Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review
Abstract
:1. Introduction
- Protection of transgene or genetic cargo from degradative action of systemic and endonucleases,
- Delivery of genetic material to the target site, i.e., either cell cytoplasm or nucleus,
- Low potential of triggering unwanted immune responses or genotoxicity,
- Economical and feasible availability for patients [3].
2. Viral Vectors for Gene Delivery
2.1. Adenoviral Vectors
2.2. Retroviruses and Lentiviruses
2.3. Adeno-Associated Viruses
2.4. Poxviruses
2.5. Other Virus
3. Nonviral Vectors for Gene Delivery
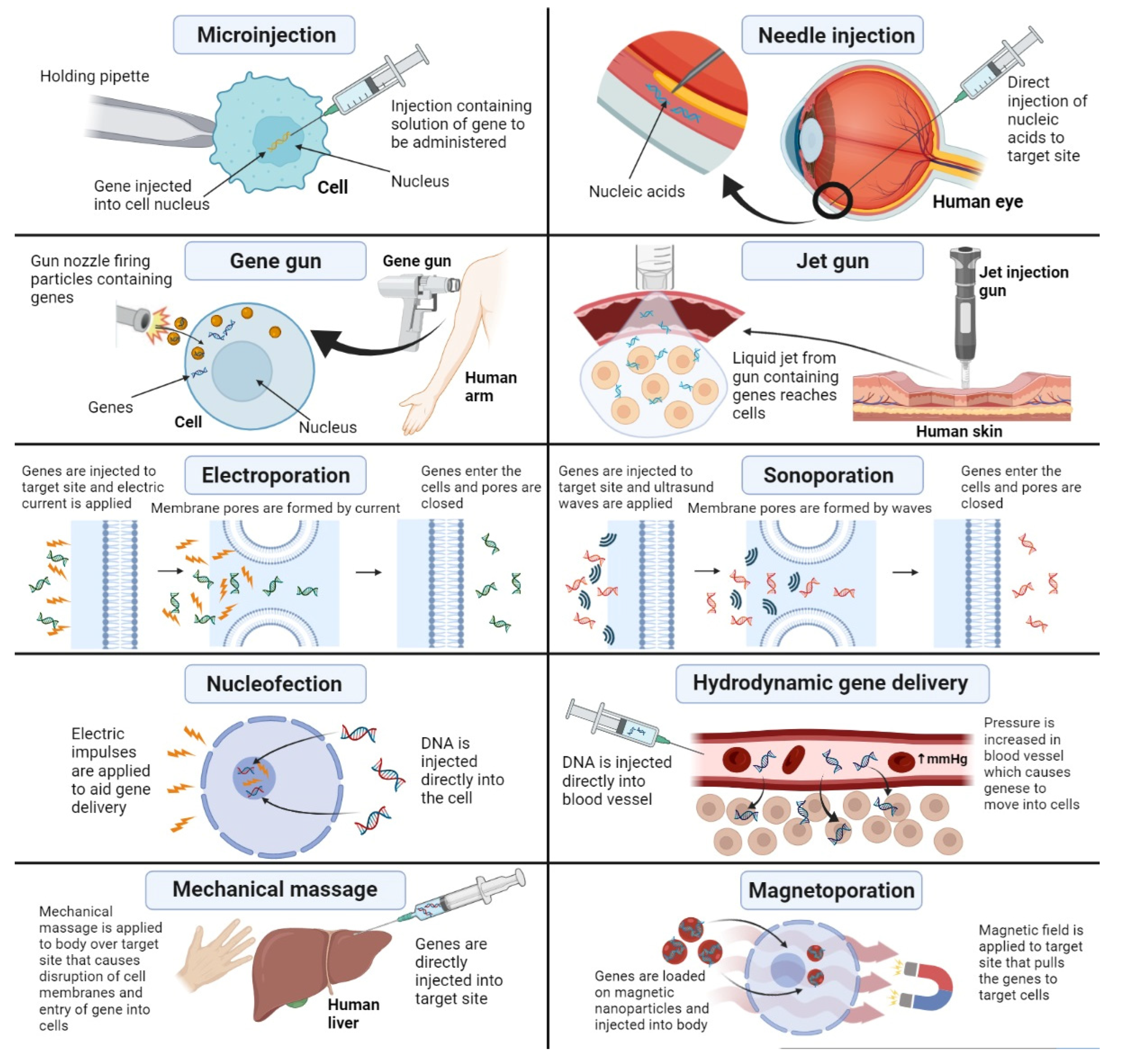
3.1. Physical Methods for Nonviral Gene Delivery
3.1.1. Microinjection
3.1.2. Needle Injection
3.1.3. Jet Gun
3.1.4. Gene Gun
3.1.5. Electroporation
3.1.6. Nucleofection
3.1.7. Sonoporation
3.1.8. Hydrodynamic Gene Transfer
3.1.9. Magnetoporation
3.1.10. Mechanical Massage
3.2. Chemical Systems for Nonviral Gene Delivery
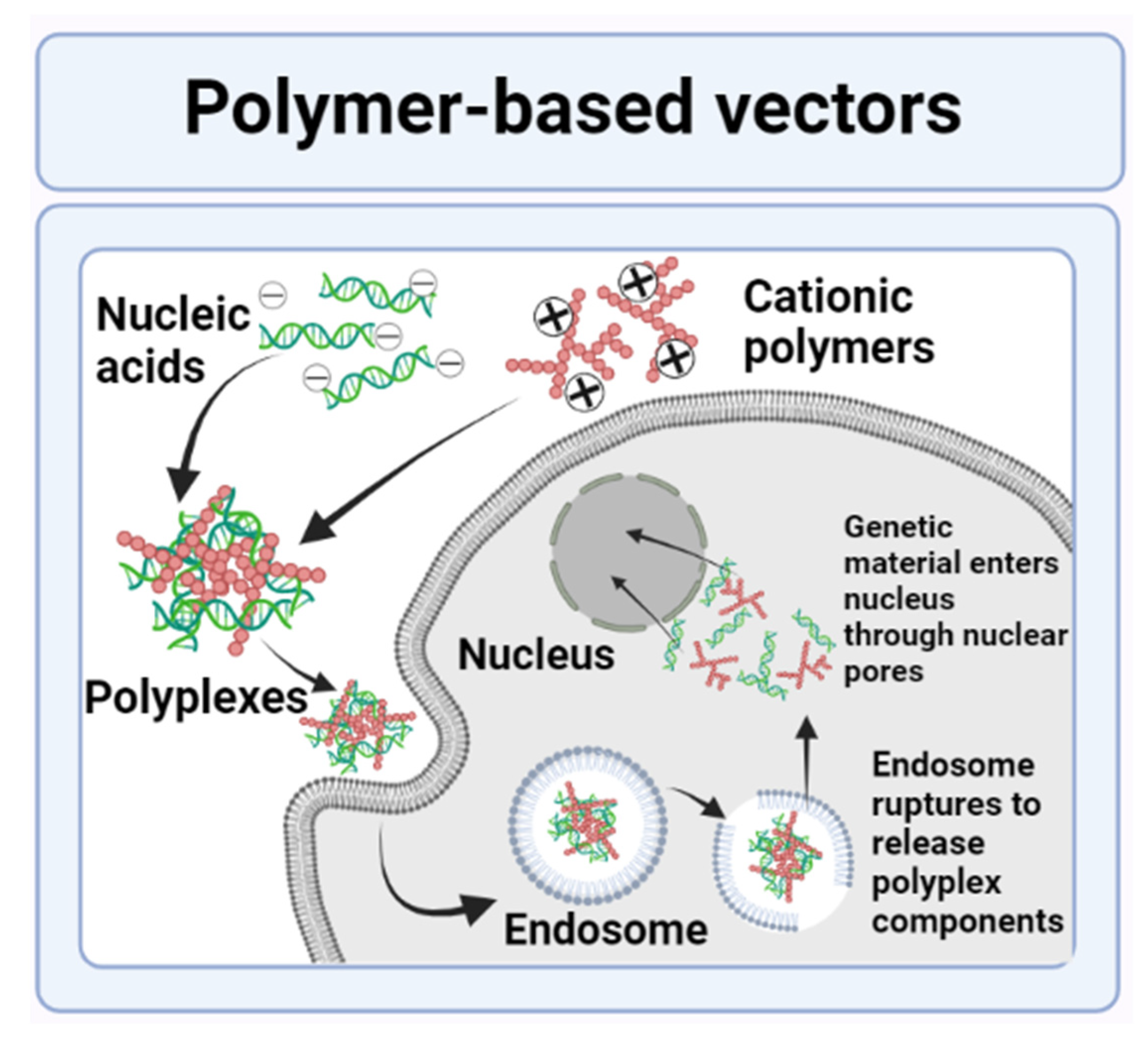
3.2.1. Polymer-Based Nanovectors
Protein-Based Vectors
Polysaccharide-Based Vectors
Polyesters
Polycarbonates
Polyurethanes
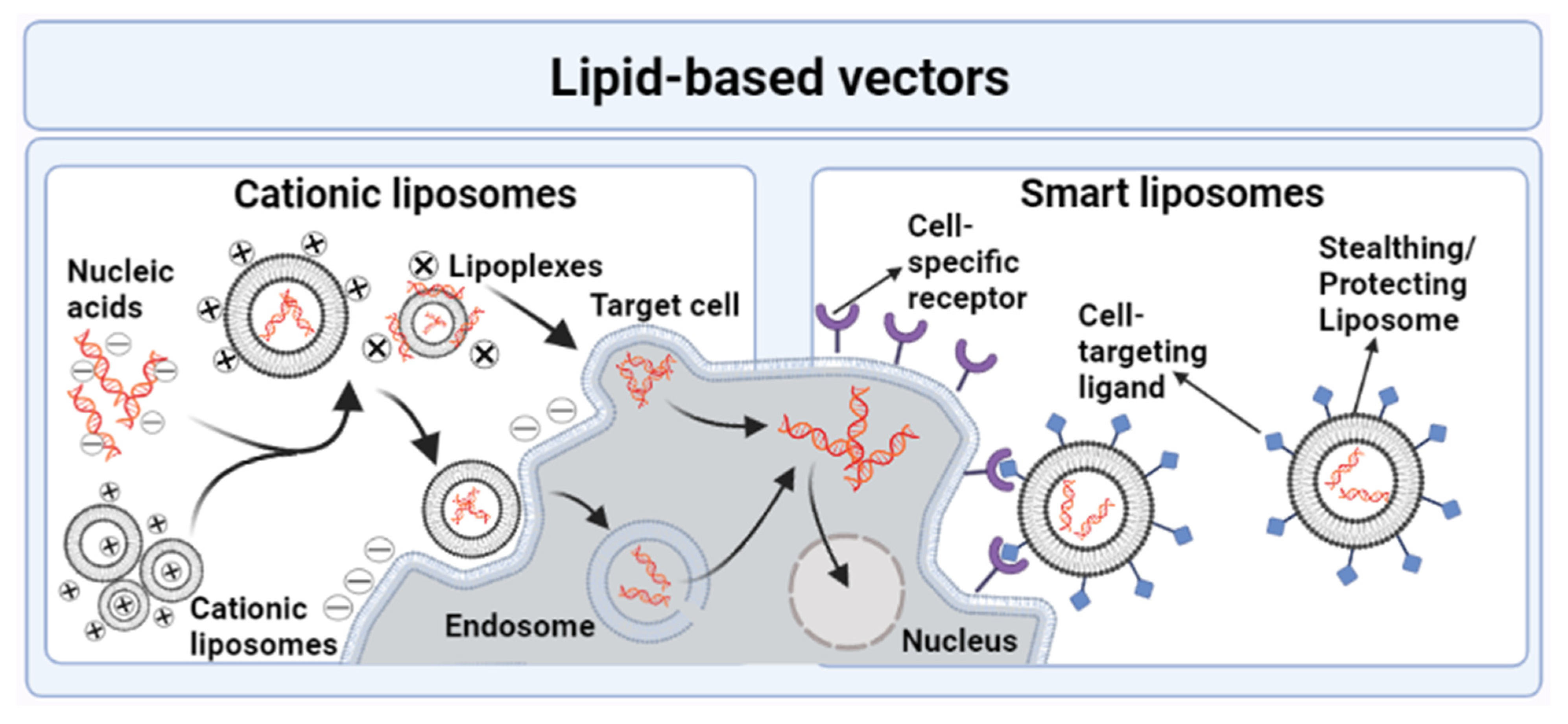
3.2.2. Lipid-Based Nanovectors
Liposomes
- Cationic Liposomes
- Smart Liposomes
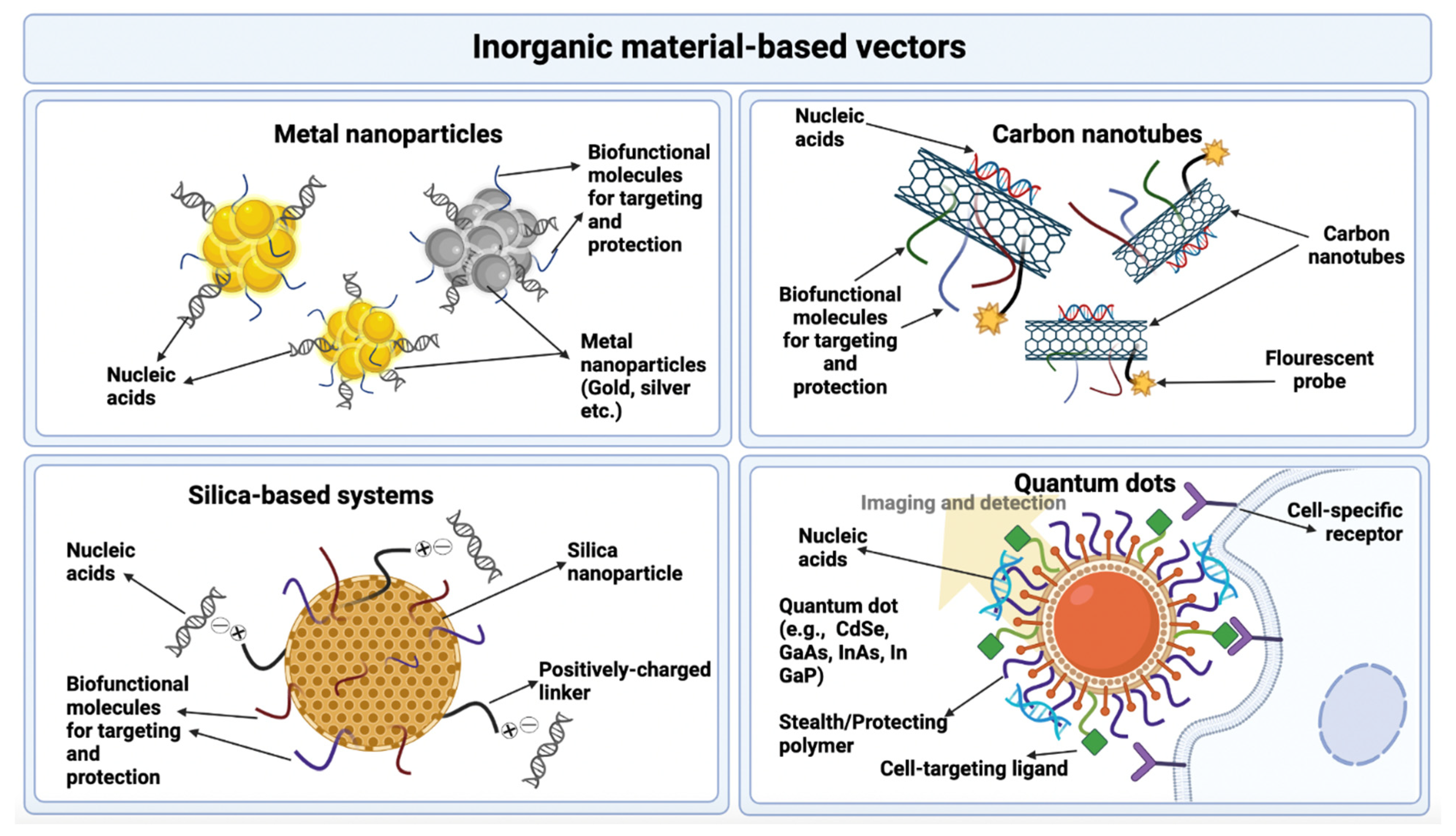
3.2.3. Inorganic Materials
Metal Nanoparticles
Quantum Dots
Carbon Nanotubes
Silica-Based Systems
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, H.; Kauffman, K.J.; Anderson, D.G. Delivery technologies for genome editing. Nat. Rev. Drug Discov. 2017, 16, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Planul, A.; Dalkara, D. Vectors and gene delivery to the retina. Annu. Rev. Vis. Sci. 2017, 3, 121–140. [Google Scholar] [CrossRef]
- Hill, A.B.; Chen, M.; Chen, C.-K.; Pfeifer, B.A.; Jones, C.H. Overcoming gene-delivery hurdles: Physiological considerations for nonviral vectors. Trends Biotechnol. 2016, 34, 91–105. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Satterlee, A.; Huang, L. In vivo gene delivery by nonviral vectors: Overcoming hurdles? Mol. Ther. 2012, 20, 1298–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginn, S.L.; Amaya, A.K.; Alexander, I.E.; Edelstein, M.; Abedi, M.R. Gene therapy clinical trials worldwide to 2017: An update. J. Gene Med. 2018, 20, e3015. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Viral vectors in gene therapy. Diseases 2018, 6, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotterman, M.A.; Chalberg, T.W.; Schaffer, D.V. Viral vectors for gene therapy: Translational and clinical outlook. Annu. Rev. Biomed. Eng. 2015, 17, 63–89. [Google Scholar] [CrossRef] [Green Version]
- Lukashev, A.; Zamyatnin, A. Viral vectors for gene therapy: Current state and clinical perspectives. Biochemistry 2016, 81, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Ju, D.; Santos, A.; Freeman, A.; Daniele, E. Neuroscience; eCampus: Toronto, ON, Canada, 2018. [Google Scholar]
- Desfarges, S.; Ciuffi, A. Viral integration and consequences on host gene expression. In Viruses: Essential Agents of Life; Springer: Berlin/Heidelberg, Germany, 2012; pp. 147–175. [Google Scholar]
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target. Ther. 2021, 6, 53. [Google Scholar] [CrossRef]
- Crenshaw, B.J.; Jones, L.B.; Bell, C.R.; Kumar, S.; Matthews, Q.L. Perspective on adenoviruses: Epidemiology, pathogenicity, and gene therapy. Biomedicines 2019, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Giacca, M. Introduction to gene therapy. In Gene Therapy; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–7. [Google Scholar]
- Tsai, S.; Schillinger, K.; Ye, X. Adenovirus-mediated transfer of regulable gene expression. Curr. Opin. Mol. Ther. 2000, 2, 515–523. [Google Scholar] [PubMed]
- Lyons, M.; Onion, D.; Green, N.K.; Aslan, K.; Rajaratnam, R.; Bazan-Peregrino, M.; Phipps, S.; Hale, S.; Mautner, V.; Seymour, L.W. Adenovirus type 5 interactions with human blood cells may compromise systemic delivery. Mol. Ther. 2006, 14, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Raper, S.E.; Chirmule, N.; Lee, F.S.; Wivel, N.A.; Bagg, A.; Gao, G.-p.; Wilson, J.M.; Batshaw, M.L. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003, 80, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Bandara, R.A.; Chen, Z.R.; Hu, J. Potential of helper-dependent Adenoviral vectors in CRISPR-cas9-mediated lung gene therapy. Cell Biosci. 2021, 11, 145. [Google Scholar] [CrossRef]
