Functional Study on Cytochrome P450 in Response to L(−)-Carvone Stress in Bursaphelenchus xylophilus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pharmaceutical Preparation and Nematode Culture
2.2. RNA Isolation and Gene Acquisition
2.3. Structural Analysis of Bx-cyp29A3
2.4. Functional Characterization of Bx-cyp29A3
2.5. Statistical Analysis
3. Results
3.1. Gene Amplification and Identification of Bx-cyp29A3
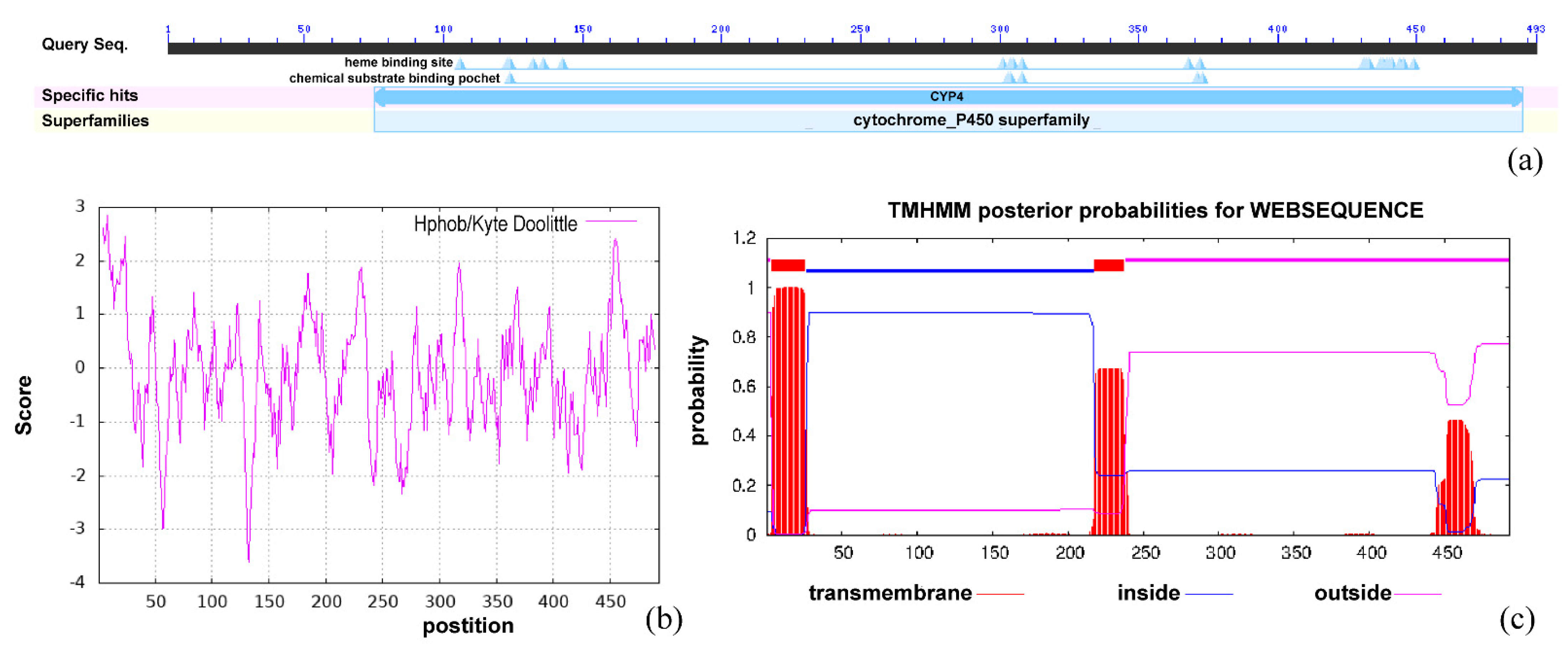
3.2. Structural and Functional Analysis of Bx-CYP29A3
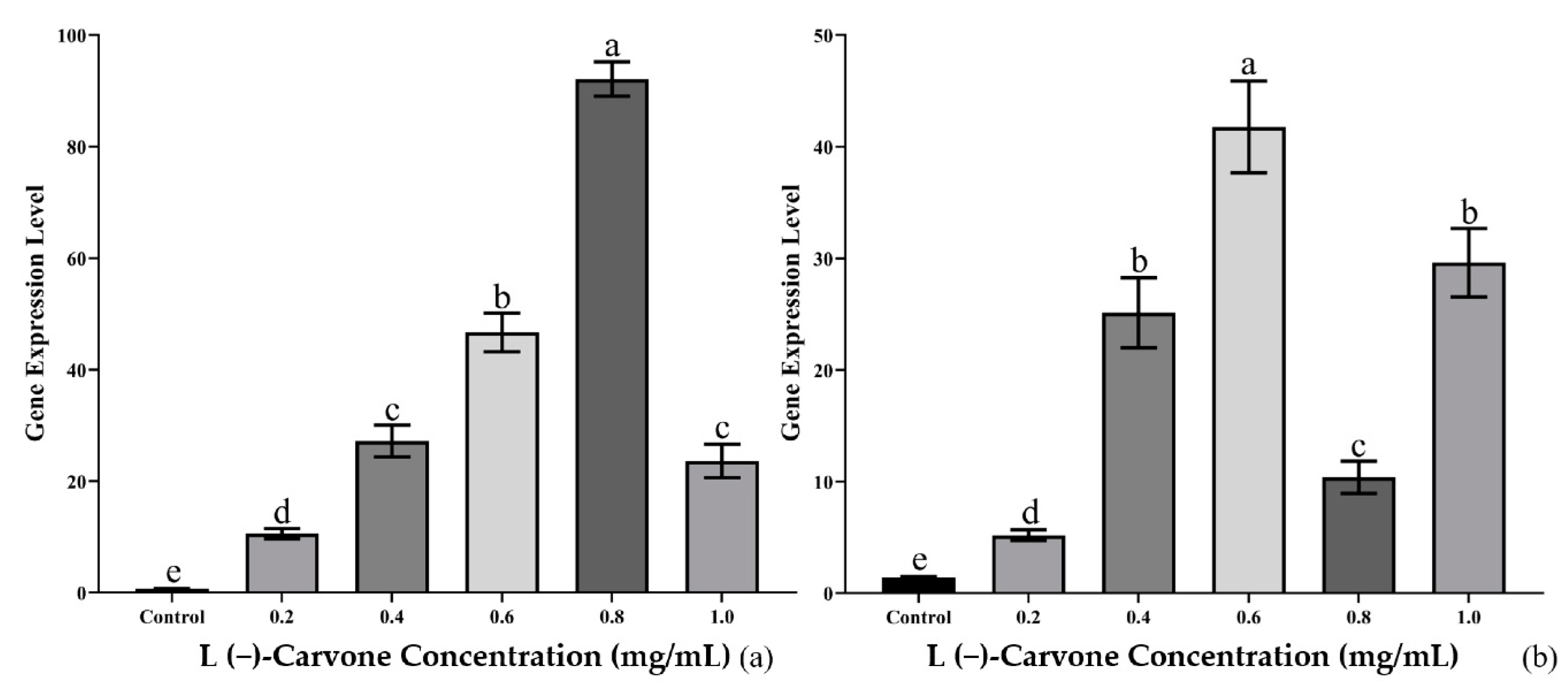
3.3. Effect of Bx-cyp29A3 Silencing on the PWN Response to L (−)-Carvone Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ugawa, S.; Fukuda, K. Effect of aerial insecticide spraying on pine wilt disease in central Japan. For. Pathol. 2008, 38, 16–28. [Google Scholar] [CrossRef]
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Pine Wilt Disease: A threat to European forestry. Eur. J. Plant Pathol. 2012, 133, 497. [Google Scholar] [CrossRef]
- Hao, Z.; Huang, J.; Li, X.; Sun, H.; Fang, G. A multi-point aggregation trend of the outbreak of pine wilt disease in China over the past 20 years. For. Ecol. Manag. 2022, 505, 119890. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, J.; Yan, J.; Fang, G. Economic Loss of Pine Wood Nematode Disease in Mainland China from 1998 to 2017. Forests 2020, 11, 1042. [Google Scholar] [CrossRef]
- Seo, S.M.; Kim, J.; Koh, S.H.; Ahn, Y.J.; Park, I.K. Nematicidal Activity of Natural Ester Compounds and Their Analogues against Pine Wood Nematode, Bursaphelenchus xylophilus. J. Agric. Food Chem. 2014, 62, 9103–9108. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Lee, H.R.; Kim, D.S.; Kwon, J.H.; Huh, M.J.; Park, I.K. Emamectin benzoate 9.7% SL as a new formulation for a trunk-injections against pine wood nematode, Bursaphelenchus xylophilus. J. For. Res. 2020, 31, 1399–1403. [Google Scholar] [CrossRef]
- Pina, L.T.S.; Serafini, M.R.; Oliveira, M.A.; Sampaio, L.A.; Guimaraes, J.O.; Guimaraes, A.G. Carvone and its pharmacological activities: A systematic review. Phytochemistry 2022, 196, 113080. [Google Scholar] [CrossRef]
- Cao, D.; Liu, J.; Zhao, Z.; Yan, X.; Wang, W.; Wei, J. Chemical Compounds Emitted from Mentha spicata Repel Aromia bungii Females. Insects 2022, 13, 244. [Google Scholar] [CrossRef]
- Jin, C.; Deng, X.; Zhou, Y.; Zhou, X. Design, Synthesis, Crystal Structure, and Fungicidal Activity of L-Carvone Derivatives Containing an Oxime Ester Moiety. Chin. J. Org. Chem. 2021, 41, 2008–2018. [Google Scholar] [CrossRef]
- Bouyahya, A.; Mechchate, H.; Benali, T.; Ghchime, R.; Charfi, S.; Balahbib, A.; Burkov, P.; Shariati, M.A.; Lorenzo, J.M.; El Omari, N. Health Benefits and Pharmacological Properties of Carvone. Biomolecules 2021, 11, 1803. [Google Scholar] [CrossRef]
