Prognostic Factors and Markers in Non-Small Cell Lung Cancer: Recent Progress and Future Challenges
Abstract
:1. Introduction
2. Epidemiology and Molecular/Genetic Basis of Lung Cancer
2.1. KRAS
2.2. EGFR
2.3. ALK
2.4. TP53
2.5. TAK1-NF-kB
3. Molecular Targeted Therapy in Lung Cancer
3.1. KRAS
3.2. EGFR TKIs (EGFR Tyrosine-Kinase Inhibitors)
3.3. ALK
3.4. TP53
4. Clinical Trials of Therapeutic Antibodies and TKIS
4.1. Nivolumab
4.2. Pembrolizumab
4.3. Durvalumab
4.4. Sacituzumab Govitecan
4.5. Datopotamab Deruxtecan
4.6. Afatinib
4.7. Gefitinib
4.8. Erlotinib
4.9. Dacomitinib
4.10. Osimertinib
4.11. ALK Inhibitors
4.12. MEK Inhibitors
5. Inhibitors: Mechanism of Action
5.1. ALK Inhibitors
5.2. BRAFV600E Inhibitors
5.3. NTRK Fusion Inhibitors
5.4. EFGR Inhibitors
5.5. RET Rearrangements Inhibitors
5.6. CD73-Targeted Therapy
5.7. VEGF (Vascular Endothelial Growth Factor) Inhibitors
5.8. Anti-CLDN18.2
5.9. Anti-MARCO and Anti-IL37R
5.10. KRAS Inhibitors
5.11. MET Inhibitors
5.12. ERBB2 (HER2) Inhibitors
5.13. Anti-PD-1/Anti-PD-L1 Antibodies
5.14. Anti-TROP2 Antibodies
6. How to Reverse Resistance to Therapeutic Antibodies and TKIS
6.1. Resistance to mAbs and TKIs
6.1.1. Main Genetic Alterations
6.1.2. Elucidating Epigenetics Alterations in Lung Cancer for Improved Therapeutic Interventions
6.2. Exploring Alternatives Approaches to Reverse Resistance
6.2.1. Overcoming Resistance Mechanisms in ALK+ NSCLC
6.2.2. Combining PD-1/PD-L1 Inhibitors with Targeted Therapy
6.2.3. EGFR Therapy with Exon 20 Insertion Mutant Receptor
Mobocertinib
Amivantamab
7. Novel Biomarkers for NSCLC
7.1. Epigenetics as a Source of Novel Biomarkers for Lung Cancer
7.2. Single-Cell Analysis: A Recent and Promising Strategy for Biomarker Discovery
7.2.1. Biomarkers and Single-Cell Analysis
7.2.2. RNA-Seq and Single-Cell Analysis
7.2.3. Bioinformatics and Single-Cell Analysis
7.3. Multiple Cellular Stress Responses in Lung Cancer: A Source of Novel Biomarkers?
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sankar, K.; Gadgeel, S.M.; Qin, A. Molecular Therapeutic Targets in Non-Small Cell Lung Cancer. Expert Rev. Anticancer Ther. 2020, 20, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Rodak, O.; Peris-Díaz, M.D.; Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P. Current Landscape of Non-Small Cell Lung Cancer: Epidemiology, Histological Classification, Targeted Therapies, and Immunotherapy. Cancers 2021, 13, 4705. [Google Scholar] [CrossRef]
- Atal, S.; Asokan, P.; Jhaj, R. Recent Advances in Targeted Small-molecule Inhibitor Therapy for Non–Small-cell Lung Cancer—An Update. J. Clin. Pharm. Ther. 2020, 45, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Du, Y.; Wen, R.; Yang, M.; Xu, J. Drug Resistance to Targeted Therapeutic Strategies in Non-Small Cell Lung Cancer. Pharmacol. Ther. 2020, 206, 107438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wan, J.; Guo, M.; Wang, Y.; Yang, Z.; Zhou, F.; Li, Z.; Ming, L. Expression and Prognostic Significance of M6A-related Genes in TP53-mutant Non-small-cell Lung Cancer. J. Clin. Lab. Anal. 2022, 36, e24118. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Antonia, T.; Simmons, V.N.; Sutton, S.K.; Schabath, M.B.; Alam, I.; Chiappori, A.; Quinn, G.P. Use of Biomarker Testing in Lung Cancer among Puerto Rico and Florida Physicians: Results of a Comparative Study. J. Clincal Pathw. 2019, 5, 33–40. [Google Scholar] [CrossRef]
- Heath, E.I.; Lynce, F.; Xiu, J.; Ellerbrock, A.; Reddy, S.K.; Obeid, E.; Liu, S.V.; Bollig-Fischer, A.; Separovic, D.; Vanderwalde, A. Racial Disparities in the Molecular Landscape of Cancer. Anticancer Res. 2018, 38, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Assoun, S.; Theou-Anton, N.; Nguenang, M.; Cazes, A.; Danel, C.; Abbar, B.; Pluvy, J.; Gounant, V.; Khalil, A.; Namour, C.; et al. Association of TP53 Mutations with Response and Longer Survival under Immune Checkpoint Inhibitors in Advanced Non-Small-Cell Lung Cancer. Lung Cancer 2019, 132, 65–71. [Google Scholar] [CrossRef]
- Letellier, N.; Wing, S.E.; Yang, J.-A.; Gray, S.W.; Benmarhnia, T.; Erhunmwunsee, L.; Jankowska, M.M. The Role of Neighborhood Air Pollution Exposure on Somatic Non-Small Cell Lung Cancer Mutations in the Los Angeles Basin (2013–2018). Int. J. Env. Res. Public Health 2022, 19, 11027. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, Z.; Dou, X.; Zhou, Q.; Zeng, G.; Liu, L.; Chen, W.; Lan, R.; Liu, W.; Ru, G.; et al. Inactivation of Tumor Suppressor Gene Clusterin Leads to Hyperactivation of TAK1-NF-ΚB Signaling Axis in Lung Cancer Cells and Denotes a Therapeutic Opportunity. Theranostics 2020, 10, 11520–11534. [Google Scholar] [CrossRef]