- Cao, H.; Ouyang, H.; Laselva, O.; Bartlett, C.; Zhou, Z.P.; Duan, C.; Gunawardena, T.; Avolio, J.; Bear, C.E.; Gonska, T. A helper-dependent adenoviral vector rescues CFTR to wild-type functional levels in cystic fibrosis epithelial cells harbouring class I mutations. Eur. Respir. J. 2020, 56, 2000205. [Google Scholar] [CrossRef] [PubMed]
- Ricobaraza, A.; Gonzalez-Aparicio, M.; Mora-Jimenez, L.; Lumbreras, S.; Hernandez-Alcoceba, R. High-capacity adenoviral vectors: Expanding the scope of gene therapy. Int. J. Mol. Sci. 2020, 21, 3643. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Agrawal, B. Adenoviral vector-based vaccines and gene therapies: Current status and future prospects. Adenoviruses 2019, 4, 53–91. [Google Scholar]
- Chandler, R.J.; Venditti, C.P. Gene therapy for metabolic diseases. Transl. Sci. Rare Dis. 2016, 1, 73–89. [Google Scholar] [CrossRef] [Green Version]
- Pereboev, A.V.; Nagle, J.M.; Shakhmatov, M.A.; Triozzi, P.L.; Matthews, Q.L.; Kawakami, Y.; Curiel, D.T.; Blackwell, J.L. Enhanced gene transfer to mouse dendritic cells using adenoviral vectors coated with a novel adapter molecule. Mol. Ther. 2004, 9, 712–720. [Google Scholar] [CrossRef]
- Stoff-Khalili, M.A.; Rivera, A.A.; Stoff, A.; Michael Mathis, J.; Rocconi, R.P.; Matthews, Q.L.; Numnum, M.T.; Herrmann, I.; Dall, P.; Eckhoff, D.E. Combining high selectivity of replication via CXCR4 promoter with fiber chimerism for effective adenoviral oncolysis in breast cancer. Int. J. Cancer 2007, 120, 935–941. [Google Scholar] [CrossRef]
- Matthews, Q.L.; Sibley, D.A.; Wu, H.; Li, J.; Stoff-Khalili, M.A.; Waehler, R.; Mathis, J.M.; Curiel, D.T. Genetic incorporation of a herpes simplex virus type 1 thymidine kinase and firefly luciferase fusion into the adenovirus protein IX for functional display on the virion. Mol. Imaging 2006, 5, 7290.2006.00029. [Google Scholar] [CrossRef]
- Tang, Y.; Le, L.P.; Matthews, Q.L.; Han, T.; Wu, H.; Curiel, D.T. Derivation of a triple mosaic adenovirus based on modification of the minor capsid protein IX. Virology 2008, 377, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Cattoglio, C.; Pellin, D.; Rizzi, E.; Maruggi, G.; Corti, G.; Miselli, F.; Sartori, D.; Guffanti, A.; Di Serio, C.; Ambrosi, A. High-definition mapping of retroviral integration sites identifies active regulatory elements in human multipotent hematopoietic progenitors. Blood J. Am. Soc. Hematol. 2010, 116, 5507–5517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Li, Y.; Crise, B.; Burgess, S.M. Transcription start regions in the human genome are favored targets for MLV integration. Science 2003, 300, 1749–1751. [Google Scholar] [CrossRef] [PubMed]
- Hacein-Bey-Abina, S.; Garrigue, A.; Wang, G.P.; Soulier, J.; Lim, A.; Morillon, E.; Clappier, E.; Caccavelli, L.; Delabesse, E.; Beldjord, K. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 2008, 118, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Brown, A.M.; Jenkins, C.; Campbell, K. Viral vector systems for gene therapy: A comprehensive literature review of progress and biosafety challenges. Appl. Biosaf. 2020, 25, 7–18. [Google Scholar] [CrossRef]
- Vogt, P.K. Retroviral oncogenes: A historical primer. Nat. Rev. Cancer 2012, 12, 639–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montini, E.; Cesana, D.; Schmidt, M.; Sanvito, F.; Ponzoni, M.; Bartholomae, C.; Sergi, L.S.; Benedicenti, F.; Ambrosi, A.; Di Serio, C. Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration. Nat. Biotechnol. 2006, 24, 687–696. [Google Scholar] [CrossRef]
- Ramezani, A.; Hawley, R.G. Overview of the HIV-1 lentiviral vector system. Curr. Protoc. Mol. Biol. 2002, 60, 16.21. 11–16.21. 15. [Google Scholar] [CrossRef]
- Collins, D.E.; Reuter, J.D.; Rush, H.G.; Villano, J.S. Viral vector biosafety in laboratory animal research. Comp. Med. 2017, 67, 215–221. [Google Scholar]
- Romano, G. Development of safer gene delivery systems to minimize the risk of insertional mutagenesis-related malignancies: A critical issue for the field of gene therapy. Int. Sch. Res. Not. 2012, 2012, 616310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romano, G.; Marino, I.R.; Pentimalli, F.; Adamo, V.; Giordano, A. Insertional mutagenesis and development of malignancies induced by integrating gene delivery systems: Implications for the design of safer gene-based interventions in patients. Drug News Perspect. 2009, 22, 185–196. [Google Scholar] [CrossRef] [PubMed]
