Utilization of Genetic Resources, Genetic Diversity and Genetic Variability for Selecting New Restorer Lines of Rice (Oryza sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Field Evaluation
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Test Cross Experiment
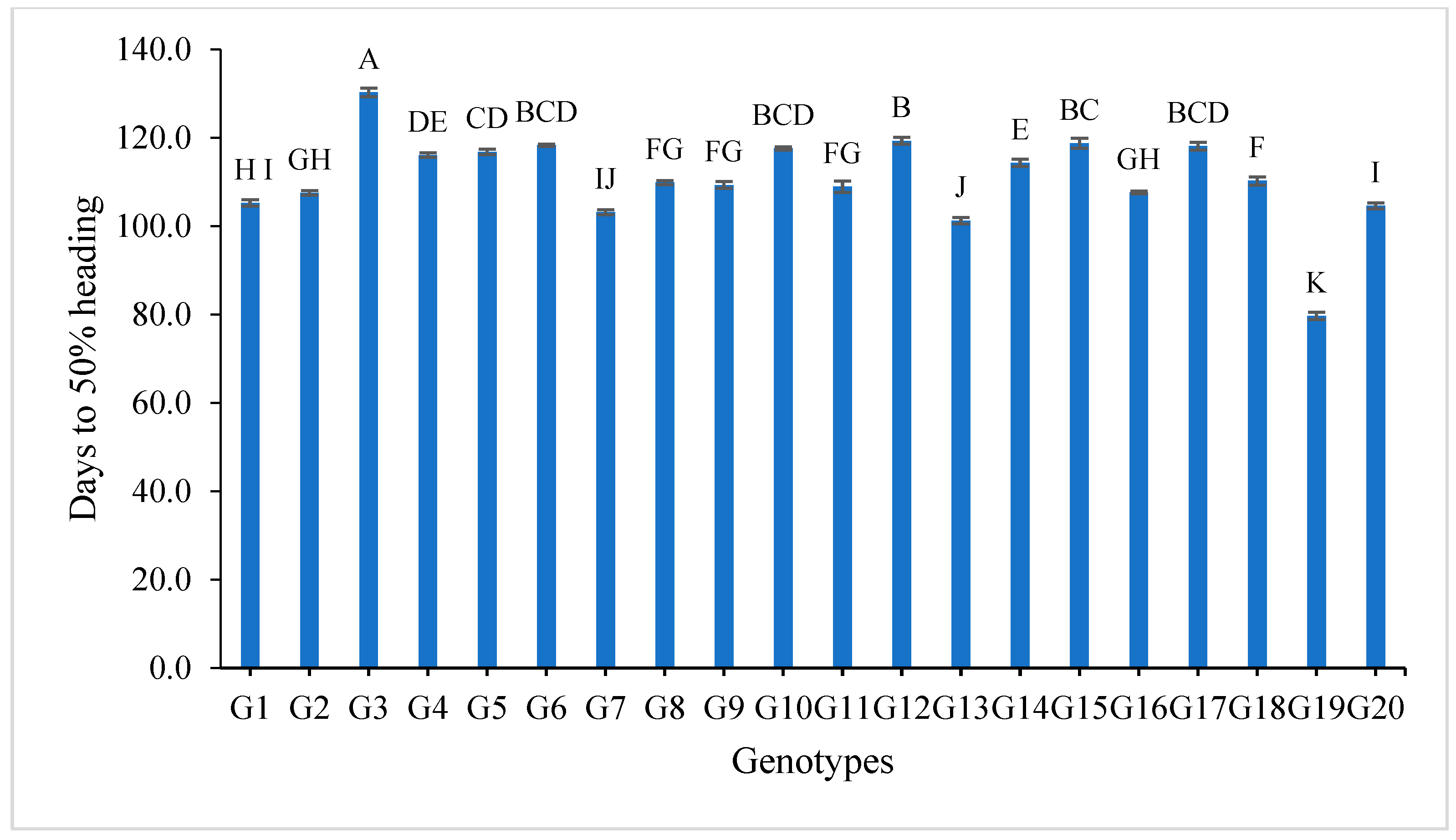
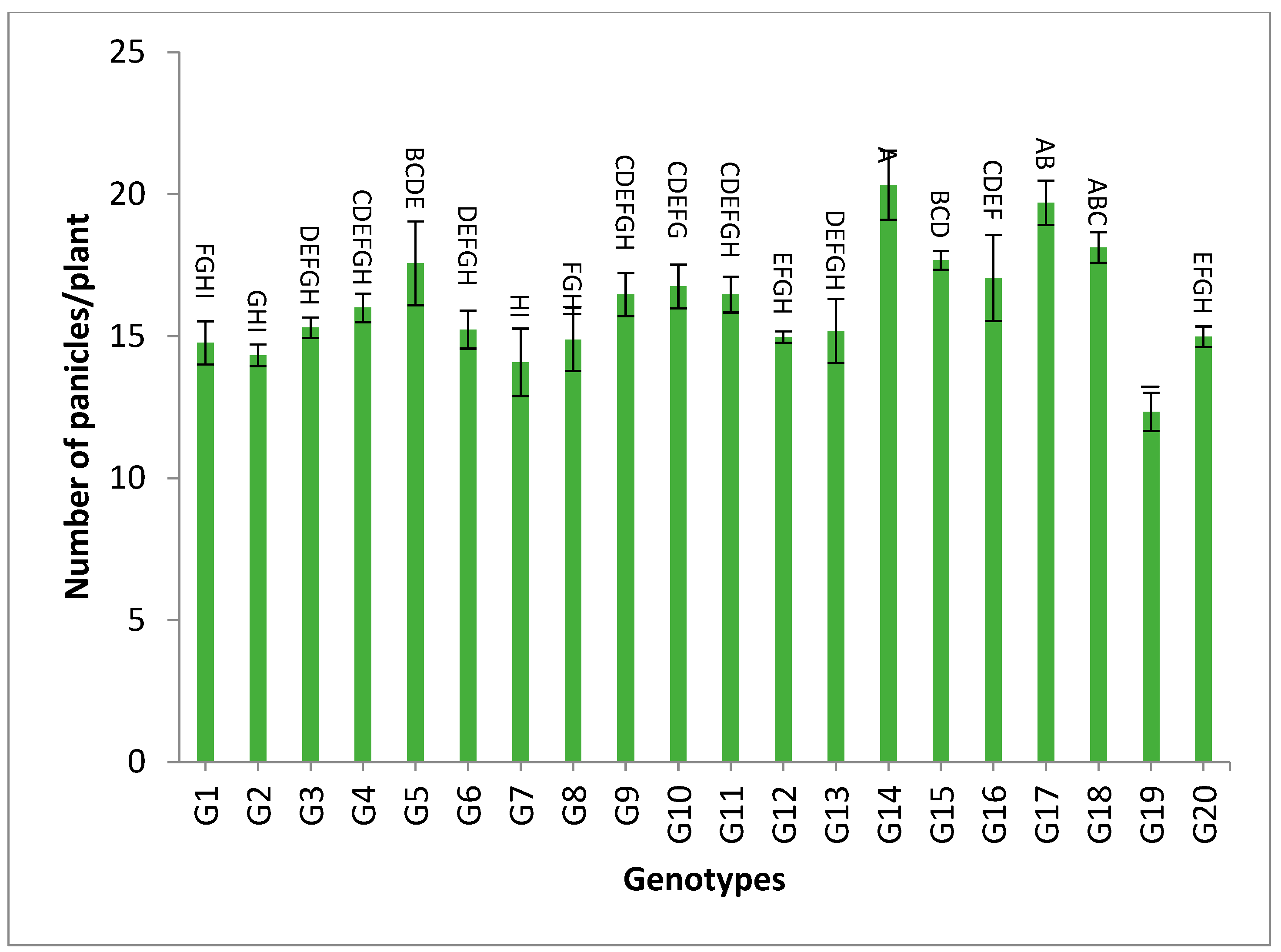
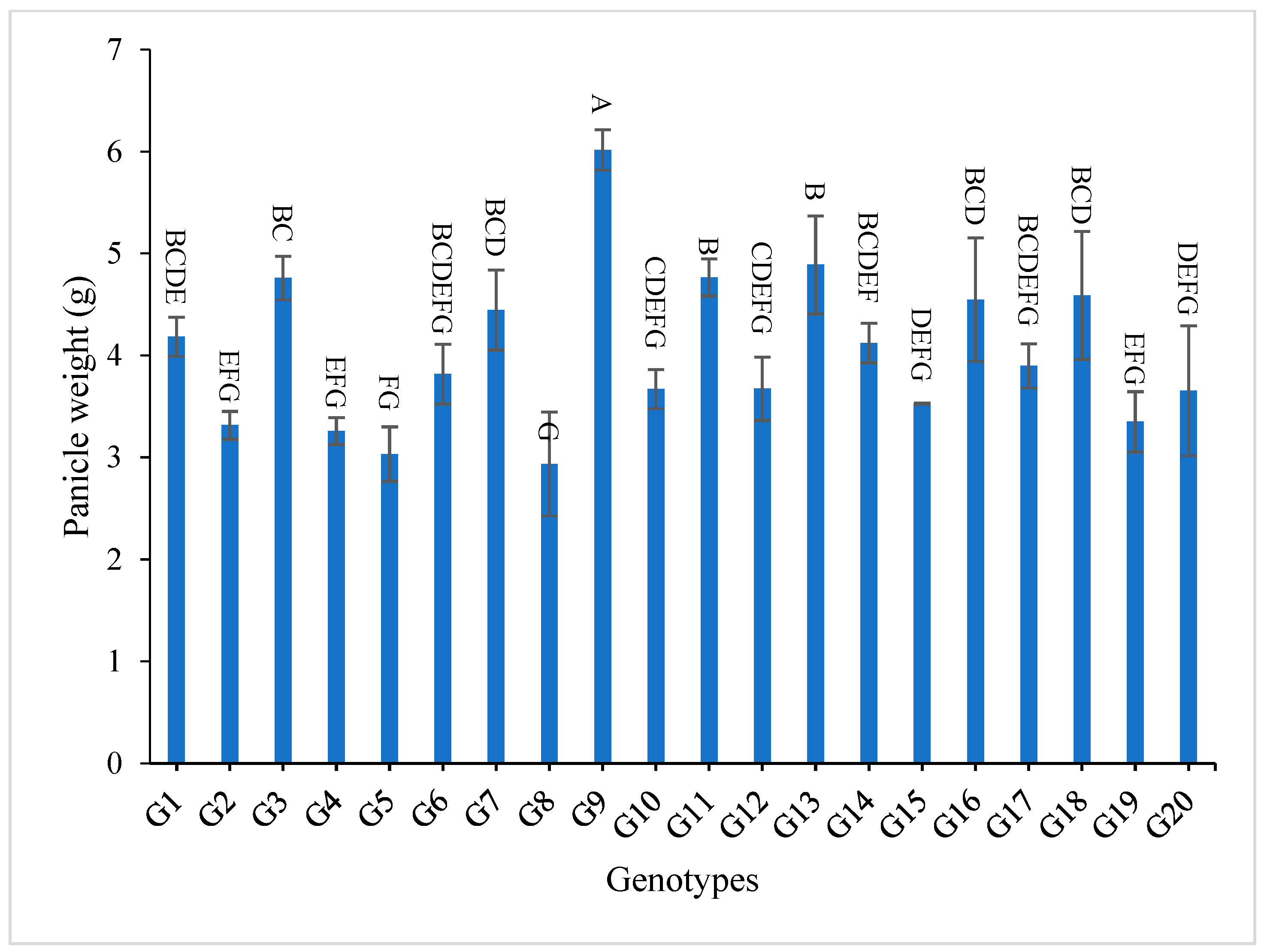
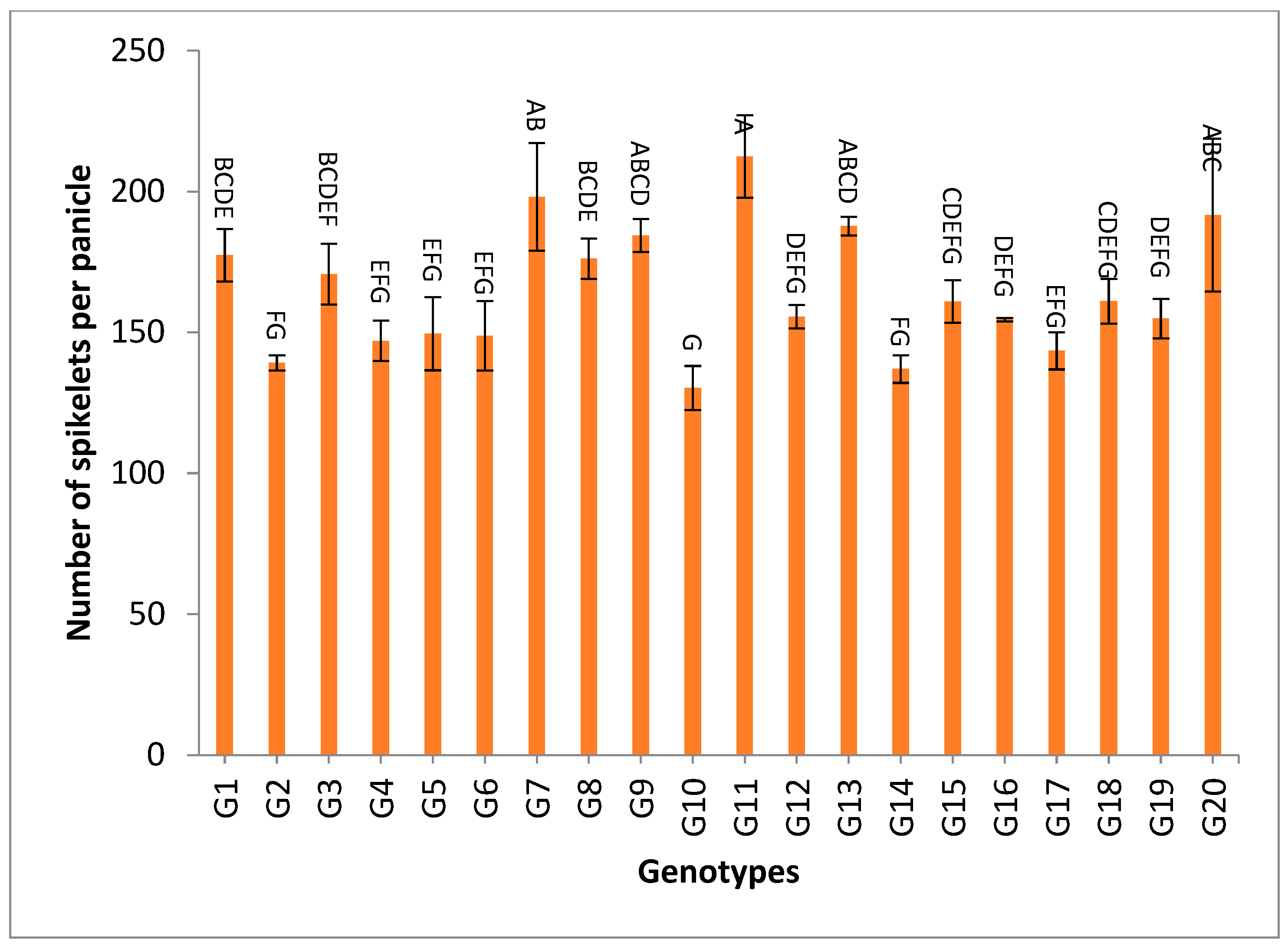
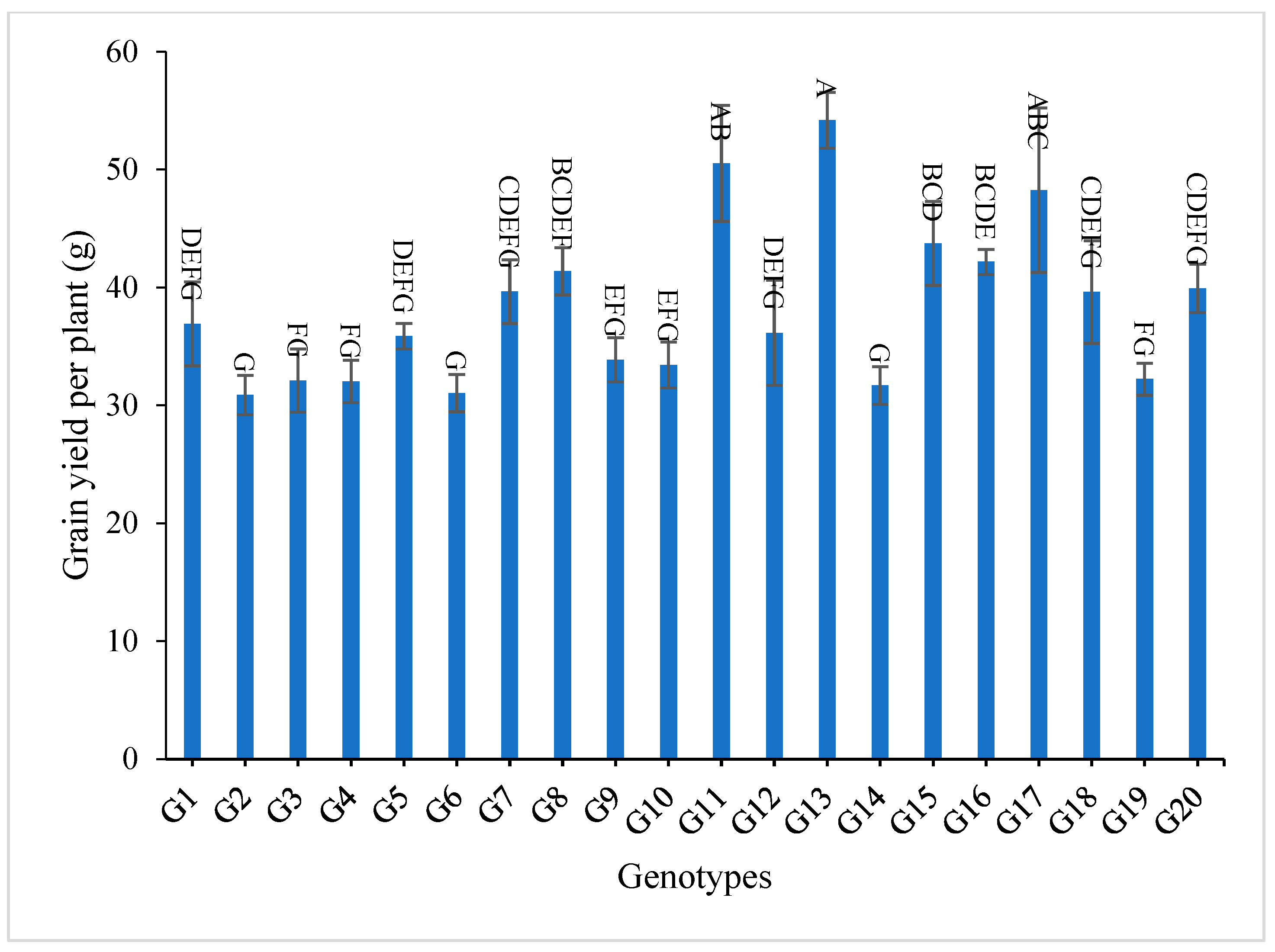
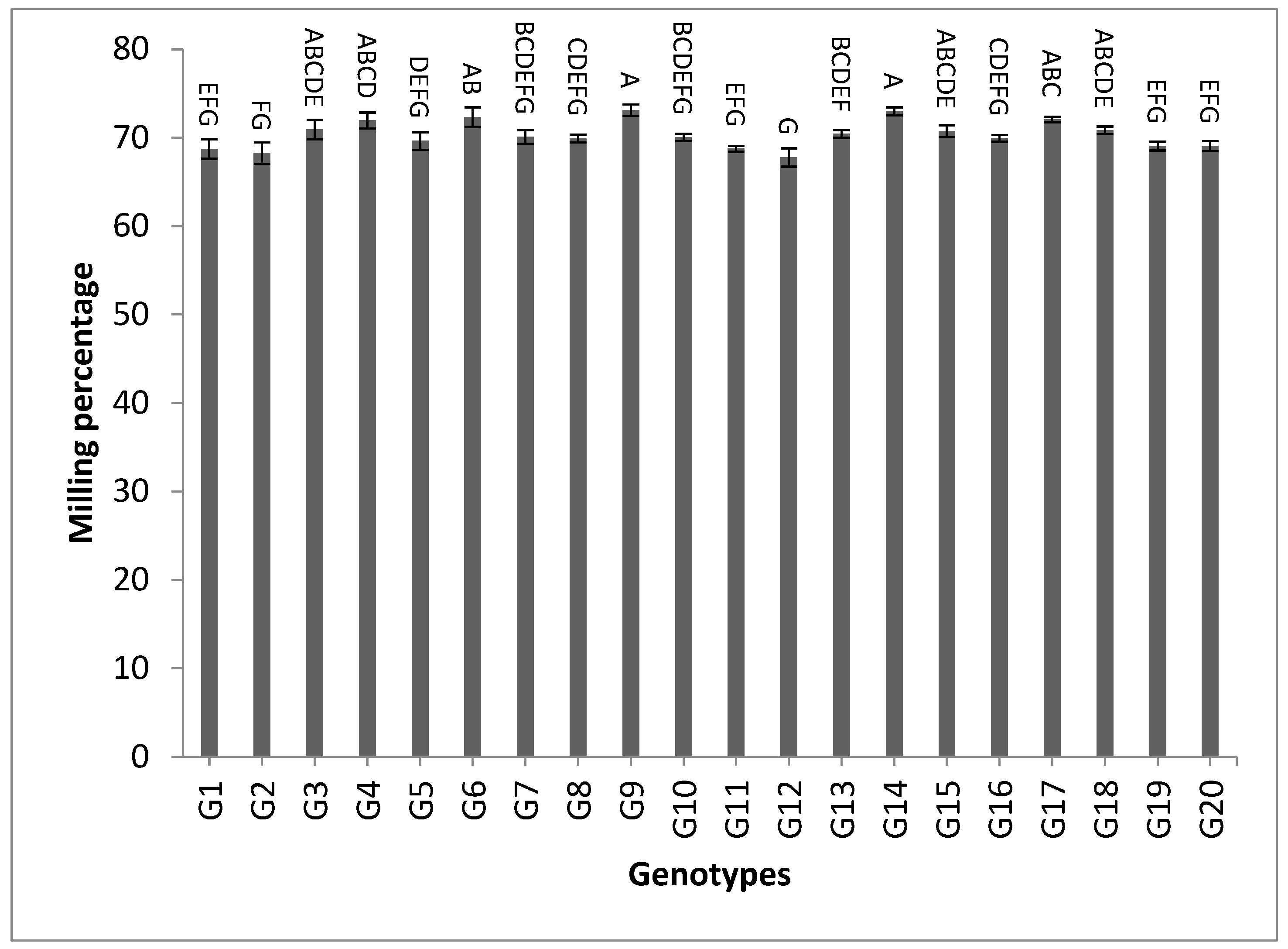
3.2. Mean Performance
3.3. Analysis of Variances
3.4. Genetic Parameters
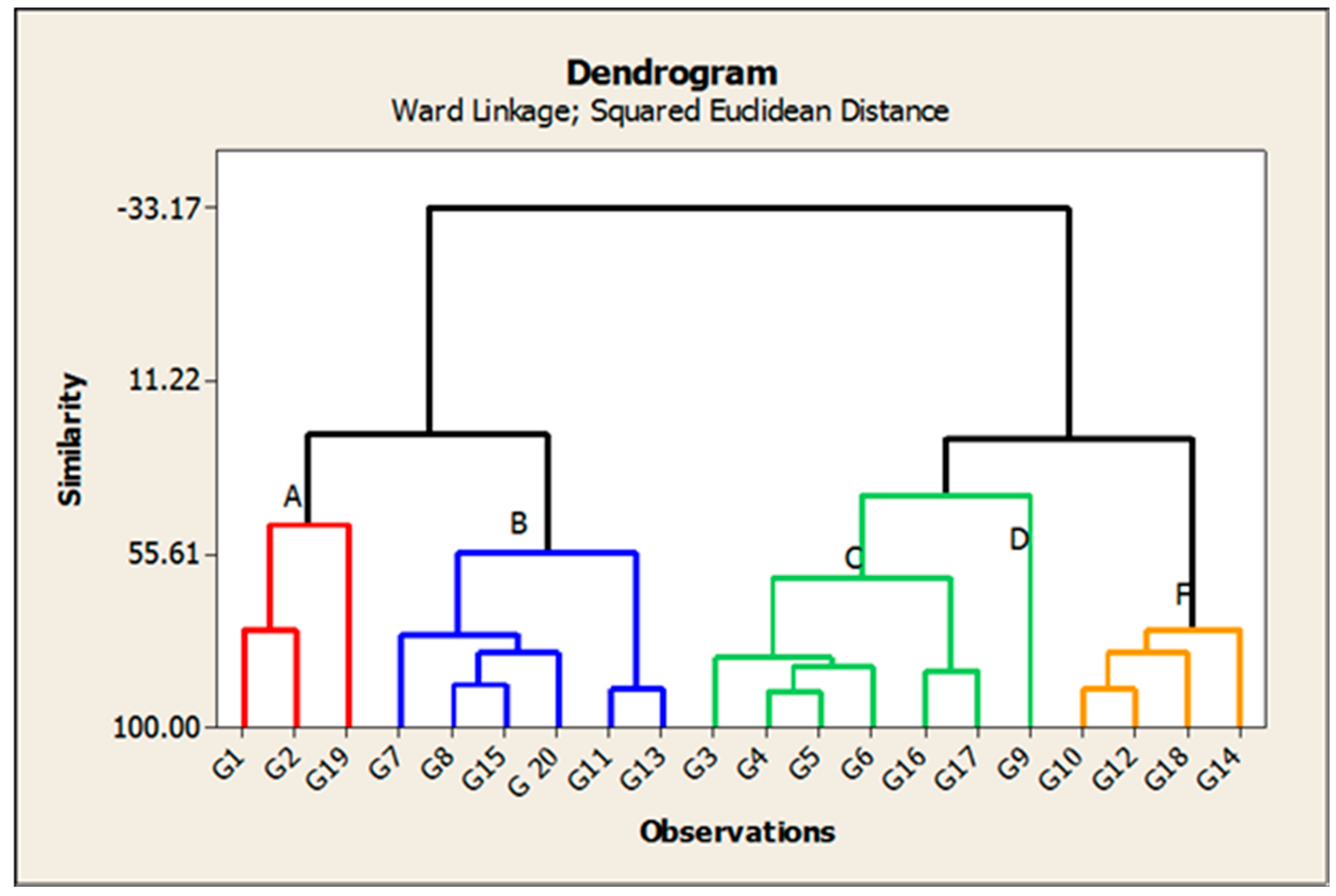
3.5. Cluster Analysis
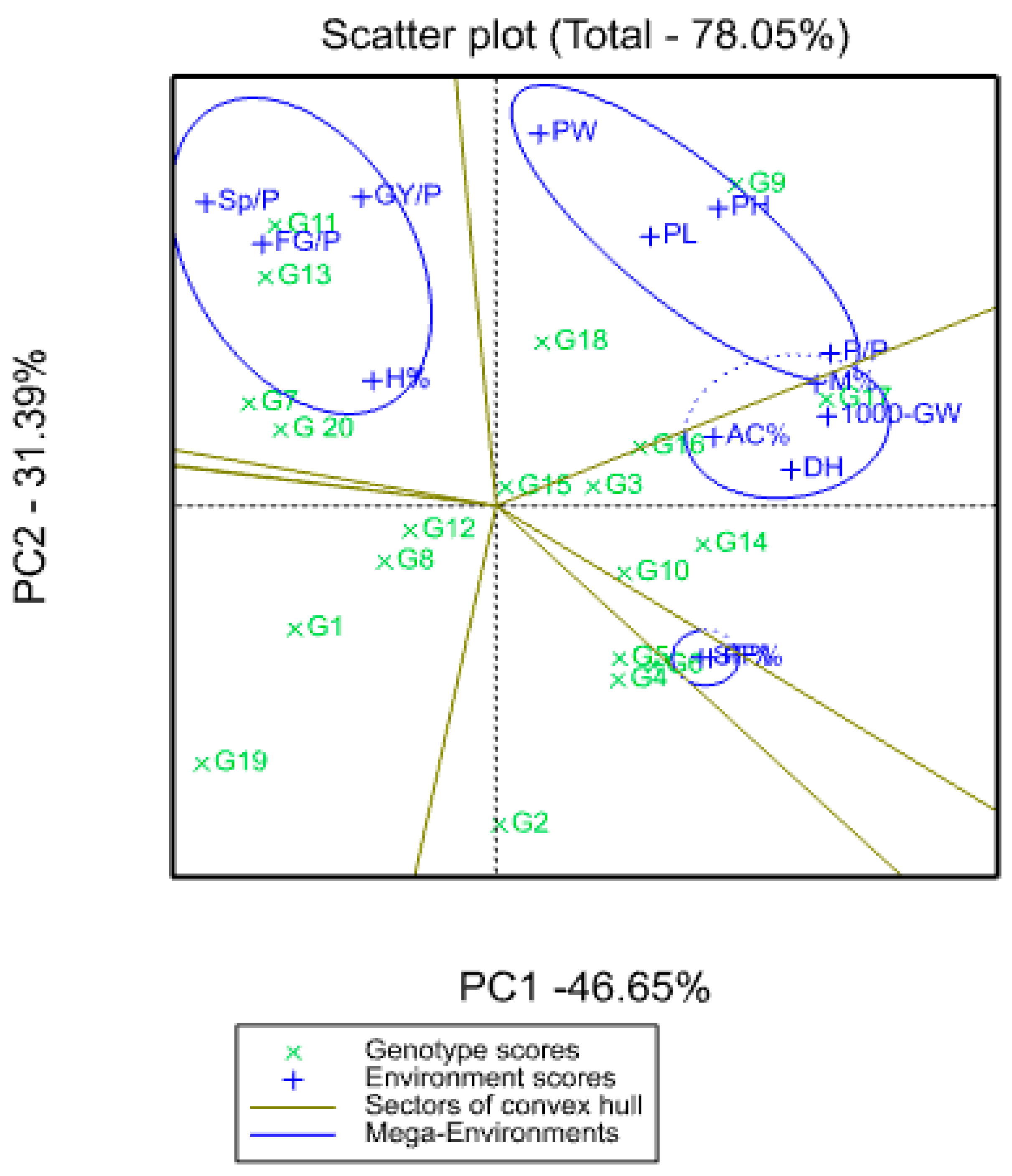
3.6. Principal Component Analysis (PCA)
4. Discussion
4.1. Selection and Evaluation
4.2. Test Cross Experiment
4.3. Mean Performance
4.4. Analysis of Variances
4.5. Genetic Parameters
4.6. Cluster Analysis
4.7. Principal Component Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awad-Allah, M.M.A.; Elekhtyar, N.M.; El-Abd, M.A.-E.-M.; Abdelkader, M.F.M.; Mahmoud, M.H.; Mohamed, A.H.; El-Diasty, M.Z.; Said, M.M.; Shamseldin, S.A.M.; Abdein, M.A. Development of New Restorer Lines Carrying Some Restoring Fertility Genes with Flowering, Yield and Grains Quality Characteristics in Rice (Oryza sativa L.). Genes 2022, 13, 458. [Google Scholar] [CrossRef]
- Awad-Allah, M.M.A.; Mohamed, A.H.; El-Bana, M.A.; El-Okkiah, S.A.F.; Abdelkader, M.F.M.; Mahmoud, M.H.; El-Diasty, M.Z.; Said, M.M.; Shamseldin, S.A.M.; Abdein, M.A. Assessment of Genetic Variability and Bran Oil Characters of New Developed Restorer Lines of Rice (Oryza sativa L.). Genes 2022, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Awad-Allah, M.M.A.; Attia, K.A.; Omar, A.A.; Dessoky, E.S.; Alzuaibr, F.M.; Alshehri, M.A.; Abdein, M.A.; Mohamed, A.H. Development of New Iso-Cytoplasmic Rice-Restorer Lines and New Rice Hybrids with Superior Grain Yield and Grain Quality Characteristics by Utilizing Restorers’ Fertility Genes. Genes 2022, 13, 808. [Google Scholar] [CrossRef] [PubMed]
- Awad-Allah, M.M.A.; Attia, K.A.; Omar, A.A.; Mohamed, A.H.; Habiba, R.M.; Alzuaibr, F.M.; Alshehri, M.A.; Alqurashi, M.; Aloufi, S.; Dessoky, E.S.; et al. Combining Ability and Gene Action Controlling Agronomic Traits for Cytoplasmic Male Sterile Line, Restorer Lines, and New Hybrids for Developing of New Drought-Tolerant Rice Hybrids. Genes 2022, 13, 906. [Google Scholar] [CrossRef] [PubMed]
- Virmani, S.S.; Mao, C.X.; Hardy, B. Hybrid Rice for Food Security, Poverty Alleviation, and Environmental Protection. In Proceedings of the 4th International Symposium on Hybrid Rice, Hanoi, Vietnam, 14–17 May 2002; International Rice Research Institute: LosBaños, Philippines, 2003. [Google Scholar]
- Pattnaik, S.; Kumar, V.; Tiwari, K.K.; Prakash, C.; Singh, A.; Singh, A.K.; Behera, N.; Panigrahi, J.; Mithra, S.V.A.; Mohapatra, T. Physiological and molecular basis of water-deficit stress tolerance in F1 hybrids and their parental lines in rice. Indian J. Genet. 2016, 76, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Virmani, S.S.; Virakamath, B.C.; Casal, C.L.; Toledo, R.S.; Lopez, M.T.; Manalo, J.O. Hybrid Rice Breeding Manual; International Rice Research Institute: LosBaños, Philippines, 1997. [Google Scholar]
- Krishnan, G.S.; Singh, A.K.; Waters, D.L.E.; Henry, R.J. Molecular Markers for Harnessing Heterosis. Mol. Markers Plants 2012, 119–136. [Google Scholar] [CrossRef]