- Judd, J.; Abdel Karim, N.; Khan, H.; Naqash, A.R.; Baca, Y.; Xiu, J.; VanderWalde, A.M.; Mamdani, H.; Raez, L.E.; Nagasaka, M.; et al. Characterization of KRAS Mutation Subtypes in Non–Small Cell Lung Cancer. Mol. Cancer Ther. 2021, 20, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Zhang, Y.; Gao, H.; Xu, Y.; Jing, P.; Wu, J.; Zhang, X.; Xiong, J.; Dong, C.; Yao, L.; et al. Activation of the Aryl Hydrocarbon Receptor Leads to Resistance to EGFR TKIs in Non–Small Cell Lung Cancer by Activating Src-Mediated Bypass Signaling. Clin. Cancer Res. 2018, 24, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.H.; Gao, Y.; Soucheray, M.; Pulido, I.; Kikuchi, E.; Rodríguez, M.L.; Gandhi, R.; Lafuente-Sanchis, A.; Aupí, M.; Alcácer Fernández-Coronado, J.; et al. CXCR7 Reactivates ERK Signaling to Promote Resistance to EGFR Kinase Inhibitors in NSCLC. Cancer Res. 2019, 79, 4439–4452. [Google Scholar] [CrossRef] [PubMed]
- Chiou, J.; Chang, Y.-C.; Jan, Y.-H.; Tsai, H.-F.; Yang, C.-J.; Huang, M.-S.; Yu, Y.-L.; Hsiao, M. Overexpression of BZW1 Is an Independent Poor Prognosis Marker and Its Down-Regulation Suppresses Lung Adenocarcinoma Metastasis. Sci. Rep. 2019, 9, 14624. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Yang, S.; Chen, Y.; Wu, Y.; Lin, C.; Chang, W.; Tseng, Y.; Lai, W.; Ho, C.; Lin, C.; et al. Real-world Outcomes of NSCLC Patients Receiving Tissue or Circulating Tumor DNA-guided Osimertinib Treatment. Cancer Med. 2019, 8, 5939–5947. [Google Scholar] [CrossRef] [PubMed]
- Rosas, G.; Ruiz, R.; Araujo, J.M.; Pinto, J.A.; Mas, L. ALK Rearrangements: Biology, Detection and Opportunities of Therapy in Non-Small Cell Lung Cancer. Crit. Rev. Oncol. Hematol. 2019, 136, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Benusiglio, P.R.; Fallet, V.; Sanchis-Borja, M.; Coulet, F.; Cadranel, J. Lung Cancer Is Also a Hereditary Disease. Eur. Respir. Rev. 2021, 30, 210045. [Google Scholar] [CrossRef]
- Fakih, M.; O’Neil, B.; Price, T.J.; Falchook, G.S.; Desai, J.; Kuo, J.; Govindan, R.; Rasmussen, E.; Morrow, P.K.H.; Ngang, J.; et al. Phase 1 Study Evaluating the Safety, Tolerability, Pharmacokinetics (PK), and Efficacy of AMG 510, a Novel Small Molecule KRASG12C Inhibitor, in Advanced Solid Tumors. J. Clin. Oncol. 2019, 37, 3003. [Google Scholar] [CrossRef]
- Hallin, J.; Engstrom, L.D.; Hargis, L.; Calinisan, A.; Aranda, R.; Briere, D.M.; Sudhakar, N.; Bowcut, V.; Baer, B.R.; Ballard, J.A.; et al. The KRAS G12C Inhibitor MRTX849 Provides Insight toward Therapeutic Susceptibility of KRAS-Mutant Cancers in Mouse Models and Patients. Cancer Discov. 2020, 10, 54–71. [Google Scholar] [CrossRef]
- Chen, R.; Manochakian, R.; James, L.; Azzouqa, A.-G.; Shi, H.; Zhang, Y.; Zhao, Y.; Zhou, K.; Lou, Y. Emerging Therapeutic Agents for Advanced Non-Small Cell Lung Cancer. J. Hematol. Oncol. 2020, 13, 58. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. Overall Survival with Osimertinib in Untreated, EGFR -Mutated Advanced NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef]
- Doebele, R.C.; Riely, G.J.; Spira, A.I.; Horn, L.; Piotrowska, Z.; Costa, D.B.; Neal, J.W.; Zhang, S.; Reichmann, W.; Kerstein, D.; et al. First Report of Safety, PK, and Preliminary Antitumor Activity of the Oral EGFR/HER2 Exon 20 Inhibitor TAK-788 (AP32788) in Non–Small Cell Lung Cancer (NSCLC). J. Clin. Oncol. 2018, 36, 9015. [Google Scholar] [CrossRef]
- Janne, P.A.; Neal, J.W.; Camidge, D.R.; Spira, A.I.; Piotrowska, Z.; Horn, L.; Costa, D.B.; Tsao, A.S.; Patel, J.D.; Gadgeel, S.M.; et al. Antitumor Activity of TAK-788 in NSCLC with EGFR Exon 20 Insertions. J. Clin. Oncol. 2019, 37, 9007. [Google Scholar] [CrossRef]
