miR-24-3p Dominates the Proliferation and Differentiation of Chicken Intramuscular Preadipocytes by Blocking ANXA6 Expression
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Cell Isolation, Culture, and Differentiation
2.3. Cell Transfection
2.4. RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR
2.5. Western Blotting
2.6. CCK-8 Assay
2.7. EdU Assay
2.8. Oil Red O Staining
2.9. Target Prediction and Dual-Luciferase Reporter Assay
2.10. Statistical Analysis
3. Results
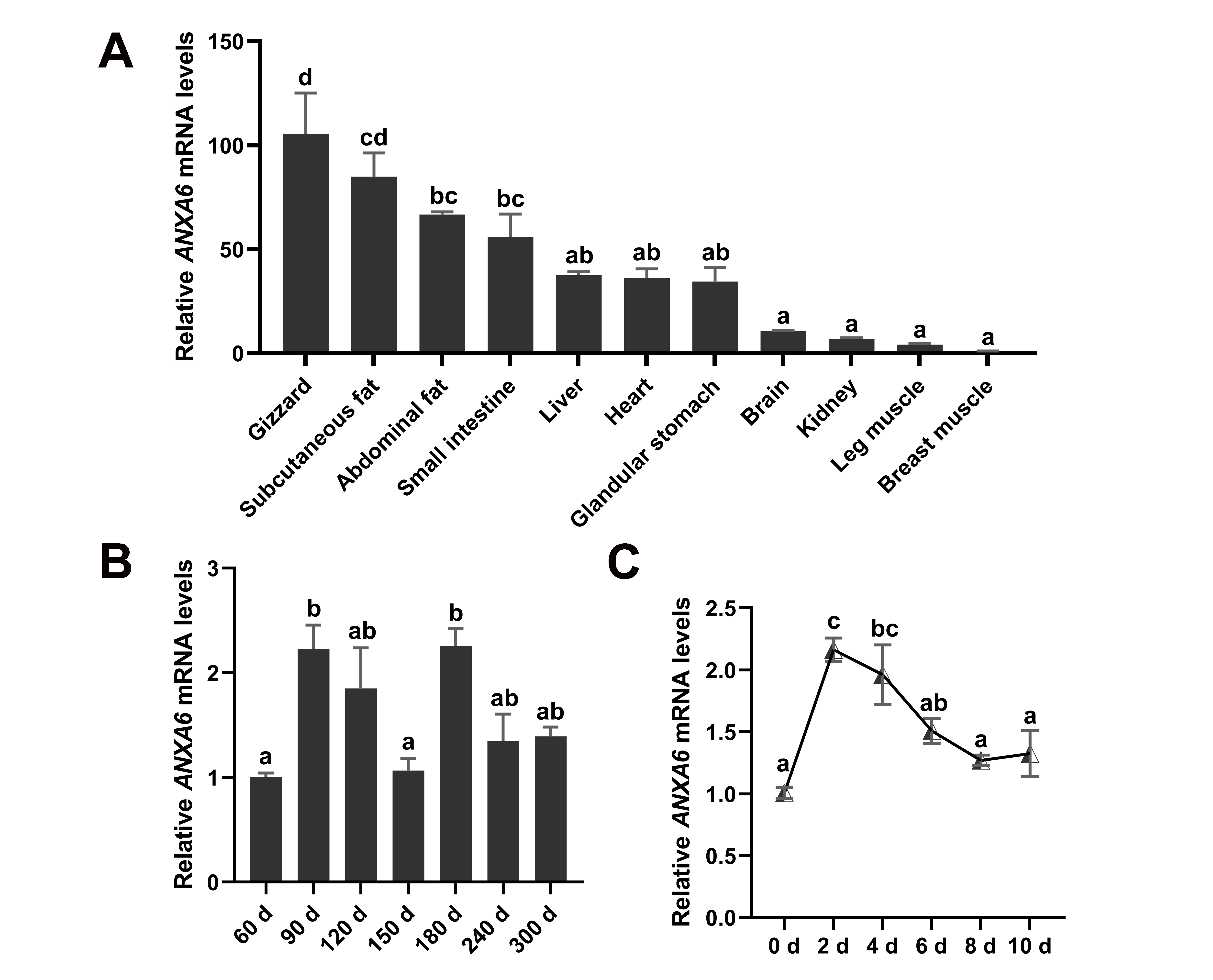
3.1. Expression Pattern of ANXA6 in Broilers
3.2. ANXA6 Inhibits the Proliferation of Chicken Intramuscular Preadipocytes
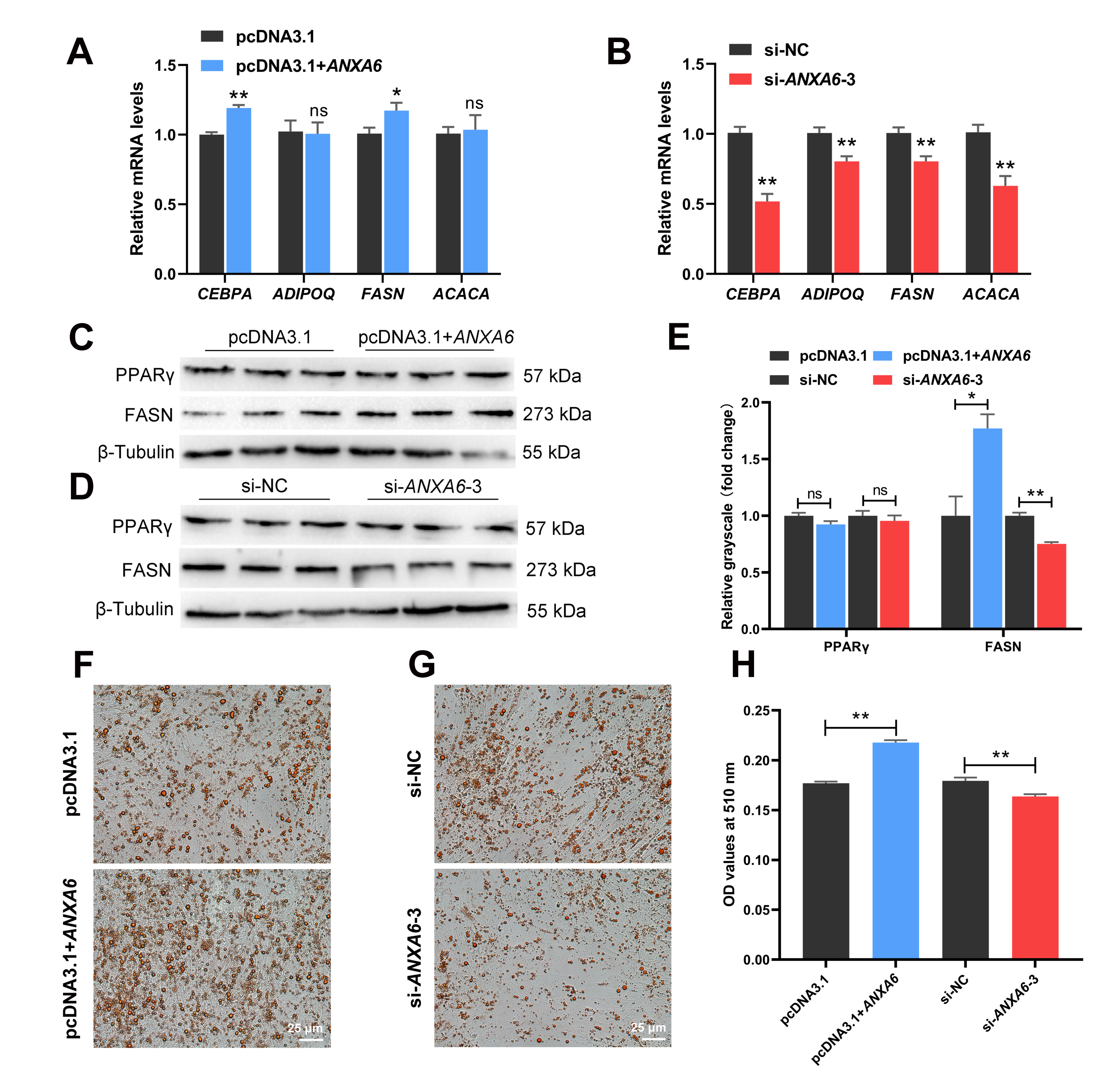
3.3. ANXA6 Promotes the Differentiation of Chicken Intramuscular Preadipocytes
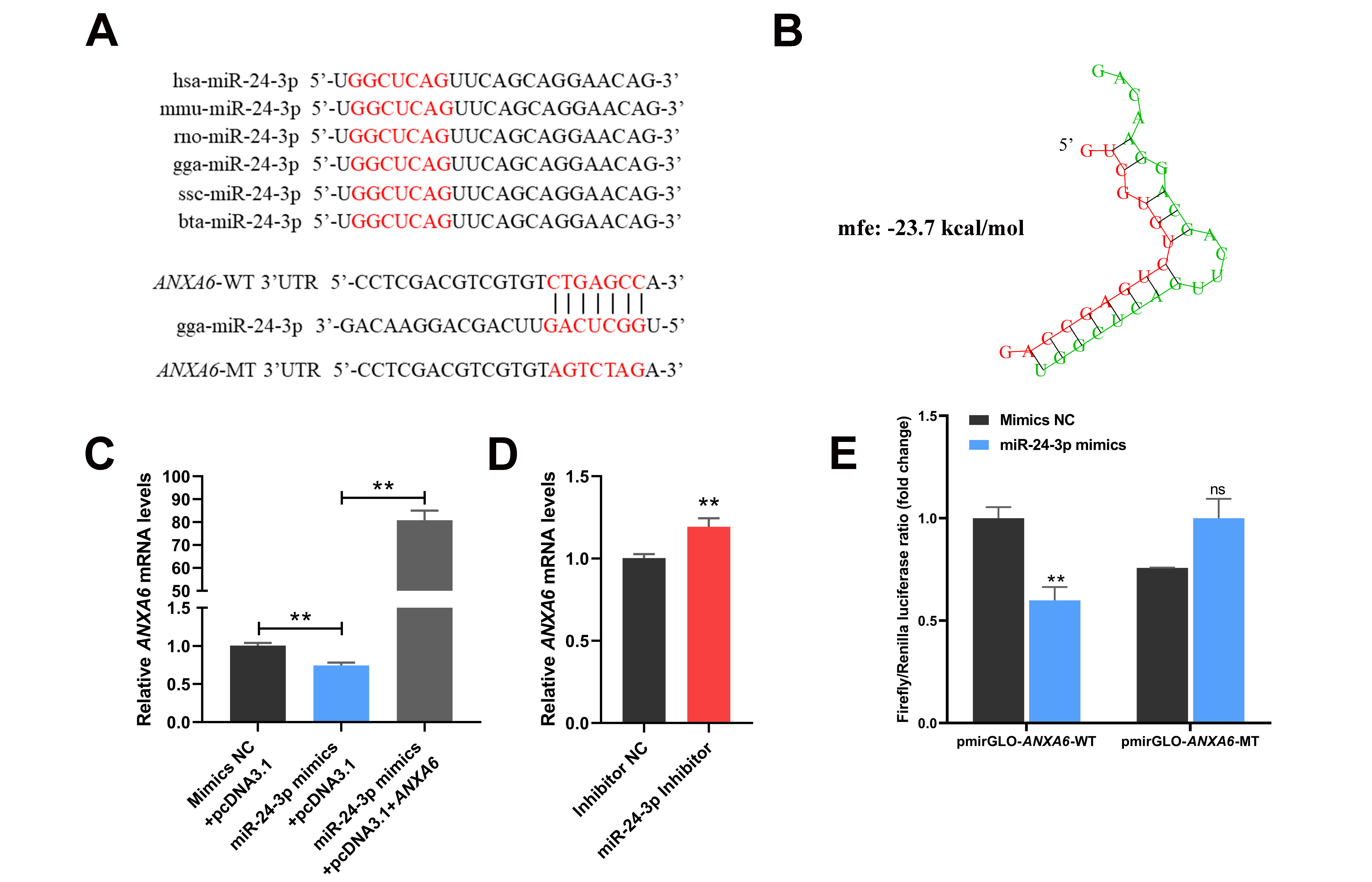
3.4. ANXA6 Is a Target Gene of miR-24-3p
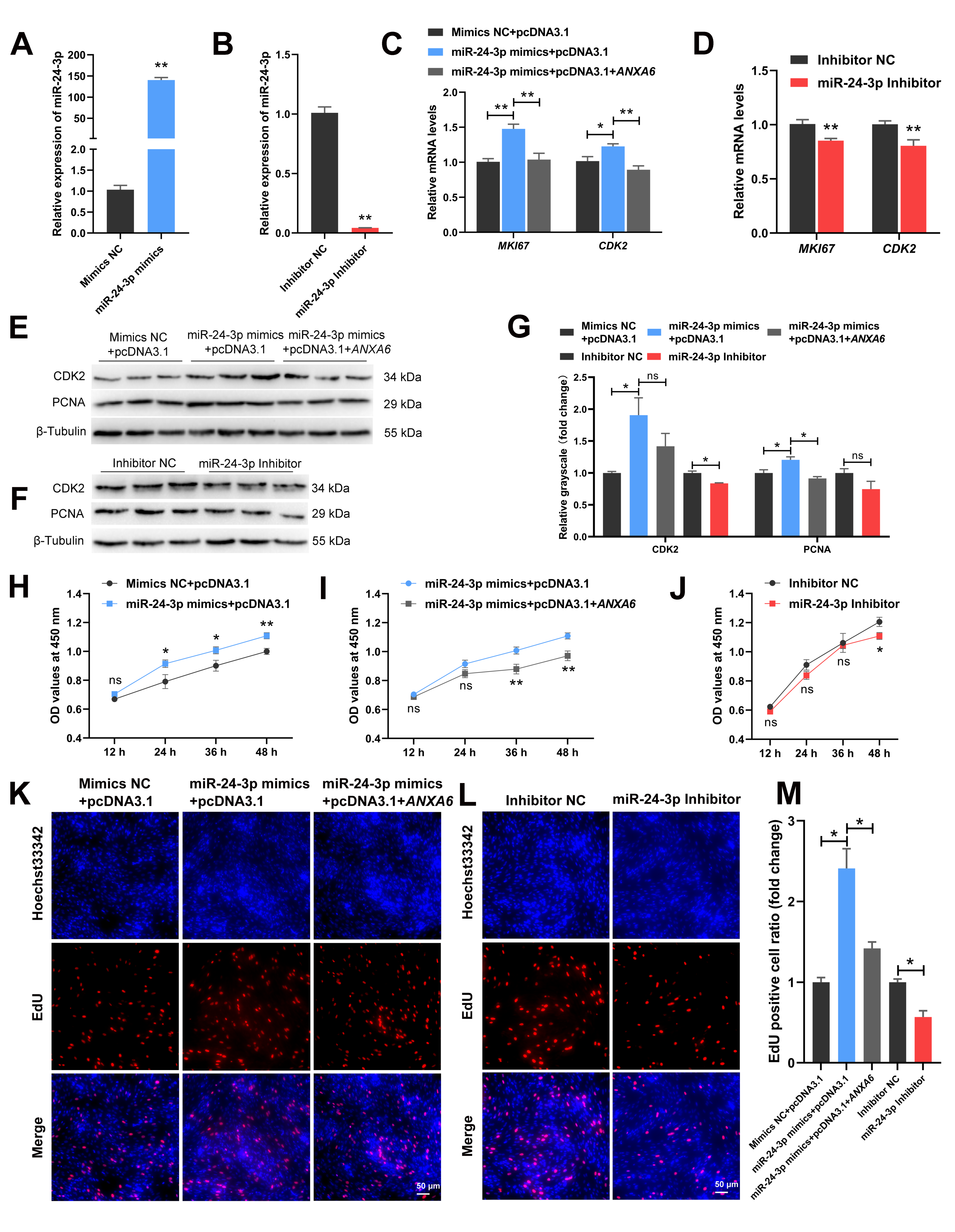
3.5. miR-24-3p Promotes the Proliferation of Chicken Intramuscular Preadipocytes through Targeting ANXA6
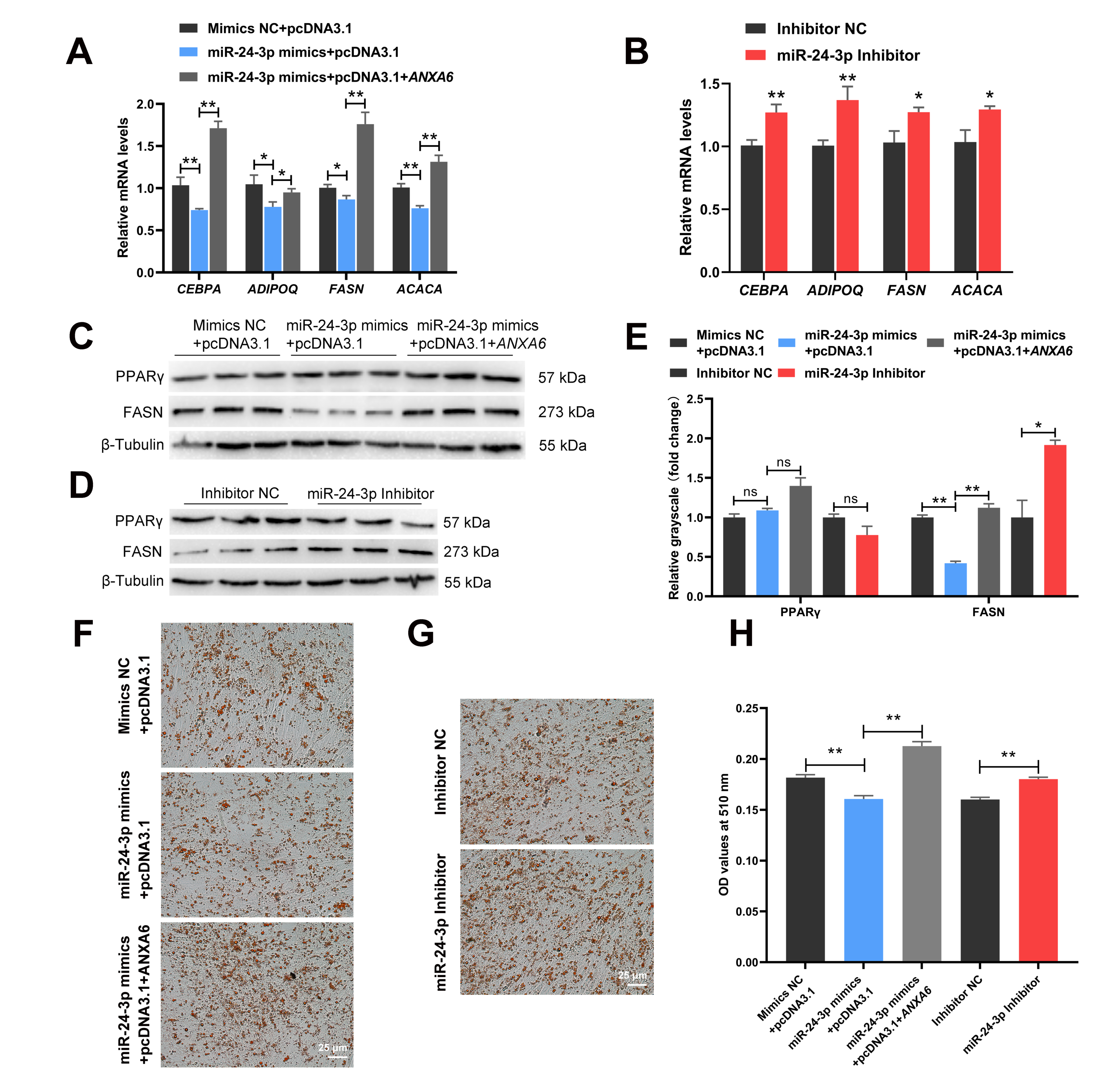
3.6. miR-24-3p Inhibits the Differentiation of Chicken Intramuscular Preadipocytes through Targeting ANXA6
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Radaelli, G.; Piccirillo, A.; Birolo, M.; Bertotto, D.; Gratta, F.; Ballarin, C.; Vascellari, M.; Xiccato, G.; Trocino, A. Effect of age on the occurrence