- David, R.M.; Doherty, A.T. Viral vectors: The road to reducing genotoxicity. Toxicol. Sci. 2017, 155, 315–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez, A.S.S.; Montes, A.M.S.; Armendáriz-Borunda, J. Viral vectors in gene therapy. Advantages of the adenoassociated vectors. Rev. Gastroenterol. Mex. 2005, 70, 192–202. [Google Scholar]
- Schlimgen, R.; Howard, J.; Wooley, D.; Thompson, M.; Baden, L.R.; Yang, O.O.; Christiani, D.C.; Mostoslavsky, G.; Diamond, D.V.; Duane, E.G. Risks associated with lentiviral vector exposures and prevention strategies. J. Occup. Environ. Med. 2016, 58, 1159. [Google Scholar] [CrossRef] [Green Version]
- Kotterman, M.A.; Schaffer, D.V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 2014, 15, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.-P.; Alvira, M.R.; Wang, L.; Calcedo, R.; Johnston, J.; Wilson, J.M. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. USA 2002, 99, 11854–11859. [Google Scholar] [CrossRef] [Green Version]
- Conrad, S.J.; Liu, J. Poxviruses as gene therapy vectors: Generating Poxviral vectors expressing therapeutic transgenes. In Viral Vectors for Gene Therapy; Springer: Berlin/Heidelberg, Germany, 2019; pp. 189–209. [Google Scholar]
- Mell, L.K.; Brumund, K.T.; Daniels, G.A.; Advani, S.J.; Zakeri, K.; Wright, M.E.; Onyeama, S.-J.; Weisman, R.A.; Sanghvi, P.R.; Martin, P.J. Phase I trial of intravenous oncolytic vaccinia virus (GL-ONC1) with cisplatin and radiotherapy in patients with locoregionally advanced head and neck carcinoma. Clin. Cancer Res. 2017, 23, 5696–5702. [Google Scholar] [CrossRef] [Green Version]
- Kantor, B.; Bailey, R.M.; Wimberly, K.; Kalburgi, S.N.; Gray, S.J. Methods for gene transfer to the central nervous system. Adv. Genet. 2014, 87, 125–197. [Google Scholar]
- Ono, C.; Okamoto, T.; Abe, T.; Matsuura, Y. Baculovirus as a tool for gene delivery and gene therapy. Viruses 2018, 10, 510. [Google Scholar] [CrossRef] [Green Version]
- Weklak, D.; Pembaur, D.; Koukou, G.; Jönsson, F.; Hagedorn, C.; Kreppel, F. Genetic and Chemical Capsid Modifications of Adenovirus Vectors to Modulate Vector–Host Interactions. Viruses 2021, 13, 1300. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, J.; Men, K.; He, Z.; Luo, M.; Qian, Z.; Wei, X.; Wei, Y. Current Status of Nonviral Vectors for Gene Therapy in China. Hum. Gene Ther. 2018, 29, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Hidai, C.; Kitano, H. Nonviral gene therapy for cancer: A review. Diseases 2018, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Jones, C.H.; Hill, A.; Chen, M.; Pfeifer, B.A. Contemporary approaches for nonviral gene therapy. Discov. Med. 2015, 19, 447–454. [Google Scholar] [PubMed]
- Mehier-Humbert, S.; Guy, R.H. Physical methods for gene transfer: Improving the kinetics of gene delivery into cells. Adv. Drug Deliv. Rev. 2005, 57, 733–753. [Google Scholar] [CrossRef] [PubMed]
- Jinturkar, K.A.; Rathi, M.N.; Misra, A. Gene delivery using physical methods. In Challenges in Delivery of Therapeutic Genomics and Proteomics; Elsevier: Amsterdam, The Netherlands, 2011; pp. 83–126. [Google Scholar]
- Hosokawa, Y.; Iguchi, S.; Yasukuni, R.; Hiraki, Y.; Shukunami, C.; Masuhara, H. Gene delivery process in a single animal cell after femtosecond laser microinjection. Appl. Surf. Sci. 2009, 255, 9880–9884. [Google Scholar] [CrossRef]
- King, R. Gene delivery to mammalian cells by microinjection. In Gene Delivery to Mammalian Cells; Springer: Berlin/Heidelberg, Germany, 2004; pp. 167–173. [Google Scholar]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Lui, V.W.Y.; Falo, L.D., Jr.; Huang, L. Systemic production of IL-12 by naked DNA mediated gene transfer: Toxicity and attenuation of transgene expression in vivo. J. Gene Med. 2001, 3, 384. [Google Scholar]
- Springer, M.L.; Rando, T.A.; Blau, H.M. Gene delivery to muscle. Curr. Protoc. Hum. Genet. 2001, 31, 13.14. 11–13.14. 19. [Google Scholar] [CrossRef]
- Wendell, D.M.; Hemond, B.D.; Hogan, N.C.; Taberner, A.J.; Hunter, I.W. The effect of jet parameters on jet injection. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 5005–5008. [Google Scholar]
- Fargnoli, A.; Katz, M.; Williams, R.; Margulies, K.; Bridges, C.R. A needleless liquid jet injection delivery method for cardiac gene therapy: A comparative evaluation versus standard routes of delivery reveals enhanced therapeutic retention and cardiac specific gene expression. J. Cardiovasc. Transl. Res. 2014, 7, 756–767. [Google Scholar] [CrossRef] [Green Version]