- Sanli, A. Caraway (Carum carvi L.) seed treatments and storage temperature influences potato tuber quality during storage. J. Appl. Bot. Food Qual. 2016, 89, 258–263. [Google Scholar] [CrossRef]
- Ali, R.; Rooman, M.; Mussarat, S.; Norin, S.; Ali, S.; Adnan, M.; Khan, S.N. A Systematic Review on Comparative Analysis, Toxicology, and Pharmacology of Medicinal Plants against Haemonchus contortus. Front. Pharmacol. 2021, 12, 644027. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, B.B.; Buzatti, A.; Chaaban, A.; Pritsch, I.C.; dos Anjos, A.; Cipriano, R.R.; Deschamps, C.; Molento, M.B. Mentha villosa Hubs., M. × piperita and their bioactives against gastrointestinal nematodes of ruminants and the potential as drug enhancers. Vet. Parasitol. 2021, 289, 109317. [Google Scholar] [CrossRef] [PubMed]
- Eloh, K.; Kpegba, K.; Sasanelli, N.; Koumaglo, H.K.; Caboni, P. Nematicidal activity of some essential plant oils from tropical West Africa. Int. J. Pest Manag. 2020, 66, 131–141. [Google Scholar] [CrossRef]
- Caboni, P.; Saba, M.; Tocco, G.; Casu, L.; Murgia, A.; Maxia, A.; Menkissoglu-Spiroudi, U.; Ntalli, N. Nematicidal Activity of Mint Aqueous Extracts against the Root-Knot Nematode Meloidogyne incognita. J. Agric. Food Chem. 2013, 61, 9784–9788. [Google Scholar] [CrossRef]
- Oka, Y.; Nacar, S.; Putievsky, E.; Ravid, U.; Yaniv, Z.; Spiegel, Y. Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology 2000, 90, 710–715. [Google Scholar] [CrossRef] [Green Version]
- Paine, M.F.; Hart, H.L.; Zeldin, D.C.; Watkins, P.B. The human intestinal cytochrome P450 “pie”. Drug Metab. Rev. 2004, 36, 90. [Google Scholar] [CrossRef] [Green Version]
- Denisov, I.G.; Makris, T.M.; Sligar, S.G.; Schlichting, I. Structure and chemistry of cytochrome P450. Chem. Rev. 2005, 105, 2253–2277. [Google Scholar] [CrossRef]
- Herzog, K.; Bracco, P.; Onoda, A.; Hayashi, T.; Hoffmann, K.; Schallmey, A. Enzyme-substrate complex structures of CYP154C5 shed light on its mode of highly selective steroid hydroxylation. Acta Crystallogr. Sect. D—Struct. Biol. 2014, 70, 2875–2889. [Google Scholar] [CrossRef]
- Monostory, K.; Dvorak, Z. Steroid Regulation of Drug-Metabolizing Cytochromes P450. Curr. Drug Metab. 2011, 12, 154–172. [Google Scholar] [CrossRef]
- Glass, S.M.; Guengerich, F.P. Cellular retinoid-binding proteins transfer retinoids to human cytochrome P450 27C1 for desaturation. J. Biol. Chem. 2021, 297, 101142. [Google Scholar] [CrossRef] [PubMed]