- Ma, C.; Wei, S.; Song, Y. T790M and Acquired Resistance of EGFR TKI: A Literature Review of Clinical Reports. J. Thorac. Dis. 2011, 3, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, Z.; Thress, K.S.; Mooradian, M.; Heist, R.S.; Azzoli, C.G.; Temel, J.S.; Rizzo, C.; Nagy, R.J.; Lanman, R.B.; Gettinger, S.N.; et al. MET Amplification (Amp) as a Resistance Mechanism to Osimertinib. J. Clin. Oncol. 2017, 35, 9020. [Google Scholar] [CrossRef]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance Mechanisms to Osimertinib in EGFR-Mutated Non-Small Cell Lung Cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Mok, T.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; et al. First-Line Crizotinib versus Chemotherapy in ALK -Positive Lung Cancer. N. Engl. J. Med. 2014, 371, 2167–2177. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.-W.; Ou, S.-H.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK -Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Camidge, D.R.; Peters, S.; Mok, T.; Gadgeel, S.M.; Cheema, P.K.; Pavlakis, N.; De Marinis, F.; Stroyakovskiy, D.L.; Cho, B.C.; Zhang, L.; et al. Updated Efficacy and Safety Data from the Global Phase III ALEX Study of Alectinib (ALC) vs. Crizotinib (CZ) in Untreated Advanced ALK+ NSCLC. J. Clin. Oncol. 2018, 36, 9043. [Google Scholar] [CrossRef]
- Camidge, D.R.; Kim, H.R.; Ahn, M.-J.; Yang, J.C.-H.; Han, J.-Y.; Lee, J.-S.; Hochmair, M.J.; Li, J.Y.-C.; Chang, G.-C.; Lee, K.H.; et al. Brigatinib versus Crizotinib in ALK -Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2027–2039. [Google Scholar] [CrossRef]
- Shaw, A.T.; Kim, T.M.; Crinò, L.; Gridelli, C.; Kiura, K.; Liu, G.; Novello, S.; Bearz, A.; Gautschi, O.; Mok, T.; et al. Ceritinib versus Chemotherapy in Patients with ALK-Rearranged Non-Small-Cell Lung Cancer Previously given Chemotherapy and Crizotinib (ASCEND-5): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Solomon, B.J.; Besse, B.; Bauer, T.M.; Felip, E.; Soo, R.A.; Camidge, D.R.; Chiari, R.; Bearz, A.; Lin, C.C.; Gadgeel, S.M.; et al. Lorlatinib in Patients with ALK-Positive Non-Small-Cell Lung Cancer: Results from a Global Phase 2 Study. Lancet Oncol. 2018, 19, 1654–1667. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, J.; Zhang, H.; Liu, J. TP53 Co-Mutations in Advanced EGFR-Mutated Non–Small Cell Lung Cancer: Prognosis and Therapeutic Strategy for Cancer Therapy. Front. Oncol. 2022, 12, 860563. [Google Scholar] [CrossRef] [PubMed]
- Hosomi, Y.; Morita, S.; Sugawara, S.; Kato, T.; Fukuhara, T.; Gemma, A.; Takahashi, K.; Fujita, Y.; Harada, T.; Minato, K.; et al. Gefitinib alone Versus Gefitinib Plus Chemotherapy for Non-Small-Cell Lung Cancer with Mutated Epidermal Growth Factor Receptor: NEJ009 Study. J. Clin. Oncol. 2019, 38, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Nadal, E.; Garon, E.B.; Nishio, M.; Seto, T.; Yamamoto, N.; Park, K.; Shih, J.-Y.; Paz-Ares, L.; Frimodt-Moller, B.; et al. RELAY Subgroup Analyses by EGFR Ex19del and Ex21L858R Mutations for Ramucirumab Plus Erlotinib in Metastatic Non-Small Cell Lung Cancer. Clin. Cancer Res. 2021, 27, 5258–5271. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ma, L.; Liu, Y.; Zhu, J.; Xin, Y.; Liu, X.; Wang, Y.; Zhang, T.; Yang, C.; Wang, S.; et al. Comprehensive Characterization and Clinical Impact of Concomitant Genomic Alterations in EGFR-Mutant NSCLCs Treated with EGFR Kinase Inhibitors. Lung Cancer 2020, 145, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus Docetaxel in Patients with Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Herbst, R.S.; Baas, P.; Kim, D.-W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.-Y.; Molina, J.; Kim, J.-H.; Arvis, C.D.; Ahn, M.-J.; et al. Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Hsu, M.L.; Naidoo, J. Principles of Immunotherapy in Non-Small Cell Lung Cancer. Thorac. Surg. Clin. 2020, 30, 187–198. [Google Scholar] [CrossRef]
- Cascone, T.; Fradette, J.; Pradhan, M.; Gibbons, D.L. Tumor Immunology and Immunotherapy of Non-Small-Cell Lung Cancer. Cold Spring Harb. Perspect. Med. 2022, 12, a037895. [Google Scholar] [CrossRef] [PubMed]
- Krzyżanowska, N.; Krawczyk, P.; Wojas-Krawczyk, K.; Kucharczyk, T.; Milanowski, J. Immunotherapy in Non-Small-Cell Lung Cancer Patients with Driver Alterations: A New Strategy? Cells 2022, 11, 3280. [Google Scholar] [CrossRef] [PubMed]