of muscle fiber degeneration associated with myopathies in broiler chickens submitted to feed restriction. Poult. Sci. 2017, 96, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Calnan, H.B.; Jacob, R.H.; Pethick, D.W.; Gardner, G.E. Selection for intramuscular fat and lean meat yield will improve the bloomed colour of Australian lamb loin meat. Meat Sci. 2017, 131, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frank, D.; Joo, S.T.; Warner, R. Consumer Acceptability of Intramuscular Fat. Korean J. Food Sci. Anim. Resour. 2016, 36, 699–708. [Google Scholar] [CrossRef]
- Wosczyna, M.N.; Perez Carbajal, E.E.; Wagner, M.W.; Paredes, S.; Konishi, C.T.; Liu, L.; Wang, T.T.; Walsh, R.A.; Gan, Q.; Morrissey, C.S.; et al. Targeting microRNA-mediated gene repression limits adipogenic conversion of skeletal muscle mesenchymal stromal cells. Cell Stem Cell 2021, 28, 1323–1334. [Google Scholar] [CrossRef]
- Li, X.; Fu, X.; Yang, G.; Du, M. Review: Enhancing intramuscular fat development via targeting fibro-adipogenic progenitor cells in meat animals. Animal 2020, 14, 312–321. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Oliveto, S.; Alfieri, R.; Miluzio, A.; Scagliola, A.; Secli, R.S.; Gasparini, P.; Grosso, S.; Cascione, L.; Mutti, L.; Biffo, S. A Polysome-Based microRNA Screen Identifies miR-24-3p as a Novel