- Nematzadeh, G.A.; Sattari, M. A study of nucleus genome of some high yielding rice (Oryza sativa L.) varieties for application in hybrid rice technology. Iranian J. Agric. Sci. 2003, 34, 213–219. [Google Scholar]
- Kumar, S.; Chauhan, M.P.; Tomar, A. Selection of Best Gene Pool Combination of the Basis of Heterotic Response Analysis for Grain Yield and Its Contributing Traits in Rice (Oryza sativa L.). Int. J. Agric. Invent. 2020, 5, 233–241. [Google Scholar] [CrossRef]
- Awad-Allah, M.M.A. Application of Genetic Engineering Tools on Rice Genome. Master’s Thesis, Al-Azhar University Faculty of Agriculture, Cairo, Egypt, 2006. [Google Scholar]
- Awad-Allah, M.M.A. Integrated Restorer Fertility and Wide Compatibility Genes for Producing Promising Restorer Lines in Rice. Ph.D. Thesis, Al-Azhar University Faculty of Agriculture, Cairo, Egypt, 2011. [Google Scholar]
- Priyadarshi, R.; Arremsetty, H.P.S.; Singh, A.K.; Khandekar, D.; Ulaganathan, K.; Shenoy, V.; Sinha, P.; Singh, V.K. Marker-Assisted Improvement of the Elite Maintainer Line of Rice, IR 58025B for Wide Compatibility (S5n) Gene. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Phetmanyseng, X.F.; Xie, J.E.; Hernandez, T.H. Boirromeo Hybrid rice heterosis and genetic diversity of IRRI and Lao rice. Field Crops Res. 2010, 117, 18–23. [Google Scholar] [CrossRef]
- Awad-Allah, M.M.A. Heterosis and Combining ability Estimates using Line x Tester Analysis to Develop Wide Compatibility and Restorer Lines in Rice. J. Agric. Chem. Biotechnol. 2020, 11, 383–393. [Google Scholar] [CrossRef]
- Khush, G.S. Challenges in rice breeding and biotechnology. In Recent Progress in Rice Research and Challenge Towards the 21st Century. Japan-IRRI Day. Tsukuba Seminar. Tsukuba 1994; Japan International Research Center for Agricultural Sciences: Tsukuba, Japan, 1994; pp. 11–19. [Google Scholar]
- Ahmed, J.; Ramaswamy, H.S.; Raghavan, V.G.S. Dielectric Properties of Indian Basmati Rice Flour Slurry. J. Food Eng. 2007, 80, 1125–1133. [Google Scholar] [CrossRef]
- Juliano, B.O.; Onate, L.U.; Imundo, A.M. Relation of starch composition, protein content and gelatinization to cooking and eating quality of milled rice. Food Technol. 1966, 19, 1006–1011. [Google Scholar]
- Azeez, M.A.; Shafi, M. Quality in Rice; Department of Agriculture, West Pakistan Technology Bulletin, 1966; No. 13; p. 50. [Google Scholar]
- Priadi, D.; Perdani, A.Y.; Sulistyowati, Y.; Pohan, F.N.; Mulyaningsih, E.S. Characterization of Carambola (Averrhoa Carambola L.) Plant Collection of Cibinong Plant Germplasm Garden Based on Phenotypic and Genetic Characters. Biosaintifika J. Biol. Biol. Educ. 2016, 8, 119. [Google Scholar] [CrossRef]
- Abd El-Mohsen, A.A.; Abo Hegazy, S.R.; Taha, M.H. Genotypic and phenotypic interrelationships among yield and yield components in Egyptian bread wheat genotypes. J. Plant Breed. Crop Sci. 2012, 4, 9–16. [Google Scholar]
- Tu Anh, T.T.; Khanh, T.D.; Dat, T.D.; Xuan, T.D. Identification of Phenotypic Variation and Genetic Diversity in Rice (Oryza sativa L.) Mutants. Agriculture 2018, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Reig-Valiente, J.L.; Viruel, J.; Sales, E.; Marqués, L.; Terol, J.; Gut, M.; Derdak, S.; Talon, M.; Domingo, C. Genetic diversity and population structure of rice varieties cultivated in temperate regions. Rice 2016, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Nachimuthu, V.V.; Muthurajan, R.; Duraialaguraja, S.; Sivakami, R.; Pandian, B.A.; Ponniah, G.; Gunasekaran, K.; Swaminathan, M.; Suji, K.K.; Sabariappan, R. Analysis of population structure and genetic diversity in rice germplasm using SSR markers: An initiative towards association mapping of agronomic traits in Oryza sativa. Rice 2015, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- F.A.O. Corporate Document Repository. Sustainable Rice Production for Food Security. In Proceedings of the 20th Session of International Rice Commission, Bangkok, Thailand, 23–25 July 2002. [Google Scholar]