- Lysakowski, C.; Dumont, L.; Tramèr, M.R.; Tassonyi, E. A needle-free jet-injection system with lidocaine for peripheral intravenous cannula insertion: A randomized controlled trial with cost-effectiveness analysis. Anesth. Analg. 2003, 96, 215–219. [Google Scholar] [PubMed]
- Chuang, Y.-C.; Chou, A.-K.; Wu, P.-C.; Chiang, P.-H.; Yu, T.-J.; Yang, L.-C.; Yoshimura, N.; Chancellor, M.B. Gene therapy for bladder pain with gene gun particle encoding pro-opiomelanocortin cDNA. J. Urol. 2003, 170, 2044–2048. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Ray, B.; Raut, S.; Sahoo, C. Nonviral gene therapy: Technology and application. Res. J. Sci. Technol. 2021, 13, 13–22. [Google Scholar] [CrossRef]
- Neumann, E.; Schaefer-Ridder, M.; Wang, Y.; Hofschneider, P. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982, 1, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Titomirov, A.V.; Sukharev, S.; Kistanova, E. In vivo electroporation and stable transformation of skin cells of newborn mice by plasmid DNA. Biochim. Et Biophys. Acta (BBA)-Gene Struct. Expr. 1991, 1088, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Heller, L.C.; Heller, R. In vivo electroporation for gene therapy. Hum. Gene Ther. 2006, 17, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Young, J.L.; Dean, D.A. Electroporation-mediated gene delivery. Adv. Genet. 2015, 89, 49–88. [Google Scholar]
- Lambricht, L.; Lopes, A.; Kos, S.; Sersa, G.; Préat, V.; Vandermeulen, G. Clinical potential of electroporation for gene therapy and DNA vaccine delivery. Expert Opin. Drug Deliv. 2016, 13, 295–310. [Google Scholar] [CrossRef]
- Aslan, H.; Zilberman, Y.; Arbeli, V.; Sheyn, D.; Matan, Y.; Liebergall, M.; Li, J.Z.; Helm, G.A.; Gazit, D.; Gazit, Z. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Eng. 2006, 12, 877–889. [Google Scholar] [CrossRef]
- Delalande, A.; Postema, M.; Mignet, N.; Midoux, P.; Pichon, C. Ultrasound and microbubble-assisted gene delivery: Recent advances and ongoing challenges. Ther. Deliv. 2012, 3, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Tang, J.; Halliwell, M. Sonoporation, drug delivery, and gene therapy. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2010, 224, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Unger, E.C.; Hersh, E.; Vannan, M.; Matsunaga, T.O.; McCreery, T. Local drug and gene delivery through microbubbles. Prog. Cardiovasc. Dis. 2001, 44, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Liu, D. Hydrodynamic gene delivery: Its principles and applications. Mol. Ther. 2007, 15, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Fabre, J.; Grehan, A.; Whitehorne, M.; Sawyer, G.; Dong, X.; Salehi, S.; Eckley, L.; Zhang, X.; Seddon, M.; Shah, A. Hydrodynamic gene delivery to the pig liver via an isolated segment of the inferior vena cava. Gene Ther. 2008, 15, 452–462. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Wang, J.; Zhou, Q.; Zhang, L.; Wang, S.; Zhang, Z.; Yao, C. Advanced physical techniques for gene delivery based on membrane perforation. Drug Deliv. 2018, 25, 1516–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Lee, M.-Y.; Hogg, M.G.; Dordick, J.S.; Sharfstein, S.T. Gene delivery in three-dimensional cell cultures by superparamagnetic nanoparticles. ACS Nano 2010, 4, 4733–4743. [Google Scholar] [CrossRef]
- Meacham, J.M.; Durvasula, K.; Degertekin, F.L.; Fedorov, A.G. Physical methods for intracellular delivery: Practical aspects from laboratory use to industrial-scale processing. J. Lab. Autom. 2014, 19, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Ma, N.; Ong, L.L.; Kaminski, A.; Skrabal, C.; Ugurlucan, M.; Lorenz, P.; Gatzen, H.H.; Lützow, K.; Lendlein, A. Enhanced thoracic gene delivery by magnetic nanobead-mediated vector. J. Gene Med. A Cross-Discip. J. Res. Sci. Gene Transf. Its Clin. Appl. 2008, 10, 897–909. [Google Scholar] [CrossRef]
- Prosen, L.; Markelc, B.; Dolinsek, T.; Music, B.; Cemazar, M.; Sersa, G. Mcam silencing with RNA interference using magnetofection has antitumor effect in murine melanoma. Mol. Ther.-Nucleic Acids 2014, 3, e205. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiang, J.; Zhang, W.; Fan, S.; Wu, M.; Li, X.; Li, G. Nanoparticle delivery of anti-metastatic NM23-H1 gene improves chemotherapy in a mouse tumor model. Cancer Gene Ther. 2009, 16, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Huang, L. Noninvasive gene delivery to the liver by mechanical massage. Hepatology 2002, 35, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Lei, J.; Vollmer, R.; Huang, L. Mechanism of liver gene transfer by mechanical massage. Mol. Ther. 2004, 9, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, W.; Ma, N.; Steinhoff, G. Non-viral gene delivery methods. Curr. Pharm. Biotechnol. 2013, 14, 46–60. [Google Scholar] [PubMed]