Promigratory miRNA in Mesothelioma. Cancer Res. 2018, 78, 5741–5753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Q.; Li, J.; He, H.; Cao, Y.; Li, D.; Amevor, F.K.; Zhang, Y.; Wang, J.; Yu, C.; Yang, C.; et al. miR-23b-3p inhibits chicken granulosa cell proliferation and steroid hormone synthesis via targeting GDF9. Theriogenology 2022, 177, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Han, Y.; Zhai, Y.; Ma, X.; An, X.; Zhang, S.; Li, Z. Integrative analysis of circRNAs, miRNAs, and mRNAs profiles to reveal ceRNAs networks in chicken intramuscular and abdominal adipogenesis. BMC Genom. 2020, 21, 594–607. [Google Scholar] [CrossRef]
- Guo, L.; Chao, X.; Huang, W.; Li, Z.; Luan, K.; Ye, M.; Zhang, S.; Liu, M.; Li, H.; Luo, W.; et al. Whole Transcriptome Analysis Reveals a Potential Regulatory Mechanism of LncRNA-FNIP2/miR-24-3p/FNIP2 Axis in Chicken Adipogenesis. Front. Cell Dev. Biol. 2021, 9, 653798–653815. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Fang, X.; Gao, M.; Mi, J.; Zhang, X.; Xia, L.; Zhao, Z.; Albrecht, E.; Maak, S.; Yang, R. Isolation and Identification of Bovine Preadipocytes and Screening of MicroRNAs Associated with Adipogenesis. Animals 2020, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Martin, W. Factors affecting households’ meat purchase and future meat consumption changes in China: A demand system approach. J. Ethn. Foods 2018, 5, 24–32. [Google Scholar] [CrossRef]