- Islam, M.Z.; Khalequzzaman, M.; Prince, M.F.R.K.; Siddique, M.A.; Rashid, E.S.M.H.; Ahmed, M.S.U.; Pittendrigh, B.R.; Ali, M.P. Diversity and population structure of red rice germplasm in Bangladesh. PLoS ONE 2018, 13, e0196096. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, U.; Subudhi, P.K. Genetic Diversity, Population Structure, and Marker-Trait Association for Drought Tolerance in US Rice Germplasm. Plants 2019, 8, 530. [Google Scholar] [CrossRef] [Green Version]
- Beena, R.; Kirubakaran, S.; Nithya, N.; Sah, R.P.; Abida, P.S.; Sreekumar, J.; Jaslam, M.P.K.; Rejeth, R.; Jayalekshmy, V.G.; Roy, S.; et al. Association Mapping of Drought Avoidance and Agronomic Traits in Rice (Oryza sativa L.) Landraces. BMC Plant Biol. 2021, 21, 484. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Rajcan, I. Biplot Analysis of Test Sites and Trait Relations of Soybean in Ontario. Crop Sci. 2002, 42, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Tinker, N.A. An Integrated Biplot Analysis System for Displaying, Interpreting, and Exploring Genotype × Environment Interaction. Crop Sci. 2005, 45, 1004–1016. [Google Scholar] [CrossRef]
- Yan, W. Genotype-by-trait data analysis and decision-making. Chapter 9. In Crop Variety Trials: Data Management and Analysis, 1st ed.; Yan, W., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2014; pp. 163–186. [Google Scholar] [CrossRef]
- Swelam, A.A. Phenotypic Stability, Biplot Analysis and Interrelationship among Agronomic Characters for Some Bread Wheat Genotypes. Egypt. J. Plant Breed. 2012, 16, 147–165. [Google Scholar] [CrossRef]
- International Rice Research Institute (IRRI). Standard Evaluation System for Rice, 5th ed.; International Rice Research Institute (IRRI): Los Banos, PA, USA, 2014; p. 57. ISBN 9789712203046. [Google Scholar]
- Xangsayasane, P.; Xie, F.; Borromeo, T.H. Borromeo. Grain quality evaluation of Lao high yielding cultivars. Lao J. Agric. For. 2009, 19, 30–45. [Google Scholar]
- Juliano, B.O. A simplified assay for milled rice amylose. Cereal Sci. Today 1971, 16, 334. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures For Agricultural Research, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1984. [Google Scholar]
- Bartlett, M.S. Properties of Sufficiency and Statistical Tests. Proceedings of the Royal Society of London. Ser. A—Math. Phys. Sci. 1937, 160, 268–282. [Google Scholar] [CrossRef]
- Johnson, H.W.; Robinson, H.F.; Comstock, R.E. Estimates of Genetic and Environmental Variability in Soybeans 1. Agron. J. 1955, 47, 314–318. [Google Scholar] [CrossRef]
- Johnson, H.W.; Robinson, H.F.; Comstock, R.E. Genotypic and Phenotypic Correlations in Soybeans and Their Implications in Selection1. Agron. J. 1955, 47, 477. [Google Scholar] [CrossRef]
- Allard, R.W. Principals of Plant Breeding, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Sivasubramanian, S.; Madhavamenon, P. Genotypic and phenotypic variability in rice. Madras Agric. J. 1973, 60, 1093–1096. [Google Scholar]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Priyanka, R.M.; Thiyagarajan, K.; Rajendran, S.P.B.R. Identification of restorers and maintainers among the locally adapted genotypes for hybrid development in rice (Oryza sativa L.). Electron. J. Plant Breed. 2016, 7, 814. [Google Scholar] [CrossRef]