- Szenasi, A.; Amrein, K.; Czeiter, E.; Szarka, N.; Toth, P.; Koller, A. Molecular Pathomechanisms of Impaired Flow-Induced Constriction of Cerebral Arteries Following Traumatic Brain Injury: A Potential Impact on Cerebral Autoregulation. Int. J. Mol. Sci. 2021, 22, 6624. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, J.C.; Giari, L.; Guerranti, C.; Tognon, M.; Castaldelli, G.; Fano, E.A.; Martini, F. Environmental doses of perfluorooctanoic acid change the expression of genes in target tissues of common carp. Environ. Toxicol. Chem. 2018, 37, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Hammerer, L.; Winkler, C.K.; Kroutil, W. Regioselective Biocatalytic Hydroxylation of Fatty Acids by Cytochrome P450s. Catal. Lett. 2018, 148, 787–812. [Google Scholar] [CrossRef] [Green Version]
- Mansuy, D. Biocatalysis and substrate chemodiversity: Adaptation of aerobic living organisms to their chemical environment. Catal. Today 2008, 138, 2–8. [Google Scholar] [CrossRef]
- Lu, K.; Song, Y.; Zeng, R. The role of cytochrome P450-mediated detoxification in insect adaptation to xenobiotics. Curr. Opin. Insect Sci. 2021, 43, 103–107. [Google Scholar] [CrossRef]
- Nauen, R.; Bass, C.; Feyereisen, R.; Vontas, J. The Role of Cytochrome P450s in Insect Toxicology and Resistance. Annu. Rev. Entomol. 2022, 67, 105–124. [Google Scholar] [CrossRef]
- Wan, L.; Zhou, A.; Xiao, W.; Zou, B.; Jiang, Y.; Xiao, J.; Deng, C.; Zhang, Y.; Huang, Z.-Y.; Bu, C.-F.; et al. Cytochrome P450 monooxygenase genes in the wild silkworm, Bombyx mandarina. PeerJ 2021, 9, 10818. [Google Scholar] [CrossRef]
- Harrop, T.W.R.; Denecke, S.; Yang, Y.T.; Chan, J.; Daborn, P.J.; Perry, T.; Batterham, P. Evidence for activation of nitenpyram by a mitochondrial cytochrome P450 in Drosophila melanogaster. Pest Manag. Sci. 2018, 74, 1616–1622. [Google Scholar] [CrossRef]
- Lu, K.; Cheng, Y.; Li, W.; Li, Y.; Zeng, R.; Song, Y. Activation of CncC pathway by ROS burst regulates cytochrome P450 CYP6AB12 responsible for lambda-cyhalothrin tolerance in Spodoptera litura. J. Hazard. Mater. 2020, 387, 121698. [Google Scholar] [CrossRef]
- Zhang, B.Z.; Su, X.; Zhen, G.A.; Lu, L.Y.; Li, Y.S.; Ge, X.; Chen, D.M.; Pei, Z.; Shi, M.W.; Chen, X.L. Silencing of Cytochrome P450 in Spodoptera frugiperda (Lepidoptera: Noctuidae) by RNA Interference Enhances Susceptibility to Chlorantraniliprole. J. Insect Sci. 2020, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, X.; Liu, Z.; Wang, F.; Fan, L.; Wu, C.; Yao, Y. Knockdown of CYP301B1 and CYP6AX1v2 increases the susceptibility of the brown planthopper to beta-asarone, a potential plant-derived insecticide. Int. J. Biol. Macromol. 