- Li, J.J.; Yang, C.W.; Peng, H.; Yin, H.D.; Wang, Y.; Hu, Y.D.; Yu, C.L.; Jiang, X.S.; Du, H.R.; Li, Q.Y.; et al. Effects of Slaughter Age on Muscle Characteristics and Meat Quality Traits of Da-Heng Meat Type Birds. Animals 2020, 10, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Yang, C.; Ren, P.; Lin, Z.; Zhang, D.; Jiang, X.; Wang, L.; Liu, Y. Transcriptomics analysis of Daheng broilers reveals that PLIN2 regulates chicken preadipocyte proliferation, differentiation and apoptosis. Mol. Biol. Rep. 2021, 48, 7985–7997. [Google Scholar] [CrossRef]
- Park, S.J.; Beak, S.H.; Jung, D.J.S.; Kim, S.Y.; Jeong, I.H.; Piao, M.Y.; Kang, H.J.; Fassah, D.M.; Na, S.W.; Yoo, S.P.; et al. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle—A review. Asian Australas. J. Anim. Sci. 2018, 31, 1043–1061. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yang, J.; Yao, Y.; Shi, X.E.; Yang, G.; Li, X. AQP3 Facilitates Proliferation and Adipogenic Differentiation of Porcine Intramuscular Adipocytes. Genes 2020, 11, 453. [Google Scholar] [CrossRef]
- Wu, W.; Ji, M.; Xu, K.; Zhang, D.; Yin, Y.; Huang, X.; Peng, Y.; Zhang, J. Knockdown of CTRP6 reduces the deposition of intramuscular and subcutaneous fat in pigs via different signaling pathways. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158729–158738. [Google Scholar] [CrossRef]