- Awad-Allah, M.M.A. Identification and Evaluation of Some New Hybrids of Rice under Egyptian Conditions. J. Agric. Chem. Biotechnol. 2016, 7, 121–133. [Google Scholar] [CrossRef] [Green Version]
- Toriyama, K.; Kazama, T.; Sato, T.; Fukuta, Y.; Oka, M. Development of cytoplasmic male sterile lines and restorer lines of various elite Indica Group rice cultivars using CW-CMS/Rf17 system. Rice 2019, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.R.; Venkanna, V.; Parimala, K.; Hari, Y.; Satish Chandra, B.; Lingaiah, N.; Prasad, K.R.; Rao, P.J.M. Developing of Heterotic Hybrids through Allocytoplasmic Restorer Improvement In Rice (Oryza sativa L.). In Proceedings of the 1st Indian Rice Congress—2020. Rice Research and Development for Achieving Sustainable Development Goals, Cuttack, India, 8–9 December 2020; ICAR-NRRI: Cuttack, India, 2020; pp. 27–28. [Google Scholar]
- Prasad, K.R.K.; Suneetha, Y. Fertility Restoration Studies of Promising Genotypes in Rice (Oryza sativa L.). In Proceedings of the 1st Indian Rice Congress—2020. Rice Research and Development for Achieving Sustainable Development Goals, Cuttack, India, 8–9 December 2020; ICAR-NRRI: Cuttack, India, 2020; pp. 40–42. [Google Scholar]
- Kumar, A.; Bhowmick, P.K.; Krishnan, S.G.; Singh, A.K. Development and evaluation of iso-cytoplasmic rice restorer lines for different agro-morphological traits. Indian J. Genet. Plant Breed. 2017, 77, 493. [Google Scholar] [CrossRef]
- Awad-Allah, M.M.A.; Attia, K.A.; El-Gohary, A.A.; Mikhael, B.B. Combining ability and gene action of grain yield and its components for some parental lines used in hybrid rice production. Egypt. J. Agric. Res. 2014, 93, 187–216. [Google Scholar]
- Bhuiyan, M.S.H.; Zahan, A.; Khatun, H.; Iqbal, M.; Alam, F.; Manir, M.R. Yield performance of newly developed test crossed hybrid rice variety. Inter. J. Agron. Agric. Res. (IJAAR) 2014, 5, 48–54. [Google Scholar]
- Awad-Allah, M.M.A. Improving Parental Lines for Hybrid Rice Development in Indica/Japonica Rice Crosses. J. Agric. Chem. Biotechnol. 2019, 10, 237–248. [Google Scholar] [CrossRef]
- Awad-Allah, M.M.A. Genetical analysis and detection for wide compatibility traits of some rice genotypes under Egyptian conditions. J. Sustain. Agric. Sci. 2016, 42, 99–125. [Google Scholar] [CrossRef]
- Rahaman, A. Study of nature and magnitude of gene action in hybrid rice (Oryza sativa L.) through experiment of line x tester mating design. Inter. J. Appl. Res. 2016, 2, 405–410. [Google Scholar]
- Dipti, S.S.; Bari, M.N.; Kabir, K.A. Grain quality characteristics of some Beruin rice varieties of Bangladesh. Pak. J. Nutr. 2003, 2, 242–245. [Google Scholar]
- Babu, P.M.; Chandramohan, Y.; Ravindrababu, V.; Arunakumari, C.H. Assessment of Genetic Variability for Yield and Bran oil Content in Segregating Generations of Rice. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 117–124. [Google Scholar] [CrossRef]
- Radha, T.; Satheesh Kumar, P.; Saravanan, K.; Suganthi, S. Genetic variabilty, association and path analysis for grain yield and quality traits in Rice (Oryza sativa L.). Plant Arch. 2019, 19, 2522–2526. Available online: http://www.plantarchives.org/19-2/2522-2526%20(4911).pdf (accessed on 7 January 2022).