- Hu-Lieskovan, S.; Lisberg, A.; Zaretsky, J.M.; Grogan, T.R.; Rizvi, H.; Wells, D.K.; Carroll, J.; Cummings, A.; Madrigal, J.; Jones, B.; et al. Tumor Characteristics Associated with Benefit from Pembrolizumab in Advanced Non–Small Cell Lung Cancer. Clin. Cancer Res. 2019, 25, 5061–5068. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Wang, H.; Liu, Y.; Guo, Q.; Zhang, L.; Li, J.; Zhou, W.; Yan, Y.; Zhou, X.; Zhang, J. Adverse Effects of Anti-PD-1/PD-L1 Therapy in Non-Small Cell Lung Cancer. Front. Oncol. 2020, 10, 554313. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Y.; Berenguer-Pina, J.J.; Mahgoub, T. The Rise of the TROP2-Targeting Agents in NSCLC: New Options on the Horizon. Oncology 2021, 99, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Sequist, L.V.; Yang, J.C.-H.; Yamamoto, N.; O’Byrne, K.; Hirsh, V.; Mok, T.; Geater, S.L.; Orlov, S.; Tsai, C.-M.; Boyer, M.; et al. Phase III Study of Afatinib or Cisplatin Plus Pemetrexed in Patients with Metastatic Lung Adenocarcinoma with EGFR Mutations. J. Clin. Oncol. 2013, 31, 3327–3334. [Google Scholar] [CrossRef] [PubMed]
- Mok, T.S.; Wu, Y.-L.; Thongprasert, S.; Yang, C.-H.; Chu, D.-T.; Saijo, N.; Sunpaweravong, P.; Han, B.; Margono, B.; Ichinose, Y.; et al. Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma. N. Engl. J. Med. 2009, 361, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Carcereny, E.; Gervais, R.; Vergnenegre, A.; Massuti, B.; Felip, E.; Palmero, R.; Garcia-Gomez, R.; Pallares, C.; Sanchez, J.M.; et al. Erlotinib versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer (EURTAC): A Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2012, 13, 239–246. [Google Scholar] [CrossRef]
- Mok, T.S.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Lee, M.; Linke, R.; Rosell, R.; Corral, J.; et al. Improvement in Overall Survival in a Randomized Study That Compared Dacomitinib with Gefitinib in Patients with Advanced Non–Small-Cell Lung Cancer and EGFR -Activating Mutations. J. Clin. Oncol. 2018, 36, 2244–2250. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Cheng, Y.; Zhou, X.; Lee, K.H.; Nakagawa, K.; Niho, S.; Tsuji, F.; Linke, R.; Rosell, R.; Corral, J.; et al. Dacomitinib versus Gefitinib as First-Line Treatment for Patients with EGFR-Mutation-Positive Non-Small-Cell Lung Cancer (ARCHER 1050): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 1454–1466. [Google Scholar] [CrossRef]
- Ramalingam, S.S.; Yang, J.C.-H.; Lee, C.K.; Kurata, T.; Kim, D.-W.; John, T.; Nogami, N.; Ohe, Y.; Mann, H.; Rukazenkov, Y.; et al. Osimertinib As First-Line Treatment of EGFR Mutation–Positive Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Correia, G.S.d.C.; Wang, J.; Manochakian, R.; Zhao, Y.; Lou, Y. Emerging Targeted Therapies in Advanced Non-Small-Cell Lung Cancer. Cancers 2023, 15, 2899. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Smit, E.F.; Groen, H.J.M.; Mazieres, J.; Besse, B.; Helland, Å.; Giannone, V.; D’Amelio, A.M.; Zhang, P.; Mookerjee, B.; et al. Dabrafenib plus Trametinib in Patients with Previously Untreated BRAFV600E-Mutant Metastatic Non-Small-Cell Lung Cancer: An Open-Label, Phase 2 Trial. Lancet Oncol. 2017, 18, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Fülöp, A.; Maciel, C.; Fischer, J.R.; Girotto, G.; Lago, S.; Smit, E.; Ostoros, G.; Eberhardt, W.E.E.; Lishkovska, P.; et al. SELECT-2: A Phase II, Double-Blind, Randomized, Placebo-Controlled Study to Assess the Efficacy of Selumetinib plus Docetaxel as a Second-Line Treatment of Patients with Advanced or Metastatic Non-Small-Cell Lung Cancer. Ann. Oncol. 2017, 28, 3028–3036. [Google Scholar] [CrossRef] [PubMed]
- Han, R.H.; Dunn, G.P.; Chheda, M.G.; Kim, A.H. The Impact of Systemic Precision Medicine and Immunotherapy Treatments on Brain Metastases. Oncotarget 2019, 10, 6739–6753. [Google Scholar] [CrossRef] [PubMed]
- Soda, M.; Takada, S.; Takeuchi, K.; Choi, Y.L.; Enomoto, M.; Ueno, T.; Haruta, H.; Hamada, T.; Yamashita, Y.; Ishikawa, Y.; et al. A Mouse Model for EML4-ALK -Positive Lung Cancer. Proc. Natl. Acad. Sci. USA 2008, 105, 19893–19897. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Yeap, B.Y.; Mino-Kenudson, M.; Digumarthy, S.R.; Costa, D.B.; Heist, R.S.; Solomon, B.; Stubbs, H.; Admane, S.; McDermott, U.; et al. Clinical Features and Outcome of Patients with Non–Small-Cell Lung Cancer Who Harbor EML4-ALK. J. Clin. Oncol. 2009, 27, 4247–4253. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non–Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Shaw, A.T.; Riely, G.J.; Bang, Y.-J.; Kim, D.-W.; Camidge, D.R.; Solomon, B.J.; Varella-Garcia, M.; Iafrate, A.J.; Shapiro, G.I.; Usari, T.; et al. Crizotinib in ROS1-Rearranged Advanced Non-Small-Cell Lung Cancer (NSCLC): Updated Results, Including Overall Survival, from PROFILE 1001. Ann. Oncol. 2019, 30, 1121–1126. [Google Scholar] [CrossRef]