- Sun, G.R.; Zhang, M.; Sun, J.W.; Li, F.; Ma, X.F.; Li, W.T.; Han, R.L.; Li, Z.J.; Jiang, R.R.; Li, G.X.; et al. Kruppel-like factor KLF9 inhibits chicken intramuscular preadipocyte differentiation. Br. Poult. Sci. 2019, 60, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Xie, L.; Ma, J.-E.; Luo, W.; Zhang, L.; Chao, Z.; Chen, S.; Nie, Q.; Lin, Z.; Zhang, X. Lower Expression of SLC27A1 Enhances Intramuscular Fat Deposition in Chicken via Down-Regulated Fatty Acid Oxidation Mediated by CPT1A. Front. Physiol. 2017, 8, 449. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Zheng, W.; Zhu, L.; Yao, D.; Wang, C.; Song, Y.; Hu, S.; Liu, H.; Bai, Y.; Pan, Y.; et al. ANXA6 Contributes to Radioresistance by Promoting Autophagy via Inhibiting the PI3K/AKT/mTOR Signaling Pathway in Nasopharyngeal Carcinoma. Front. Cell Dev. Biol. 2020, 8, 232. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Shu, Y.; Xu, M.; Jiang, J.; Wang, L.; Wang, J.; Huang, D.; Zhang, J. ANXA6 suppresses the tumorigenesis of cervical cancer through autophagy induction. Clin. Transl. Med. 2020, 10, e208. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.; Fischer, A.W.; Blanco-Munoz, P.; Alvarez-Guaita, A.; Meneses-Salas, E.; Egert, A.; Buechler, C.; Hoy, A.J.; Heeren, J.; Enrich, C.; et al. Altered hepatic glucose homeostasis in AnxA6-KO mice fed a high-fat diet. PLoS ONE 2018, 13, e0201310. [Google Scholar] [CrossRef]