- Adjah, K.L.; Abe, A.; Adetimirin, V.O.; Asante, M.D. Genetic variability, heritability and correlations for milling and grain appearance qualities in some accessions of rice (Oryza sativa L.). Physiol. Mol. Biol. Plants 2020, 26, 1309–1317. Available online: https://link.springer.com/content/pdf/10.1007/s12298-020-00826-x.pdf (accessed on 7 January 2022). [CrossRef]
- Nirmaladevi, G.; Padmavathi, G.; Kota, S.; Babu, V.R. Genetic variability, heritability and correlation coefficients of grain quality characters in rice (Oryza sativa L.). SABRAO J. Breed. Genet. 2015, 47, 424–433. [Google Scholar]
- Qiu, C.; Cao, J.; Xiong, L.; Sun, Q. Differences in Physicochemical, Morphological, and Structural Properties between Rice Starch and Rice Flour Modified by Dry Heat Treatment. Starch-Stärke 2015, 67, 756–764. [Google Scholar] [CrossRef]
- Babu, V.R.; Shreya, K.; Dangi, K.S.; Usharani, G.; Shankar, A.S. Correlation and path analysis studies in popular rice hybrids of India. Int. J. Sci. Res. Publ. 2012, 2, 1–5. [Google Scholar]
- Karuppaiyan, R.; Kapoor, C.; Gopi, R. Variability, heritability and genetic divergence in lowland rice genotypes under the mid-hills of Sikkim. Oryza 2013, 50, 81–84. [Google Scholar]
- Tiwari, D.N.; Tripathi, S.R.; Tripathi, M.P.; Khatri, N.; Bastola, B.R. Genetic Variability and Correlation Coefficients of Major Traits in Early Maturing Rice under Rainfed Lowland Environments of Nepal. Adv. Agric. 2019, 5975901. [Google Scholar] [CrossRef] [Green Version]
- Dutta, P.; Nath Dutta, P.; Borua, P.K. Morphological Traits as Selection Indices in Rice: A Statistical View. Univers. J. Agric. Res. 2013, 1, 85–96. [Google Scholar] [CrossRef]
- Ahmadikhah, A. Study on selection effect, genetic advance and genetic parameters in rice. Ann. Biol. Res. 2010, 1, 45–51. [Google Scholar]
- Kumar, M.; Sharma, P.R.; Krakash, N.; Singh, P.K. Selection criteria for high yielding genotypes in early generations of rice. SAARC J. Agric. 2009, 7, 37–42. [Google Scholar]
- Seyoum, M.; Alamerew, S.; Bantte, K. Genetic Variability, Heritability, Correlation Coefficient and Path Analysis for Yield and Yield Related Traits in Upland Rice (Oryza sativa L.). J. Plant Sci. 2011, 7, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Pal Atanu, K.; Sabesan, T. Studies on genetic variability for lodging related traits in rice (Oryza sativa L.). Electron. J. Plant Breed. 2010, 1, 301–304. [Google Scholar]
- Lal, M.; Chauhan Devendra, K. Studies of genetic variability, heritability and genetic advance in relation to yield traits in rice. Agric. Sci. Dig.-A Res. J. 2011, 31, 220–222. [Google Scholar]
- Prajapati, M.K.; Singh, C.M.; Suresh Babu, G.; Roopa Lavanya, G.; Jadhav, P. Genetic parameters for grain yield and its component characters in rice. Electron. J. Plant Breed. 2011, 2, 235–238. [Google Scholar]
- Ahmad, H.; Razvi, S.M.; Ashraf Bhat, A.; Najeeb, S.; Wani, N.; Habib, M.; Mir, M.R.; Gupta, B.B. Genetic variability and genetic divergence of important rice (Oryza sativa L.) varieties. Int. J. Curr. Res. 2010, 4, 33–37. [Google Scholar]
- Vange, T. Biometrical studies on genetic diversity of some upland rice (Oryza sativa L.) accessions. Nat. Sci. 2009, 7, 21–27. [Google Scholar]
- Singh, S.K.; Singh, C.M.; Lal, G.M. Assessment of genetic variability for yield and its component characters in rice (Oryza sativa L.). Res. Plant Biol. 2011, 1, 73–76. [Google Scholar]
- Bisne, R.; Sarawgi, K.; Verulkar, S.B. Study of heritability, genetic advance and variability for yield contributing characters in rice Bangladesh. J. Agric. Res. 2009, 34, 175–179. [Google Scholar] [CrossRef]
- Nayak, A.R.; Chaudhary, D.; Reddy, J.N. Genetic divergence in scented rice. Oryza 2004, 41, 79–82. [Google Scholar]