- Huo, K.-G.; Notsuda, H.; Fang, Z.; Liu, N.F.; Gebregiworgis, T.; Li, Q.; Pham, N.-A.; Li, M.; Liu, N.; Shepherd, F.A.; et al. Lung Cancer Driven by BRAFG469V Mutation Is Targetable by EGFR Kinase Inhibitors. J. Thorac. Oncol. 2022, 17, 277–288. [Google Scholar] [CrossRef]
- Nguyen-Ngoc, T.; Bouchaab, H.; Adjei, A.A.; Peters, S. BRAF Alterations as Therapeutic Targets in Non–Small-Cell Lung Cancer. J. Thorac. Oncol. 2015, 10, 1396–1403. [Google Scholar] [CrossRef] [PubMed]
- Harada, G.; Santini, F.C.; Wilhelm, C.; Drilon, A. NTRK Fusions in Lung Cancer: From Biology to Therapy. Lung Cancer 2021, 161, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Haratake, N.; Seto, T. NTRK Fusion-Positive Non–Small-Cell Lung Cancer: The Diagnosis and Targeted Therapy. Clin. Lung Cancer 2021, 22, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wei, Y.; Zhang, H.; Jiang, J.; Zhang, P.; Chu, Q. NTRK Fusion in Non-Small Cell Lung Cancer: Diagnosis, Therapy, and TRK Inhibitor Resistance. Front. Oncol. 2022, 12, 864666. [Google Scholar] [CrossRef]
- Riely, G.J.; Politi, K.A.; Miller, V.A.; Pao, W. Update on Epidermal Growth Factor Receptor Mutations in Non–Small Cell Lung Cancer. Clin. Cancer Res. 2006, 12, 7232–7241. [Google Scholar] [CrossRef] [PubMed]
- O’Kane, G.M.; Bradbury, P.A.; Feld, R.; Leighl, N.B.; Liu, G.; Pisters, K.-M.; Kamel-Reid, S.; Tsao, M.S.; Shepherd, F.A. Uncommon EGFR Mutations in Advanced Non-Small Cell Lung Cancer. Lung Cancer 2017, 109, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-H.; Yang, J.C.-H.; Mok, T.S.; Loong, H.H. Overview of Current Systemic Management of EGFR-Mutant NSCLC. Ann. Oncol. 2018, 29, i3–i9. [Google Scholar] [CrossRef]
- Park, K.; Haura, E.B.; Leighl, N.B.; Mitchell, P.; Shu, C.A.; Girard, N.; Viteri, S.; Han, J.-Y.; Kim, S.-W.; Chee, L.; et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: Initial Results From the CHRYSALIS Phase I Study. J. Clin. Oncol. 2021, 39, 3391–3402. [Google Scholar] [CrossRef]
- Zhou, C.; Ramalingam, S.S.; Tae, K.M.; Kim, S.-W.; Yang, J.C.-H.; Riely, G.J.; Mekhail, T.; Nguyen, D.; Campelo, M.R.G.; Felip, E.; et al. Treatment Outcomes and Safety of Mobocertinib in Platinum-Pretreated Patients with EGFR Exon 20 Insertion-Positive Metastatic Non-Small Cell Lung Cancer A Phase 1/2 Open-Label Nonrandomized Clinical Trial Supplemental Content. JAMA Oncol. 2021, 7, 214761. [Google Scholar] [CrossRef]
- Cascetta, P.; Sforza, V.; Manzo, A.; Carillio, G.; Palumbo, G.; Esposito, G.; Montanino, A.; Costanzo, R.; Sandomenico, C.; De Cecio, R.; et al. RET Inhibitors in Non-Small-Cell Lung Cancer. Cancers 2021, 13, 4415. [Google Scholar] [CrossRef]
- Subbiah, V.; Yang, D.; Velcheti, V.; Drilon, A.; Meric-Bernstam, F. State-of-the-Art Strategies for Targeting RET -Dependent Cancers. J. Clin. Oncol. 2020, 38, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Liu, L.; Xing, Y.; Meng, T.; Ma, L.; Pei, J.; Cong, Y.; Zhang, X.; Ren, Z.; Wang, X.; et al. Dual Mechanisms of Novel CD73-Targeted Antibody and Antibody–Drug Conjugate in Inhibiting Lung Tumor Growth and Promoting Antitumor Immune-Effector Function. Mol. Cancer Ther. 2020, 19, 2340–2352. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.M.; Lupo, K.B.; Wang, J.; Cao, J.; Utturkar, S.; Lanman, N.; Bernal-Crespo, V.; Jalal, S.; Pine, S.R.; Torregrosa-Allen, S.; et al. Engineered Natural Killer Cells Impede the Immunometabolic CD73-Adenosine Axis in Solid Tumors. Elife 2022, 11, e73699. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, S.; Deng, J.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; Li, X.; et al. VEGF/VEGFR-Targeted Therapy and Immunotherapy in Non-Small Cell Lung Cancer: Targeting the Tumor Microenvironment. Int. J. Biol. Sci. 2022, 18, 3845–3858. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in Cancer Treatment: A Review of 15 Years of Clinical Experience and Future Outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Xing, H.; Li, Y.; Tian, W.; Song, Y.; Jiang, Z.; Yu, J. Claudin18.2 Is a Novel Molecular Biomarker for Tumor-Targeted Immunotherapy. Biomark. Res. 2022, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- La Fleur, L.; Botling, J.; He, F.; Pelicano, C.; Zhou, C.; He, C.; Palano, G.; Mezheyeuski, A.; Micke, P.; Ravetch, J.V.; et al. Targeting MARCO and IL37R on Immunosuppressive Macrophages in Lung Cancer Blocks Regulatory T Cells and Supports Cytotoxic Lymphocyte Function. Cancer Res. 2021, 81, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Eberhard, D.A.; Johnson, B.E.; Amler, L.C.; Goddard, A.D.; Heldens, S.L.; Herbst, R.S.; Ince, W.L.; Jänne, P.A.; Januario, T.; Johnson, D.H.; et al. Mutations in the Epidermal Growth Factor Receptor and in KRAS Are Predictive and Prognostic Indicators in Patients with Non–Small-Cell Lung Cancer Treated with Chemotherapy Alone and in Combination with Erlotinib. J. Clin. Oncol. 2005, 23, 5900–5909. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Vansteenkiste, J.F.; Van De Kerkhove, C.; Wauters, E.; Van Mol, P. Capmatinib for the Treatment of Non-Small Cell Lung Cancer. Expert Rev. Anticancer Ther. 2019, 19, 659–671. [Google Scholar] [CrossRef]