- Qimuge, N.; He, Z.; Qin, J.; Sun, Y.; Wang, X.; Yu, T.; Dong, W.; Yang, G.; Pang, W. Overexpression of DNMT3A promotes proliferation and inhibits differentiation of porcine intramuscular preadipocytes by methylating p21 and PPARg promoters. Gene 2019, 696, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wu, M.; Liu, X.; Wang, F.; Li, M.; An, X.; Bai, F.; Lei, C.; Dang, R. MiR-24-3p Conservatively Regulates Muscle Cell Proliferation and Apoptosis by Targeting Common Gene CAMK2B in Rat and Cattle. Animals 2022, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Ma, J.; Zhu, Y.; Zan, J.; Wang, Z.; Ling, L.; Li, Q.; Lv, J.; Qi, S.; Cao, Y.; et al. miR-24-3p promotes cell migration and proliferation in lung cancer by targeting SOX7. J. Cell. Biochem. 2018, 119, 3989–3998. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Xing, Y.; Ren, L.; Wang, Y.; Li, Q.; Fu, X.; Yang, Q.; Xu, L.; Willems, L.; Li, J.; et al. Bta-miR-24-3p Controls the Myogenic Differentiation and Proliferation of Fetal Bovine Skeletal Muscle-Derived Progenitor Cells by Targeting ACVR1B. Animals 2019, 9, 859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Xu, K.; Li, M.; Zhang, J.; Wang, Y. MicroRNA-29b/29c