- Khalid, M.; El-Sawy, H.S. Polymeric nanoparticles: Promising platform for drug delivery. Int. J. Pharm. 2017, 528, 675–691. [Google Scholar]
- Rose, V.L.; Mastrotto, F.; Mantovani, G. Phosphonium polymers for gene delivery. Polym. Chem. 2017, 8, 353–360. [Google Scholar] [CrossRef]
- Nóbrega, C.; Mendonça, L.; Matos, C.A. A Handbook of Gene and Cell Therapy; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Park, T.G.; Jeong, J.H.; Kim, S.W. Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 467–486. [Google Scholar] [CrossRef]
- Jäger, M.; Schubert, S.; Ochrimenko, S.; Fischer, D.; Schubert, U.S. Branched and linear poly (ethylene imine)-based conjugates: Synthetic modification, characterization, and application. Chem. Soc. Rev. 2012, 41, 4755–4767. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Zhou, C.; Jiao, Y. Hydrophobic modifications of cationic polymers for gene delivery. Prog. Polym. Sci. 2010, 35, 1144–1162. [Google Scholar] [CrossRef]
- Cavalu, S.; Damian, G.; Dansoreanu, M. EPR study of non-covalent spin labeled serum albumin and hemoglobin. Biophys. Chem. 2002, 99, 181–188. [Google Scholar] [CrossRef]
- Luten, J.; van Nostrum, C.F.; De Smedt, S.C.; Hennink, W.E. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J. Control. Release 2008, 126, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Pashuck, E.T.; Stevens, M.M. Designing regenerative biomaterial therapies for the clinic. Sci. Transl. Med. 2012, 4, 160sr4. [Google Scholar] [CrossRef] [PubMed]
- Vert, M. Degradable polymers in medicine: Updating strategies and terminology. Int. J. Artif. Organs 2011, 34, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762. [Google Scholar] [CrossRef]
- Liu, X.; Holzwarth, J.M.; Ma, P.X. Functionalized synthetic biodegradable polymer scaffolds for tissue engineering. Macromol. Biosci. 2012, 12, 911–919. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Cavalu, S.; Antoniac, I.V.; Mohan, A.; Bodog, F.; Doicin, C.; Mates, I.; Ulmeanu, M.; Murzac, R.; Semenescu, A. Nanoparticles and Nanostructured Surface Fabrication for Innovative Cranial and Maxillofacial Surgery. Materials 2020, 13, 5391. [Google Scholar] [CrossRef]
- Lee, S.J.; Yhee, J.Y.; Kim, S.H.; Kwon, I.C.; Kim, K. Biocompatible gelatin nanoparticles for tumor-targeted delivery of polymerized siRNA in tumor-bearing mice. J. Control. Release 2013, 172, 358–366. [Google Scholar] [CrossRef]
- Youngren, S.R.; Tekade, R.K.; Gustilo, B.; Hoffmann, P.R.; Chougule, M.B. STAT6 siRNA matrix-loaded gelatin nanocarriers: Formulation, characterization, and ex vivo proof of concept using adenocarcinoma cells. BioMed Res. Int. 2013, 2013, 858946. [Google Scholar] [CrossRef] [Green Version]
- Syga, M.-I.; Nicolì, E.; Kohler, E.; Shastri, V.P. Albumin incorporation in polyethylenimine–DNA polyplexes influences transfection efficiency. Biomacromolecules 2016, 17, 200–207. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Xu, F.-J. Versatile functionalization of polysaccharides via polymer grafts: From design to biomedical applications. Acc. Chem. Res. 2017, 50, 281–292. [Google Scholar] [CrossRef]
- Cavalu, S.; Bisboaca, S.; Mates, I.M.; Pasca, P.M.; Laslo, V.; Costea, T.; Fritea, L.; Vicas, S. Novel Formulation Based on Chitosan-Arabic Gum Nanoparticles Entrapping Propolis Extract Production, physico-chemical and structural characterization. Rev. Chim. 2018, 69, 3756–3760. [Google Scholar] [CrossRef]
- Kean, T.; Roth, S.; Thanou, M. Trimethylated chitosans as non-viral gene delivery vectors: Cytotoxicity and transfection efficiency. J. Control. Release 2005, 103, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Lee, W.-C.; Shieh, M.-J. Hyaluronic acid conjugated micelles possessing CD44 targeting potential for gene delivery. Carbohydr. Polym. 2017, 155, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jiang, L.; Shi, R.; Zhang, L. Synthesis, preparation, in vitro degradation, and application of novel degradable bioelastomers—A review. Prog. Polym. Sci. 2012, 37, 715–765. [Google Scholar] [CrossRef]