- Soni, S.K.; Yadav, V.K.; Ram, T. Genetic Divergence Analysis in Tropical Japonica and Indica Rice Lines (Oryza sativa L.). Plant Arch. 2014, 14, 449–453. [Google Scholar]
- Chandra, R.; Pradhan, S.K.; Singh, S.; Bose, L.K.; Singh, O.N. Maltivariate analysis in upland rice genotypes. World J. Agric. Sci. 2007, 3, 295–300. [Google Scholar]
- Banumathi, S.; Manimaran, R.; Sheeba, A.; Manivannan, N.; Ramya, B.; Kumar, D.; Ramasubramanian, G.V. Genetic diversity analysis of rice germplasm lines for yield attributing traits. Electron. J. Plant Breed. 2010, 1, 500–504. [Google Scholar]
- Dushyantha, K.B.M.; Kantti, A. Generic divergence in nice accessions of hill zone of Karnataka. Electron. J. Plant Breed. 2010, 1, 1329–1331. [Google Scholar]
- Mattjik, A.A.; Sumertajaya, I.; Wibawa, G.N.A.; Hadi, A.F. Sidik Peubah Ganda Dengan Menggunakan SAS; IPB Press: Bogor, Indonesia, 2011. [Google Scholar]
- Rehman, F.; Saeed, A.; Yaseen, M.; Shakeel, A.; Ziaf, K.; Munir, H.; Tariq, S.A.; Raza, M.A.; Riaz, A. Genetic Evaluation and Characterization Using Cluster Heat Map to Assess NaCl Tolerance in Tomato Germplasm at the Seedling Stage. Chil. J. Agric. Res. 2019, 79, 56–65. [Google Scholar] [CrossRef]
- Akbar, M.R.; Purwoko, B.S.; Dewi, I.S.; Suwarno, W.B. Sugiyanta. Short Communication: Selection of Doubled Haploid Lines of Rainfed Lowland Rice in Preliminary Yield Trial. Biodiversitas J. Biol. Divers. 2019, 20, 2796–2801. [Google Scholar] [CrossRef] [Green Version]
- Anshori, M.F.; Purwoko, B.S.; Dewi, I.S.; Ardie, S.W.; Suwarno, W.B. Selection index based on multivariate analysis for selecting doubled-haploid rice lines in lowland saline prone area. SABRAO J. Breed. Genet. 2019, 51, 161–174. [Google Scholar]
- Farid, M.; Anshori, M.F.; Musa, Y.; Iswoyo, H.; Sakinah, A.I. Interaction of Rice Salinity Screening in Germination and Seedling Phase through Selection Index Based on Principal Components. Chil. J. Agric. Res. 2021, 81, 368–377. [Google Scholar] [CrossRef]



| ID | Name | Origin |
|---|---|---|
| G1 | Giza 178 | Egypt |
| G2 | Giza 181 | Egypt |
| G3 | IR38 | IRRI |
| G4 | IR64 | IRRI |
| G5 | IR65 | IRRI |
| G6 | Tox 502-1-SLR1-LS3-B | Brazil |
| G7 | Maybelle | USA |
| G8 | H 156 | China |
| G9 | BR27 | Bangladesh |
| G10 | Xiangzi 3150 | China |
| G11 | IR71144-176-3-2-3-2 | IRRI |
| G12 | Oryzica 1-266-M | Colombia |
| G13 | IR74052-184-3-3 | IRRI |
| G14 | IR72864-80 | IRRI |
| G15 | IR72868-11-1 | IRRI |
| G16 | PR2 | India |
| G17 | SPR85163 | Thailand |
| G18 | TOX 3133-59-1-2-4 | IITA |
| G19 | G46A,B | IRRI |
| G 20 | IR79156A,B | IRRI |
| Mean Squares | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source of Variance | d.f. | Days to 50% Heading | Plant Height (cm) | Number of Panicles/Plant | Panicle Length (cm) | ||||
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| Reps. | 2 | 0.01 | 0.05 | 8.78 | 15.23 | 1.73 | 1.46 | 0.10 | 0.27 |
| Genotypes | 19 | 309.61 ** | 309.36 ** | 547.89 ** | 407.04 ** | 14.21 ** | 9.06 ** | 7.85 ** | 8.08 ** |
| Error | 38 | 0.88 | 0.45 | 4.10 | 7.74 | 0.85 | 0.79 | 0.86 | 1.05 |
| Mean Squares | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source of Variance | d.f. | Panicle Weight (g) | Pollen Fertility (%) | Number of Spikelets/Panicle | Spikelet Fertility (%) | ||||
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| Reps. | 2 | 0.20 | 0.11 | 1.15 | 2.46 | 20.2 | 2.82 | 1.15 | 2.46 |
| Genotypes | 19 | 1.61 ** | 2.30 ** | 63.10 ** | 35.59 ** | 1872.98 ** | 2208.71 ** | 63.33 ** | 34.95 ** |