targeting CTRP6 influences porcine adipogenesis via the AKT/PKA/MAPK Signalling pathway. Adipocyte 2021, 10, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Xu, Y.; Zhang, P.W.; Zhao, X.; Gan, M.L.; Li, Q.; Ma, J.D.; Tang, G.Q.; Jiang, Y.Z.; Wang, J.Y.; et al. MicroRNA-125a-5p Affects Adipocytes Proliferation, Differentiation and Fatty Acid Composition of Porcine Intramuscular Fat. Int. J. Mol. Sci. 2018, 19, 501. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Cai, R.; Tang, G.; Zhang, W.; Pang, W. MiR-146a-5p targeting SMAD4 and TRAF6 inhibits adipogenensis through TGF-β and AKT/mTORC1 signal pathways in porcine intramuscular preadipocytes. J. Anim. Sci. Biotechnol. 2021, 12, 12–27. [Google Scholar] [CrossRef]
- Chen, F.-F.; Xiong, Y.; Peng, Y.; Gao, Y.; Qin, J.; Chu, G.-Y.; Pang, W.-J.; Yang, G.-S. miR-425-5p Inhibits Differentiation and Proliferation in Porcine Intramuscular Preadipocytes. Int. J. Mol. Sci. 2017, 18, 2101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Fu, S.; Chen, Y.; Jin, W.; Zhai, B.; Li, Y.; Sun, G.; Han, R.; Wang, Y.; Tian, Y.; et al. MicroRNA-15a Regulates the Differentiation of Intramuscular Preadipocytes by Targeting ACAA1, ACOX1 and SCP2 in Chickens. Int. J. Mol. Sci. 2019, 20, 4063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, G.; Li, F.; Ma, X.; Sun, J.; Jiang, R.; Tian, Y.; Han, R.; Li, G.; Wang, Y.; Li, Z.; et