2021, 171, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Martis, M.M.; Tarbiat, B.; Tyden, E.; Jansson, D.S.; Hoglund, J. RNA-Seq de novo assembly and differential transcriptome analysis of the nematode Ascaridia galli in relation to in vivo exposure to flubendazole. PLoS ONE 2017, 12, e0185182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.Y.M.; Alshagga, M.; Kong, C.; Alshawsh, M.A.; Alshehade, S.A.; Pan, Y. CYP35 family in Caenorhabditis elegans biological processes: Fatty acid synthesis, xenobiotic metabolism, and stress responses. Arch. Toxicol. 2022, 96, 3163–3174. [Google Scholar] [CrossRef] [PubMed]
- Blomquist, G.J.; Tittiger, C.; MacLean, M.; Keeling, C.I. Cytochromes P450: Terpene detoxification and pheromone production in bark beetles. Curr. Opin. Insect Sci. 2021, 43, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hao, X.; Xu, J.; Wang, B.; Ma, W.; Liu, X.; Ma, L. Cytochrome P450 metabolism mediates low-temperature resistance in pinewood nematode. FEBS Open Bio 2020, 10, 1171–1179. [Google Scholar] [CrossRef]
- Larigot, L.; Mansuy, D.; Borowski, I.; Coumoul, X.; Dairou, J. Cytochromes P450 of Caenorhabditis elegans: Implication in Biological Functions and Metabolism of Xenobiotics. Biomolecules 2022, 12, 342. [Google Scholar] [CrossRef]
- Harris, T.W.; Arnaboldi, V.; Cain, S.; Chan, J.; Chen, W.J.; Cho, J.; Davis, P.; Gao, S.; Grove, C.A.; Kishore, R.; et al. WormBase: A modern Model Organism Information Resource. Nucleic Acids Res. 2020, 48, 762–767. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Wang, X.; Cui, J.; Deng, X.; Zhang, X. Adaptation of pine wood nematode Bursaphelenchus xylophilus to beta-pinene stress. BMC Genom. 2020, 21, 478. [Google Scholar] [CrossRef]
- Chen, J.; Hao, X.; Wang, B.; Ma, L. Transcriptomics and coexpression network profiling of the effects of levamisole hydrochloride on Bursaphelenchus xylophilus. Pestic. Biochem. Physiol. 2022, 181, 105019. [Google Scholar] [CrossRef]
- Qiu, X.; Yang, L.; Ye, J.; Wang, W.; Zhao, T.; Hu, H.; Zhou, G. Silencing of cyp-33C9 Gene Affects the Reproduction and Pathogenicity of the Pine Wood Nematode, Bursaphelenchus xylophilus. Int. J. Mol. Sci. 2019, 20, 4520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, V.D.; Carletto, J.S.; Carasek, E.; Stambuk, B.U.; Nascimento, M.d.G. Asymmetric reduction of (4S)-(+)-carvone catalyzed by baker’s yeast: A green method for monitoring the conversion based on liquid-liquid-liquid microextraction with polypropylene hollow fiber membranes. Process Biochem. 2013, 48, 1159–1165. [Google Scholar] [CrossRef] [Green Version]
- Hammadeh, H.H.; Serrano, A.; Wernet, V.; Stomberg, N.; Hellmeier, D.; Weichert, M.; Brandt, U.; Sieg, B.; Kanofsky, K.; Hehl, R.; et al. A dialogue-like cell communication mechanism is conserved in filamentous ascomycete fungi and mediates interspecies interactions. Proc. Natl. Acad. Sci. USA 2022, 119, e2112518119. [Google Scholar] [CrossRef] [PubMed]