| Error | 38 | 0.21 | 0.14 | 4.28 | 3.84 | 197.91 | 132.29 | 4.28 | 3.84 |
| Mean Squares | |||||||
|---|---|---|---|---|---|---|---|
| Source of Variance | d.f. | Number of Filled Grains/Panicle | 1000 g Grain Weight (g) | Grain Yield/Plant (g) | |||
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| Reps. | 2 | 21.12 | 1.55 | 0.33 | 0.09 | 6.44 | 6.44 |
| Genotypes | 19 | 1286.26 ** | 1674.52 ** | 13.53 ** | 13.43 ** | 145.59 ** | 133.34 ** |
| Error | 38 | 157.20 | 94.42 | 1.09 | 0.49 | 9.54 | 9.54 |
| Mean Squares | |||||||
|---|---|---|---|---|---|---|---|
| Source of Variance | d.f. | Hulling Percentage | Milling Percentage | Amylase Content % | |||
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | ||
| Reps. | 2 | 0.12 | 0.12 | 0.22 | 0.22 | 0.13 | 0.13 |
| Genotypes | 19 | 3.36 ** | 2.82 ** | 7.65 ** | 6.85 ** | 41.83 ** | 39.66 ** |
| Error | 38 | 0.71 | 0.71 | 0.59 | 0.59 | 0.61 | 0.61 |
| Mean Squares | |||||||
|---|---|---|---|---|---|---|---|
| Source of Variance | d.f. | Days to 50% Heading | Plant Height (cm) | Number of Panicles/Plant | Panicle Length (cm) | Panicle Weight (g) | Pollen Fertility (%) |
| Year (Y) | 1 | 7.65 ** | 1860.39 ** | 47.38 ** | 18.33 ** | 0.01 | 169.39 ** |
| Error | 4 | 0.026 | 12.01 | 1.6 | 0.19 | 0.155 | 1.81 |
| Genotypes (G) | 19 | 618.96 ** | 905.58 ** | 21.49 ** | 13.20 ** | 3.48 ** | 72.09 ** |
| Y × G | 19 | 0.008 | 49.36 ** | 1.78 ** | 2.73 ** | 0.43 ** | 26.60 ** |
| Error | 76 | 0.665 | 5.92 | 0.82 | 0.96 | 0.175 | 4.06 |
| Mean Squares | ||||||
|---|---|---|---|---|---|---|
| Source of Variance | d.f. | Number of Spikelets/Panicle | Spikelet Fertility (%) | Number of Filled Grains/Panicle | 1000 g Grain Weight (g) | Grain Yield/Plant (g) |
| Year (Y) | 1 | 253.75 ** | 169.39 ** | 64.59 * | 0.31 | 241.97 ** |
| Error | 4 | 11.51 | 1.81 | 11.33 | 0.21 | 6.44 |
| Genotypes (G) | 19 | 3007.83 ** | 71.68 ** | 2250.11 ** | 26.40 ** | 278.61 ** |
| Y × G | 19 | 1073.85 ** | 26.60 ** | 710.67 ** | 0.57 | 0.32 |
| Error | 76 | 165.10 | 4.06 | 125.81 | 0.79 | 9.54 |
| Mean Squares | ||||
|---|---|---|---|---|
| Source of Variance | d.f. | Hulling Percentage | Milling Percentage | Amylase Content % |
| Year (Y) | 1 | 30.88 ** | 30.88 ** | 28.78 ** |
| Error | 4 | 0.12 | 0.22 | 0.13 |
| Genotypes (G) | 19 | 6.00 ** | 14.33 ** | 81.24 ** |
| Y × G | 19 | 0.18 | 0.18 | 0.25 |
| Error | 76 | 0.71 | 0.59 | 0.62 |
| Traits | Genotypic Coefficient of Variation (G.C.V %) | Phenotypic Coefficient of Variation (P.C.V %) | Heritability in a Broad Sense | Genetic Advance in Percentage (Expected) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 | 2020 | Combined | 2019 | 2020 | Combined | 2019 | 2020 | Combined | 2019 | 2020 | Combined | |
| Days to 50% heading | 9.17 | 9.13 | 9.16 | 9.21 | 9.15 | 9.18 | 99.15 | 99.57 | 99.43 | 18.82 | 18.78 | 18.81 |
| Plant height (cm) | 12.14 | 11.20 | 11.44 | 12.28 | 11.52 | 11.58 | 97.79 | 94.51 | 97.49 | 24.37 | 22.42 | 23.26 |
| Number of panicles/plant | 12.65 | 10.77 | 13.09 | 13.81 | 12.21 | 14.71 | 83.92 | 77.79 | 79.15 | 23.88 | 19.57 | 21.70 |
| Panicle length (cm) | 6.01 | 6.21 | 5.64 | 7.03 | 7.48 | 6.46 | 72.96 | 69.00 | 76.05 | 10.57 | 10.63 | 10.13 |
| Panicle weight (g) | 16.99 | 21.12 | 18.24 | 20.39 | 23.12 | 20.22 | 69.41 | 83.39 | 81.38 | 29.16 | 39.73 | 33.9 |
| Pollen fertility (%) | 4.98 | 3.56 | 3.74 | 5.50 | 4.16 | 4.05 | 82.07 | 73.39 | 85.17 | 9.29 | 6.29 | 7.11 |
| Number of spikelets per panicle | 14.28 | 16.18 | 13.09 | 16.62 | 17.66 | 14.71 | 73.83 | 83.95 | 79.15 | 25.27 | 30.54 | 23.98 |
