and of the ace-1 duplication in West African Anopheles gambiae s. s. PLoS ONE 2008, 3, e2172. [Google Scholar] [CrossRef] [PubMed]
- Djogbénou, L.; Labbé, P.; Chandre, F.; Pasteur, N.; Weill, M. Ace-1 duplication in Anopheles gambiae: A challenge for malaria control. Malar. J. 2009, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Labbé, P.; Berthomieu, A.; Berticat, C.; Alout, H.; Raymond, M.; Lenormand, T.; Weill, M. Independent duplications of the acetylcholinesterase gene conferring insecticide resistance in the mosquito Culex pipiens. Mol. Biol. Evol. 2007, 24, 1056–1067. [Google Scholar] [CrossRef] [PubMed]






| 2n | S | Ka | Ks | h | hd | π | D | D* | Fs | |
|---|---|---|---|---|---|---|---|---|---|---|
| Alive | 16 | 25 | 1 | 8 | 10 | 0.825 | 0.01 | −0.384 ns | −0.801 ns | 0.561 ns |
| Dead | 16 | 3 | 0 | 1 | 4 | 0.650 | 0.001 | 0.467 ns | −0.038 ns | −0.151 ns |
| F0 | 24 | 29 | 1 | 12 | 10 | 0.757 | 0.009 | −0.755 ns | −1.721 ns | 0.588 ns |
| Total | 56 | 35 | 1 | 14 | 23 | 0.853 | 0.01 | −0.507 ns | −2 ns | −3.695 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elanga-Ndille, E.; Nouage, L.; Ndo, C.; Binyang, A.; Assatse, T.; Nguiffo-Nguete, D.; Djonabaye, D.; Irving, H.; Tene-Fossog, B.; Wondji, C.S. The G119S Acetylcholinesterase (Ace-1) Target Site Mutation Confers Carbamate Resistance in the Major Malaria Vector Anopheles gambiae from Cameroon: A Challenge for the Coming IRS Implementation. Genes 2019, 10, 790. https://doi.org/10.3390/genes10100790
Elanga-Ndille E, Nouage L, Ndo C, Binyang A, Assatse T, Nguiffo-Nguete D, Djonabaye D, Irving H, Tene-Fossog B, Wondji CS. The G119S Acetylcholinesterase (Ace-1) Target Site Mutation Confers Carbamate Resistance in the Major Malaria Vector Anopheles gambiae from Cameroon: A Challenge for the Coming IRS Implementation. Genes. 2019; 10(10):790. https://doi.org/10.3390/genes10100790
Chicago/Turabian StyleElanga-Ndille, Emmanuel, Lynda Nouage, Cyrille Ndo, Achille Binyang, Tatiane Assatse, Daniel Nguiffo-Nguete, Doumani Djonabaye, Helen Irving, Billy Tene-Fossog, and Charles S. Wondji. 2019. "The G119S Acetylcholinesterase (Ace-1) Target Site Mutation Confers Carbamate Resistance in the Major Malaria Vector Anopheles gambiae from Cameroon: A Challenge for the Coming IRS Implementation" Genes 10, no. 10: 790. https://doi.org/10.3390/genes10100790