- Giblin-Davis, R.M.; Mundo-Ocampo, M.; Baldwin, J.G.; Norden, B.B.; Batra, S.W. Description of Bursaphelenchus abruptusn sp. (Nemata: Aphelenchoididae), an Associate of a Digger Bee. J. Nematol. 1993, 25, 161–172. [Google Scholar] [PubMed]
- Kanzaki, N.; Futai, K. Description and phylogeny of Bursaphelenchus luxuriosaen sp. (Nematoda: Aphelenchoididae) isolated from Acalolepta luxuriosa (Coleoptera: Cerambycidae). Nematology 2003, 5, 565–572. [Google Scholar] [CrossRef]
- Tintori, S.C.; Sloat, S.A.; Rockman, M.V. Rapid Isolation of Wild Nematodes by Baermann Funnel. Jove—J. Vis. Exp. 2022, 179, e63287. [Google Scholar] [CrossRef]
- Martinez, M.A.Q.; Matus, D.Q. Auxin-mediated Protein Degradation in Caenorhabditis elegans. Bio-Protocol 2020, 10, e3589. [Google Scholar] [CrossRef]
- Hu, X.; Xu, S.; Chen, Y.; Gao, Z.; Li, Y.; Hu, J.; Huang, X.; Zhang, Y.; Jiang, X.; Li, L.; et al. Depletion of Ars2 inhibits cell proliferation and leukemogenesis in acute myeloid leukemia by modulating the miR-6734-3p/p27 axis. Leukemia 2019, 33, 1090–1101. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.; Wang, B.; Chen, J.; Wang, B.; Xu, J.; Pan, J.; Ma, L. Molecular characterization and functional analysis of multidrug resistance-associated genes of Pinewood nematode (Bursaphelenchus xylophilus) for nematicides. Pestic. Biochem. Physiol. 2021, 177, 104902. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, 200–203. [Google Scholar] [CrossRef]
- Kurotani, A.; Yamada, Y.; Sakurai, T. Alga-PrAS (Algal Protein Annotation Suite): A Database of Comprehensive Annotation in Algal Proteomes. Plant Cell Physiol. 2017, 58, e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girija, A.S.S. Delineating the Immuno-Dominant Antigenic Vaccine Peptides Against gacS-Sensor Kinase in Acinetobacter baumannii: Anin silicoInvestigational Approach. Front. Microbiol. 2020, 11, 2078. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; He, X.; Liu, X.; Zhang, H.; Shen, Z.; Shi, Y.; Liu, X. A novel missense variant in cathepsin C gene leads to PLS in a Chinese patient: A case report and literature review. Mol. Genet. Genom. Med. 2021, 9, e1686. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhan, C.; Varney, K.; Yuan, W.; Zhao, L.; Lu, W. Interrogation of MDM2 Phosphorylation in p53 Activation Using Native Chemical Ligation: The Functional Role of Ser17 Phosphorylation in MDM2 Reexamined. J. Am. Chem. Soc. 2012, 134, 6855–6864. [Google Scholar] [CrossRef] [Green Version]
- Sevindik, E. In Silico Analysis of Putative Polyphenol Oxidases in Olive Using Bioinformatics Tools. Bangladesh J. Bot. 2019, 48, 17–24. [Google Scholar] [CrossRef]