- Wolf, J.; Seto, T.; Han, J.-Y.; Reguart, N.; Garon, E.B.; Groen, H.J.M.; Tan, D.S.W.; Hida, T.; de Jonge, M.; Orlov, S.V.; et al. Capmatinib in MET Exon 14–Mutated or MET -Amplified Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Clark, J.W.; Weiss, J.; Ou, S.-H.I.; Camidge, D.R.; Solomon, B.J.; Otterson, G.A.; Villaruz, L.C.; Riely, G.J.; Heist, R.S.; et al. Antitumor Activity of Crizotinib in Lung Cancers Harboring a MET Exon 14 Alteration. Nat. Med. 2020, 26, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Paik, P.K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot, A.B.; Garassino, M.C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef]
- Pillai, R.N.; Behera, M.; Berry, L.D.; Rossi, M.R.; Kris, M.G.; Johnson, B.E.; Bunn, P.A.; Ramalingam, S.S.; Khuri, F.R. HER2 Mutations in Lung Adenocarcinomas: A Report from the Lung Cancer Mutation Consortium. Cancer 2017, 123, 4099–4105. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Shen, R.; Buonocore, D.; Olah, Z.T.; Ni, A.; Ginsberg, M.S.; Ulaner, G.A.; Offin, M.; Feldman, D.; Hembrough, T.; et al. Ado-Trastuzumab Emtansine for Patients with HER2 -Mutant Lung Cancers: Results From a Phase II Basket Trial. J. Clin. Oncol. 2018, 36, 2532–2537. [Google Scholar] [CrossRef] [PubMed]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab Deruxtecan in HER2 -Mutant Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, K.; Imanishi, N.; Ichiki, Y.; Tanaka, F. Immune Checkpoint Inhibitors (ICIs) in Non-Small Cell Lung Cancer (NSCLC). J. UOEH 2018, 40, 173–189. [Google Scholar] [CrossRef]
- To, K.K.W.; Fong, W.; Cho, W.C.S. Immunotherapy in Treating EGFR-Mutant Lung Cancer: Current Challenges and New Strategies. Front. Oncol. 2021, 11, 635007. [Google Scholar] [CrossRef]
- Zerunian, M.; Caruso, D.; Zucchelli, A.; Polici, M.; Capalbo, C.; Filetti, M.; Mazzuca, F.; Marchetti, P.; Laghi, A. CT Based Radiomic Approach on First Line Pembrolizumab in Lung Cancer. Sci. Rep. 2021, 11, 6633. [Google Scholar] [CrossRef]
- Kanazu, M.; Edahiro, R.; Krebe, H.; Nishida, K.; Ishijima, M.; Uenami, T.; Akazawa, Y.; Yano, Y.; Yamaguchi, T.; Mori, M. Hyperprogressive Disease in Patients with Non-Small Cell Lung Cancer Treated with Nivolumab: A Case Series. Thorac. Cancer 2018, 9, 1782–1787. [Google Scholar] [CrossRef]
- Choi, H.; Deng, J.; Li, S.; Silk, T.; Dong, L.; Brea, E.J.; Houghton, S.; Redmond, D.; Zhong, H.; Boiarsky, J.; et al. Pulsatile MEK Inhibition Improves Anti-Tumor Immunity and T Cell Function in Murine Kras Mutant Lung Cancer. Cell Rep. 2019, 27, 806–819.e5. [Google Scholar] [CrossRef] [PubMed]
- Meira, D.D.; Casotti, M.C.; Braga, R.F.R.; Filho, L.C.G.S.; Guimarães, A.P.; Campanharo, C.V.; Duque, D.A.; Barbosa, D.G.; Lopes, L.M.; Kohls, V.N.G.; et al. Targeted Cancer Therapy: The Future of Drug Combinations. In Novel Sensitizing Agents for Therapeutic Anti-EGFR Antibodies; Elsevier: Amsterdam, The Netherlands, 2023; pp. 189–217. [Google Scholar]
- Araghi, M.; Mannani, R.; Maleki, A.H.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent advances in non-small cell lung cancer targeted therapy; an update review. Cancer Cell Int. 2023, 23, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Vyse, S.; Huang, P.H. Amivantamab for the Treatment of EGFR Exon 20 Insertion Mutant Non-Small Cell Lung Cancer. Expert Rev. Anticancer Ther. 2022, 22, 3–16. [Google Scholar] [CrossRef]
- Li, J.-X.; Huang, J.-M.; Jiang, Z.-B.; Li, R.-Z.; Sun, A.; Lai-Han Leung, E.; Yan, P.-Y. Current Clinical Progress of PD-1/PD-L1 Immunotherapy and Potential Combination Treatment in Non–Small Cell Lung Cancer. Integr. Cancer Ther. 2019, 18, 153473541989002. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Wu, J.-Z.; Cao, H.-X.; Ma, R.; Wu, J.-Q.; Zhong, Y.-J.; Feng, J.-F. Blockade of DNA Methylation Enhances the Therapeutic Effect of Gefitinib in Non-Small Cell Lung Cancer Cells. Oncol. Rep. 2013, 29, 1975–1982. [Google Scholar] [CrossRef] [PubMed]