al. gga-miRNA-18b-3p Inhibits Intramuscular Adipocytes Differentiation in Chicken by Targeting the ACOT13 Gene. Cells 2019, 8, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Li, D.; Zhang, M.; Sun, J.; Li, W.; Jiang, R.; Han, R.; Wang, Y.; Tian, Y.; Kang, X.; et al. miRNA-223 targets the GPAM gene and regulates the differentiation of intramuscular adipocytes. Gene 2019, 685, 106–113. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Tang, Y.; Li, Z.; Li, J.; Yu, C.; Yang, C.; Liu, L.; Wang, Y.; Liu, Y. miR-24-3p Dominates the Proliferation and Differentiation of Chicken Intramuscular Preadipocytes by Blocking ANXA6 Expression. Genes 2022, 13, 635. https://doi.org/10.3390/genes13040635
Lin Z, Tang Y, Li Z, Li J, Yu C, Yang C, Liu L, Wang Y, Liu Y. miR-24-3p Dominates the Proliferation and Differentiation of Chicken Intramuscular Preadipocytes by Blocking ANXA6 Expression. Genes. 2022; 13(4):635. https://doi.org/10.3390/genes13040635
Chicago/Turabian StyleLin, Zhongzhen, Yuan Tang, Zhiqiang Li, Jingjing Li, Chunlin Yu, Chaowu Yang, Li Liu, Yan Wang, and Yiping Liu. 2022. "miR-24-3p Dominates the Proliferation and Differentiation of Chicken Intramuscular Preadipocytes by Blocking ANXA6 Expression" Genes 13, no. 4: 635. https://doi.org/10.3390/genes13040635