- Reul, R.; Nguyen, J.; Kissel, T. Amine-modified hyperbranched polyesters as non-toxic, biodegradable gene delivery systems. Biomaterials 2009, 30, 5815–5824. [Google Scholar] [CrossRef]
- Liang, S.; Yang, X.Z.; Du, X.J.; Wang, H.X.; Li, H.J.; Liu, W.W.; Yao, Y.D.; Zhu, Y.H.; Ma, Y.C.; Wang, J. Optimizing the size of micellar nanoparticles for efficient siRNA delivery. Adv. Funct. Mater. 2015, 25, 4778–4787. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Xiong, M.-H.; Shu, X.-T.; Tang, R.-Z.; Wang, J. Therapeutic delivery of siRNA silencing HIF-1 α with micellar nanoparticles inhibits hypoxic tumor growth. Mol. Pharm. 2012, 9, 2863–2874. [Google Scholar] [CrossRef]
- Sun, T.-M.; Du, J.-Z.; Yao, Y.-D.; Mao, C.-Q.; Dou, S.; Huang, S.-Y.; Zhang, P.-Z.; Leong, K.W.; Song, E.-W.; Wang, J. Simultaneous delivery of siRNA and paclitaxel via a “two-in-one” micelleplex promotes synergistic tumor suppression. ACS Nano 2011, 5, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Meng, F.; Cheng, R.; Deng, C.; Feijen, J.; Zhong, Z. Advanced drug and gene delivery systems based on functional biodegradable polycarbonates and copolymers. J. Control. Release 2014, 190, 398–414. [Google Scholar] [CrossRef]
- Frère, A.; Kawalec, M.; Tempelaar, S.; Peixoto, P.; Hendrick, E.; Peulen, O.; Evrard, B.; Dubois, P.; Mespouille, L.; Mottet, D. Impact of the structure of biocompatible aliphatic polycarbonates on siRNA transfection ability. Biomacromolecules 2015, 16, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Ong, Z.Y.; Yang, Y.Y.; Ee, P.L.R.; Hedrick, J.L. Novel biodegradable block copolymers of poly (ethylene glycol)(PEG) and cationic polycarbonate: Effects of PEG configuration on gene delivery. Macromol. Rapid Commun. 2011, 32, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-P.; Chien, Y.; Chiou, G.-Y.; Cherng, J.-Y.; Wang, M.-L.; Lo, W.-L.; Chang, Y.-L.; Huang, P.-I.; Chen, Y.-W.; Shih, Y.-H. Inhibition of cancer stem cell-like properties and reduced chemoradioresistance of glioblastoma using microRNA145 with cationic polyurethane-short branch PEI. Biomaterials 2012, 33, 1462–1476. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Malabadi, R.B.; Meti, N.T.; Chalannavar, R.K. Applications of nanotechnology in vaccine development for coronavirus (SARS-CoV-2) disease (COVID-19). Int. J. Res. Sci. Innov. 2021, 8, 191–198. [Google Scholar]
- Drummond, D.C.; Noble, C.O.; Hayes, M.E.; Park, J.W.; Kirpotin, D.B. Pharmacokinetics and in vivo drug release rates in liposomal nanocarrier development. J. Pharm. Sci. 2008, 97, 4696–4740. [Google Scholar] [CrossRef]
- Ewert, K.K.; Zidovska, A.; Ahmad, A.; Bouxsein, N.F.; Evans, H.M.; McAllister, C.S.; Samuel, C.E.; Safinya, C.R. Cationic liposome–nucleic acid complexes for gene delivery and silencing: Pathways and mechanisms for plasmid DNA and siRNA. Nucleic Acid Transfection 2010, 296, 191–226. [Google Scholar]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Mendonça, L.; de Lima, M.P.; Simões, S. Targeted lipid-based systems for siRNA delivery. J. Drug Deliv. Sci. Technol. 2012, 22, 65–73. [Google Scholar] [CrossRef]
- Karmali, P.P.; Chaudhuri, A. Cationic liposomes as non-viral carriers of gene medicines: Resolved issues, open questions, and future promises. Med. Res. Rev. 2007, 27, 696–722. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Üstündağ Okur, N.; Karavas, E.; Bikiaris, D.N. Surface modified multifunctional and stimuli responsive nanoparticles for drug targeting: Current status and uses. Int. J. Mol. Sci. 2016, 17, 1440. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, L.S.; Moreira, J.N.; de Lima, M.C.P.; Simoes, S. Co-encapsulation of anti-BCR-ABL siRNA and imatinib mesylate in transferrin receptor-targeted sterically stabilized liposomes for chronic myeloid leukemia treatment. Biotechnol. Bioeng. 2010, 107, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Kanamala, M.; Palmer, B.D.; Jamieson, S.M.; Wilson, W.R.; Wu, Z. Dual pH-sensitive liposomes with low pH-triggered sheddable PEG for enhanced tumor-targeted drug delivery. Nanomedicine 2019, 14, 1971–1989. [Google Scholar] [CrossRef]
- Zangabad, P.S.; Mirkiani, S.; Shahsavari, S.; Masoudi, B.; Masroor, M.; Hamed, H.; Jafari, Z.; Taghipour, Y.D.; Hashemi, H.; Karimi, M. Stimulus-responsive liposomes as smart nanoplatforms for drug delivery applications. Nanotechnol. Rev. 2018, 7, 95–122. [Google Scholar] [CrossRef] [Green Version]