| Spikelet fertility (%) | 5.10 | 3.60 | 3.81 | 5.63 | 4.22 | 4.13 | 82.12 | 72.99 | 85.09 | 9.52 | 6.34 | 7.24 |
| Number of filled grains/panicle | 13.51 | 15.82 | 12.79 | 16.09 | 17.18 | 14.59 | 70.54 | 84.80 | 76.88 | 23.38 | 30.01 | 23.1 |
| 1000 g grain weight (g) | 8.03 | 8.22 | 8.15 | 9.02 | 8.67 | 8.54 | 79.11 | 89.79 | 81.38 | 14.71 | 16.04 | 16.02 |
| Grain yield per plant (g) | 18.27 | 16.18 | 17.18 | 20.10 | 17.96 | 18.98 | 82.62 | 81.22 | 81.93 | 30.04 | 34.21 | 32.04 |
| Hulling percentage | 1.19 | 1.05 | 1.10 | 1.59 | 1.48 | 1.52 | 55.59 | 49.95 | 51.99 | 1.82 | 1.52 | 1.63 |
| Milling percentage | 2.20 | 2.04 | 2.11 | 2.46 | 2.31 | 2.37 | 80.10 | 78.11 | 78.94 | 4.05 | 3.72 | 3.86 |
| Amylase content % | 15.61 | 14.60 | 15.07 | 15.96 | 14.94 | 15.42 | 95.72 | 95.49 | 95.59 | 31.47 | 29.38 | 30.36 |
| Cluster ID | No. of Genotypes | GEN | DH | PH | P/P | PL | PW | PF% | Sp/P | SF% | FG/P | 1000-GW | GY/P | H% | M% | AC% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 3 | G1, G2, G19 | 97.5 | 95.6 | 13.8 | 23. | 3.62 | 92.3 | 157.2 | 90.4 | 142.2 | 23.9 | 33.3 | 79.5 | 68.7 | 20.30 |
| B | 6 | G7, G8, G15, G20, G11, G13 | 107.7 | 106.8 | 15.3 | 25. | 4.04 | 88.0 | 187.8 | 86.2 | 161.8 | 24.1 | 44.9 | 80.2 | 69.8 | 22.74 |
| C | 6 | G3, G4, G5, G6, G16, G17 | 117.9 | 107.9 | 16.8 | 25.2 | 3.88 | 93 | 152.3 | 90.9 | 138.4 | 26.4 | 36.9 | 79.1 | 71.1 | 26.01 |
| D | 1 | G9 | 109.3 | 145.2 | 16.5 | 25.5 | 6.01 | 93.2 | 184.4 | 91.3 | 167.8 | 29.0 | 33.9 | 80.1 | 73.1 | 28.96 |
| F | 4 | G10, G12, G18, G14 | 115.4 | 104.9 | 17.5 | 26.2 | 4.01 | 86.5 | 146 | 84.5 | 122.8 | 25.7 | 35.2 | 80.3 | 70.4 | 25.55 |
| Variable | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| DH | 0.331 | 0.044 | −0.018 | −0.21 |
| PH | 0.25 | 0.364 | −0.154 | 0.093 |
| P/P | 0.375 | 0.186 | 0.169 | −0.214 |
| PL | 0.173 | 0.329 | 0.233 | −0.438 |
| PW | 0.049 | 0.455 | −0.16 | 0.199 |
| PF% | 0.24 | −0.186 | −0.526 | −0.054 |
| Sp/P | −0.326 | 0.371 | −0.243 | 0.041 |
| SF% | 0.23 | −0.186 | −0.535 | −0.049 |
| FG/P | −0.262 | 0.32 | −0.401 | 0.036 |
| 1000-GW | 0.372 | 0.109 | −0.008 | 0.125 |
| GY/P | −0.15 | 0.377 | 0.017 | −0.386 |
| H% | −0.138 | 0.153 | 0.16 | 0.502 |
| M% | 0.36 | 0.15 | −0.046 | 0.304 |
| AC% | 0.244 | 0.084 | 0.237 | 0.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad-Allah, M.M.A.; Shafie, W.W.M.; Alsubeie, M.S.; Alatawi, A.; Safhi, F.A.; ALshamrani, S.M.; Albalawi, D.A.; Al-Amrah, H.; Alshehri, D.; Alshegaihi, R.M.; et al. Utilization of Genetic Resources, Genetic Diversity and Genetic Variability for Selecting New Restorer Lines of Rice (Oryza sativa L.). Genes 2022, 13, 2227. https://doi.org/10.3390/genes13122227
Awad-Allah MMA, Shafie WWM, Alsubeie MS, Alatawi A, Safhi FA, ALshamrani SM, Albalawi DA, Al-Amrah H, Alshehri D, Alshegaihi RM, et al. Utilization of Genetic Resources, Genetic Diversity and Genetic Variability for Selecting New Restorer Lines of Rice (Oryza sativa L.). Genes. 2022; 13(12):2227. https://doi.org/10.3390/genes13122227
Chicago/Turabian StyleAwad-Allah, Mamdouh M. A., Wafaa W. M. Shafie, Moodi Saham Alsubeie, Aishah Alatawi, Fatmah Ahmed Safhi, Salha Mesfer ALshamrani, Doha A. Albalawi, Hadba Al-Amrah, Dikhnah Alshehri, Rana M. Alshegaihi, and et al. 2022. "Utilization of Genetic Resources, Genetic Diversity and Genetic Variability for Selecting New Restorer Lines of Rice (Oryza sativa L.)" Genes 13, no. 12: 2227. https://doi.org/10.3390/genes13122227