- Greve, G.; Schiffmann, I.; Pfeifer, D.; Pantic, M.; Schüler, J.; Lübbert, M. The Pan-HDAC Inhibitor Panobinostat Acts as a Sensitizer for Erlotinib Activity in EGFR-Mutated and -Wildtype Non-Small Cell Lung Cancer Cells. BMC Cancer 2015, 15, 947. [Google Scholar] [CrossRef]
- Abuhelwa, Z.; Alloghbi, A.; Nagasaka, M. A Comprehensive Review on Antibody-Drug Conjugates (ADCs) in the Treatment Landscape of Non-Small Cell Lung Cancer (NSCLC). Cancer Treat. Rev. 2022, 106, 102393. [Google Scholar] [CrossRef]
- Singhi, E.K.; Horn, L.; Sequist, L.V.; Heymach, J.; Langer, C.J. Advanced Non–Small Cell Lung Cancer: Sequencing Agents in the EGFR-Mutated/ALK-Rearranged Populations. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e187–e197. [Google Scholar] [CrossRef]
- Solomon, B.J.; Kim, D.-W.; Wu, Y.-L.; Nakagawa, K.; Mekhail, T.; Felip, E.; Cappuzzo, F.; Paolini, J.; Usari, T.; Tang, Y.; et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2251–2258. [Google Scholar] [CrossRef]
- Zou, H.Y.; Friboulet, L.; Kodack, D.P.; Engstrom, L.D.; Li, Q.; West, M.; Tang, R.W.; Wang, H.; Tsaparikos, K.; Wang, J.; et al. PF-06463922, an ALK/ROS1 Inhibitor, Overcomes Resistance to First and Second Generation ALK Inhibitors in Preclinical Models. Cancer Cell 2015, 28, 70–81. [Google Scholar] [CrossRef]
- Pakkala, S.; Ramalingam, S.S. Personalized Therapy for Lung Cancer: Striking a Moving Target. JCI Insight 2018, 3, e120858. [Google Scholar] [CrossRef] [PubMed]
- Vyse, S.; Huang, P.H. Targeting EGFR Exon 20 Insertion Mutations in Non-Small Cell Lung Cancer. Signal Transduct. Target. Ther. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Gonzalvez, F.; Zhu, X.; Huang, W.-S.; Baker, T.E.; Ning, Y.; Wardwell, S.D.; Nadworny, S.; Zhang, S.; Das, B.; Gong, Y.; et al. Abstract 2644: AP32788, a Potent, Selective Inhibitor of EGFR and HER2 Oncogenic Mutants, Including Exon 20 Insertions, in Preclinical Models. Cancer Res. 2016, 76, 2644. [Google Scholar] [CrossRef]
- Neijssen, J.; Cardoso, R.M.F.; Chevalier, K.M.; Wiegman, L.; Valerius, T.; Anderson, G.M.; Moores, S.L.; Schuurman, J.; Parren, P.W.H.I.; Strohl, W.R.; et al. Discovery of Amivantamab (JNJ-61186372), a Bispecific Antibody Targeting EGFR and MET. J. Biol. Chem. 2021, 296, 100641. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, H. miRNAs as biomarkers and for the early detection of non-small cell lung cancer (NSCLC). J. Thorac. Dis. 2018, 10, 3119. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Mencaroni, C.; Paglialunga, L.; Paciullo, F.; Crinò, L.; Chiari, R.; Metro, G. Long noncoding RNAs: New insights into non-small cell lung cancer biology, diagnosis and therapy. Med. Oncol. 2016, 33, 18. [Google Scholar] [CrossRef] [PubMed]
- Vardarlı, A.T.; Ozgur, S.; Goksel, T.; Korba, K.; Karakus, H.S.; Asık, A.; Pelit, L.; Gunduz, C. Conversion of specific lncRNAs to biomarkers in exhaled breath condensate samples of patients with advanced stage non-small-cell lung cancer. Front. Genet. 2023, 14, 1200262. [Google Scholar] [CrossRef]
- Guo, S.; Yan, F.; Xu, J.; Bao, Y.; Zhu, J.; Wang, X.; Wu, J.; Li, Y.; Pu, W.; Liu, Y.; et al. Identification and validation of the methylation biomarkers of non-small cell lung cancer (NSCLC). Clin. Epigenetics 2015, 7, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Han, X.; Gao, C.; Xing, Y.; Qi, Z.; Liu, R.; Wang, Y.; Zhang, X.; Yang, Y.G.; Li, X.; et al. 5-Hydroxymethylome in Circulating Cell-free DNA as A Potential Biomarker for Non-small-cell Lung Cancer. Genom. Proteom. Bioinform. 2018, 16, 187–199. [Google Scholar] [CrossRef]
- Szejniuk, W.M.; Robles, A.I.; McCulloch, T.; Falkmer, U.G.I.; Røe, O.D. Epigenetic predictive biomarkers for response or outcome to platinum-based chemotherapy in non-small cell lung cancer, current state-of-art. Pharmacogenomics J. 2019, 19, 5–14. [Google Scholar] [CrossRef]