- Hossen, S.; Hossain, M.K.; Basher, M.; Mia, M.; Rahman, M.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Dastidar, D.G.; Chakrabarti, G. Thermoresponsive drug delivery systems, characterization and application. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 133–155. [Google Scholar]
- Mollazadeh, S.; Mackiewicz, M.; Yazdimamaghani, M. Recent advances in the redox-responsive drug delivery nanoplatforms: A chemical structure and physical property perspective. Mater. Sci. Eng. C 2021, 118, 111536. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Bharadwaj, P.; Bilal, M.; Barani, M.; Rahdar, A.; Taboada, P.; Bungau, S.; Kyzas, G.Z. Stimuli-responsive polymeric nanocarriers for drug delivery, imaging, and theragnosis. Polymers 2020, 12, 1397. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The smart drug delivery system and its clinical potential. Theranostics 2016, 6, 1306. [Google Scholar] [CrossRef] [PubMed]
- Riley, M.K.; Vermerris, W. Recent advances in nanomaterials for gene delivery—a review. Nanomaterials 2017, 7, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.-H.; Niu, J.; Zhang, C.-Z.; Yu, W.; Wu, J.-H.; Shan, Y.-H.; Wang, X.-R.; Shen, Y.-Q.; Mao, Z.-W.; Liang, W.-Q. TAT conjugated cationic noble metal nanoparticles for gene delivery to epidermal stem cells. Biomaterials 2014, 35, 5605–5618. [Google Scholar] [CrossRef]
- Kundu, S. Gold nanoparticles: Their application as antimicrobial agents and vehicles of gene delivery. Adv. Biotechnol. Microbiol. 2017, 4, 555–658. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, A.C.; Mitragotri, S. A review of clinical translation of inorganic nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, A.; Hashemi, E. A pseudohomogeneous nanocarrier based on carbon quantum dots decorated with arginine as an efficient gene delivery vehicle. Sci. Rep. 2021, 11, 13790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, T.-H. Quantum dot enabled molecular sensing and diagnostics. Theranostics 2012, 2, 631. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Perez, V.; Cifuentes, A.; Coronas, N.; de Pablo, A.; Borrós, S. Modification of carbon nanotubes for gene delivery vectors. In Nanomaterial Interfaces in Biology; Springer: Berlin/Heidelberg, Germany, 2013; pp. 261–268. [Google Scholar]
- Mebert, A.M.; Baglole, C.J.; Desimone, M.F.; Maysinger, D. Nanoengineered silica: Properties, applications and toxicity. Food Chem. Toxicol. 2017, 109, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Banica, F.; Gruian, C.; Vanea, E.; Goller, G.; Simon, V. Microscopic and spectroscopic investigation of bioactive glasses for antibiotic controlled release. J. Mol. Struct. 2013, 1040, 47–52. [Google Scholar] [CrossRef]
- Huang, X.; Zhuang, J.; Teng, X.; Li, L.; Chen, D.; Yan, X.; Tang, F. The promotion of human malignant melanoma growth by mesoporous silica nanoparticles through decreased reactive oxygen species. Biomaterials 2010, 31, 6142–6153. [Google Scholar] [CrossRef]



| Advantages | Disadvantages |
|---|---|
| Provide greater gene transfer efficiency in both in vivo and in vitro environments | Can trigger severe immune responses and inflammatory reactions |
| Persist for longer periods of time in most cases | Their cloning capacity is very limited |
| Can target a large number of cells | Produced by complex production methods |
| A large variety of viruses are available to choose from | Low capability of tropism to some specific target cells |
| Innate ability of tropism toward infection | Can cause mutagenesis by inserting their exogenous DNA into the host genome |
| Capable of evading endosomes by various mechanisms learned by evolution of viruses | Research is needed to further understand the mechanisms of molecular infection by viruses |
| Techniques | Advantages | Disadvantages |
|---|---|---|
| Microinjection | Allows delivery of large amount of genetic material, convenient, simple, cost-effective, less toxic, and reproducible | Special handling technique is required and cannot be used for large number of cells transfection |
| Needle injection | Simple to perform and requires small amount of DNA | Therapeutic efficacy is quite low and is difficult to conduct |
| Jet gun | Noninvasive, safe, and easily controllable | Causes local tissue damage and efficiency is low |
| Gene gun | Nontoxic, highly effective, and allows gene delivery to cells that are difficult to transfect | Limited to superficial cells and cannot be used for gene delivery to cells where deep penetration is required |