- Geourjon, C.; Deleage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput. Appl. Biosci. 1995, 11, 681–684. [Google Scholar] [CrossRef]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Y.; Pan, D.; Xv, Y.; Shen, W. Genetic Analysis of a Pedigree With Antithrombin and Prothrombin Compound Mutations and Antithrombin Heterozygotes. Front. Genet. 2022, 13, 832582. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, R.; Li, D.; Wang, F.; Jiang, S.; Wang, J. Trehalose in pine wood nematode participates in DJ3 formation and confers resistance to low-temperature stress. BMC Genom. 2021, 22, 524. [Google Scholar] [CrossRef]
- Wang, B.; Ma, L.; Wang, F.; Wang, B.; Hao, X.; Xu, J.; Ma, Y. Low Temperature Extends the Lifespan of Bursaphelenchus xylophilus through the cGMP Pathway. Int. J. Mol. Sci. 2017, 18, 2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, G.K.; Hsu, M.H.; Shock, L.S.; Johnson, E.F.; Hackett, J.C. Noncovalent interactions dominate dynamic heme distortion in cytochrome P450 4B1. J. Biol. Chem. 2018, 293, 11433–11446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Chen, Q.; Zhang, R.; Li, D.; Ling, Y.; Song, R. The anti-phytoalexin gene Bx-cathepsin W supports the survival of Bursaphelenchus xylophilus under Pinus massoniana phytoalexin stress. BMC Genom. 2019, 20, 779. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Liu, W.; Wan, F.; Lv, Z.; Guo, J. The Role of Cytochrome P450 4C1 and Carbonic Anhydrase 3 in Response to Temperature Stress in Bemisia tabaci. Insects 2021, 12, 1071. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Wu, X.Q.; Ye, J.R.; Huang, L. Molecular Characterization and Functional Analysis of Three Pathogenesis-Related Cytochrome P450 Genes from Bursaphelenchus xylophilus (Tylenchida: Aphelenchoidoidea). Int. J. Mol. Sci. 2015, 16, 5216–5234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Carvalho, C.; da Fonseca, M.M.R. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006, 95, 413–422. [Google Scholar] [CrossRef]
- Yang, X.; Han, H.; Li, B.; Zhang, D.; Zhang, Z.; Xie, Y. Fumigant toxicity and physiological effects of spearmint (Mentha spicata, Lamiaceae) essential oil and its major constituents against Reticulitermes dabieshanensis. Ind. Crops Prod. 2021, 171, 113894. [Google Scholar] [CrossRef]
- Jayaram, C.S.; Chauhan, N.; Dolma, S.K.; Reddy, S.G.E. Chemical Composition and Insecticidal Activities of Essential Oils against the Pulse Beetle. Molecules 2022, 27, 568. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Prajapati, V.; Kumar, S. Bioactivities of l-carvone, d-carvone, and dihydrocarvone toward three stored product beetles. J. Econ. Entomol. 2003, 96, 1594–1601. [Google Scholar] [CrossRef]
- Klys, M.; Izdebska, A.; Malejky-Klusek, N. Repellent Effect of the Caraway Carum carvi L. on the Rice Weevil Sitophilus oryzae L. (Coleoptera, Dryophthoridae). Insects 2020, 11, 836. [Google Scholar] [CrossRef]
- Kroona, L.; Warfvinge, G.; Isaksson, M.; Ahlgren, C.; Dahlin, J.; Sorensen, O.; Bruze, M. Quantification of L-carvone in toothpastes available on the Swedish market. Contact Dermat. 2017, 77, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, E.; Andersen, K.E.; Carlsen, L.; Egsgaard, H. Carvone—An Overlooked Contact Allergen Cross-Reacting with Sesquiterpene Lactones. Contact Dermat. 1993, 29, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.M.; dos Santos, G.F.; Saraiva, N.N.; Trapp, M.A.; de Mattos, M.C.; Maria da Conceição, F.O.; Rodrigues-Filho, E. New fungi for whole-cell biotransformation of carvone enantiomers. Novel p-menthane-2,8,9-triols production. Appl. Catal. A—Gen. 2013, 468, 88–94. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; Dekant, W.; et al. RIFM fragrance ingredient safety assessment, l-carvone, CAS Registry Number 6485-40-1. Food Chem. Toxicol. 2022, 163 (Suppl. S1), 112975. [Google Scholar] [CrossRef]




| Scientific Name | Accession | Score | E-Value | Per. Ident |
|---|---|---|---|---|
| D. destructor | KAI1718381.1 | 379 | 3.00 × 10−125 | 39.69% |
| A. avenae | KAH7708527.1 | 373 | 4.00 × 10−123 | 43.78% |
| Caenorhabditis brenneri | EGT47445.1 | 348 | 1.00 × 10−113 | 38.45% |
| C. remanei | XP_003098490.1 | 345 | 2.00 × 10−112 | 39.48% |
| C. briggsae | XP_045095724.1 | 337 | 6.00 × 10−109 | 38.26% |
| C. elegans | NP_505490.2 | 333 | 1.00 × 10−107 | 38.81% |
| Strongyloides ratti | XP_024510577.1 | 321 | 7.00 × 10−103 | 37.30% |
| Ancylostoma caninum | RCN40602.1 | 308 | 6.00 × 10−98 | 37.23% |
| Toxocara canis | KHN86065.1 | 300 | 9.00 × 10−95 | 38.06% |
| Pristionchus pacificus | KAF8363934.1 | 293 | 6.00 × 10−92 | 34.84% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Hao, X.; Tan, R.; Li, Y.; Wang, B.; Pan, J.; Ma, W.; Ma, L. Functional Study on Cytochrome P450 in Response to L(−)-Carvone Stress in Bursaphelenchus xylophilus. Genes 2022, 13, 1956. https://doi.org/10.3390/genes13111956
Chen J, Hao X, Tan R, Li Y, Wang B, Pan J, Ma W, Ma L. Functional Study on Cytochrome P450 in Response to L(−)-Carvone Stress in Bursaphelenchus xylophilus. Genes. 2022; 13(11):1956. https://doi.org/10.3390/genes13111956
Chicago/Turabian StyleChen, Jie, Xin Hao, Ruina Tan, Yang Li, Bowen Wang, Jialiang Pan, Wei Ma, and Ling Ma. 2022. "Functional Study on Cytochrome P450 in Response to L(−)-Carvone Stress in Bursaphelenchus xylophilus" Genes 13, no. 11: 1956. https://doi.org/10.3390/genes13111956