| Electroporation | Fast, effective, reproducible, and allows delivery of large quantities of DNA | Requires surgery, risk of DNA damage due to exposure to high voltage, and highly localized |
| Nucleofection | Fast and efficient in cases where cell membranes are difficult to permeate | Very limited application for in vivo gene delivery and can be highly toxic |
| Sonoporation | Noninvasive, capable of reaching deep tissues and organs, can be used for specific local targets, and capable of crossing blood–brain barrier | Efficiency is relatively low and target cells can be damaged |
| Hydrodynamic gene transfer | Simple and very efficient in deliver of gene to liver cells | Injection volume required is very large and clinically not feasible |
| Magnetoporation | Noninvasive and capable of reaching cells that are deep and demand complex transfection | Special equipment is required, preparation of magnetic vectors is complex, and magnetic reagent can cause toxicity after removal of magnetic field |
| Mechanical Massage | Simple, noninvasive, and easy to apply | Efficiency is low and application is not yet available for humans |
| Polymer-Based Vector | Advantages | Disadvantages |
|---|---|---|
| Protein-based vectors | Highly biocompatible, biodegradable, and non-toxic | Have low mechanical strength and are vulnerable to rapid degradation by biological components |
| Polysaccharide-based vectors | Highly biocompatible, biodegradable, hydrophilic, nontoxic, and easily modifiable with ligands and functional groups | Lack cationic groups of their own and need to be modified to make them interact with genetic materials |
| Polyesters | Have a compatible and biologically recognizable backbone that is analogous to nucleic acids | Complex molecular structure that is difficult to study and modify |
| Polycarbonates | Nontoxic, highly biocompatible, and controllable mechanical properties | Need to be modified with ligands to avoid unwanted immune reactions |
| Polyurethanes | Highly elastic, flexible, and biocompatible | Need strict control of molecular weight to form complex with DNA |
| Lipid-Based Vector | Advantages | Disadvantages |
|---|---|---|
| Cationic liposomes | Fusogenic, compatible with DNA, and can carry large amount of genetic material | Are unstable and can result in aggregation, micelle formation, poor distribution, and toxicity via intravenous route |
| Smart liposomes | Stealth properties and ability to circulate in bloodstream for longer periods | Surface needs to be coated with polymers or protective substances to prevent interaction with or degradation by blood components |
| Inorganic Material | Advantages | Disadvantages |
|---|---|---|
| Metal nanoparticles | Allow molecular tracking after administration, can be modified to achieve targeting, biocompatible, and easy manufacturing | Special equipment is required for preparation |
| Quantum dots | Capable of conjugating with many ligands and biomolecules for specialized targeting, and very sensitive to tracking probes | Production mechanism is complex and requires sensitive handling |
| Carbon nanotubes | Nanosized, amazing capacity to load drugs, remarkable efficiency, and chemically inert | Poor aqueous solubility, complex manufacturing, and expensive |
| Silica-based systems | Allow vast chemical modification, low cytotoxicity, good storage capacity, stable, and amazing capacity to load drugs | Can cause disruption in metabolic process, toxicity, and hemolysis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butt, M.H.; Zaman, M.; Ahmad, A.; Khan, R.; Mallhi, T.H.; Hasan, M.M.; Khan, Y.H.; Hafeez, S.; Massoud, E.E.S.; Rahman, M.H.; et al. Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review. Genes 2022, 13, 1370. https://doi.org/10.3390/genes13081370
Butt MH, Zaman M, Ahmad A, Khan R, Mallhi TH, Hasan MM, Khan YH, Hafeez S, Massoud EES, Rahman MH, et al. Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review. Genes. 2022; 13(8):1370. https://doi.org/10.3390/genes13081370
Chicago/Turabian StyleButt, Muhammad Hammad, Muhammad Zaman, Abrar Ahmad, Rahima Khan, Tauqeer Hussain Mallhi, Mohammad Mehedi Hasan, Yusra Habib Khan, Sara Hafeez, Ehab El Sayed Massoud, Md. Habibur Rahman, and et al. 2022. "Appraisal for the Potential of Viral and Nonviral Vectors in Gene Therapy: A Review" Genes 13, no. 8: 1370. https://doi.org/10.3390/genes13081370