- Yang, S.; Huang, Y.; Zhao, Q. Epigenetic alterations and inflammation as emerging use for the advancement of treatment in non-small cell lung cancer. Front. Immunol. 2022, 13, 878740. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.; Shackelford, R.E.; El-Osta, H. Epigenetics in non-small cell lung cancer: From basics to therapeutics. Transl. Lung Cancer Res. 2016, 5, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, R.; Song, H.; Huang, G.; Yi, J.; Zheng, Y.; Wang, J.; Chen, L. Methylation of multiple genes as a candidate biomarker in non-small cell lung cancer. Cancer Lett. 2011, 303, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Vrba, L.; Oshiro, M.M.; Kim, S.S.; Garland, L.L.; Placencia, C.; Mahadevan, D.; Nelson, M.A.; Futscher, B.W. DNA methylation biomarkers discovered in silico detect cancer in liquid biopsies from non-small cell lung cancer patients. Epigenetics 2020, 15, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Shames, D.S.; Girard, L.; Gao, B.; Sato, M.; Lewis, C.M.; Shivapurkar, N.; Jiang, A.; Perou, C.M.; Kim, Y.H.; Pollack, J.R.; et al. A genome-wide screen for promoter methylation in lung cancer identifies novel methylation markers for multiple malignancies. PLoS Med. 2006, 3, e486. [Google Scholar] [CrossRef] [PubMed]
- De Fraipont, F.; Levallet, G.; Creveuil, C.; Bergot, E.; Beau-Faller, M.; Mounawar, M.; Richard, N.; Antoine, M.; Rouquette, I.; Favrot, M.-C.; et al. An apoptosis methylation prognostic signature for early lung cancer in the IFCT-0002 trial. Clin. Cancer Res. 2012, 18, 2976–2986. [Google Scholar] [CrossRef] [PubMed]
- Liloglou, T.; Bediaga, N.G.; Brown, B.R.; Field, J.K.; Davies, M.P. Epigenetic biomarkers in lung cancer. Cancer Lett. 2014, 342, 200–212. [Google Scholar] [CrossRef]
- Begum, S.; Brait, M.; Dasgupta, S.; Ostrow, K.L.; Zahurak, M.; Carvalho, A.L.; Califano, J.A.; Goodman, S.N.; Westra, W.H.; Hoque, M.O.; et al. An epigenetic marker panel for detection of lung cancer using cell-free serum DNA. Clin. Res. Câncer 2011, 17, 4494–4503. [Google Scholar] [CrossRef]
- Dietrich, D.; Hasinger, O.; Liebenberg, V.; Field, J.K.; Kristiansen, G.; Soltermann, A. DNA methylation of the homeobox genes PITX2 and SHOX2 predicts outcome in non-small-cell lung cancer patients. Diagn. Mol. Pathol. 2012, 21, 93–104. [Google Scholar] [CrossRef]
- Yi, M.; Liao, Z.; Deng, L.; Xu, L.; Tan, Y.; Liu, K.; Chen, Z.; Zhang, Y. High diagnostic value of miRNAs for NSCLC: Quantitative analysis for both single and combined miRNAs in lung cancer. Ann. Med. 2021, 53, 2178–2193. [Google Scholar] [CrossRef]
- Zhu, J.; Qi, Y.; Wu, J.; Shi, M.; Feng, J.; Chen, L. Evaluation of plasma microRNA levels to predict insensitivity of patients with advanced lung adenocarcinomas to pemetrexed and platinum. Oncol. Lett. 2016, 12, 4829–4837. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Wang, Y.; Su, G.; Zhao, S. Decreased plasma let-7c and miR-152 as noninvasive biomarker for non-small-cell lung cancer. Int. J. Clin. Exp. Med. 2015, 8, 9291–9298. [Google Scholar] [PubMed]
- Wu, L.; Hu, B.; Zhao, B.; Liu, Y.-N.; Yang, Y.; Zhang, L.-J.; Chen, J.-F. Circulating microRNA-422a is associated with lymphatic metastasis in lung cancer. Oncotarget 2017, 26, 42173–42188. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Gupta, R.C. Exosomal miRNAs as biomarkers of recurrent lung cancer. Tumor Biol. 2016, 37, 10703–10714. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Nicassio, F.; Veronesi, G.; Di Fiore, P. Circulating microRNAs: Next-generation biomarkers for early lung cancer detection. Ecancermedicalscience 2012, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Navarro, A.; Vinolas, N.; Marrades, R.M.; Diaz, T.; Gel, B.; Quera, A.; Bandres, E.; Garcia-Foncillas, J.; Ramirez, J.; et al. miR-34a as a prognostic marker of relapse in surgically resected non-small-cell lung cancer. Carcinogênese 2009, 30, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Franchina, T.; Amodeo, V.; Bronte, G.; Savio, G.; Ricciardi, G.R.; Picciotto, M.; Russo, A.; Giordano, A.; Adamo, V. Circulating miR-22, miR-24 and miR-34a as novel predictive biomarkers to pemetrexed-based chemotherapy in advanced non-small cell lung cancer. J. Cell Physiol. 2014, 229, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.L.; Liu, W.L.; Chang, J.M.; Chen, Y.H.; Liu, Y.P.; Kuo, H.F.; Hsieh, C.C.; Ding, Y.S.; Chen, W.W.; Chong, I.W. MicroRNA-200c inhibits epithelial-mesenchymal transition, invasion, and migration of lung cancer by targeting HMGB1. PLoS ONE 2017, 12, e0180844. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ding, M.; Xia, M.; Chen, S.; Van Le, A.; Soto-Gil, R.; Shen, Y.; Wang, N.; Wang, J.; Gu, W.; et al. A Five-miRNA Panel Identified from a multicentric case-control study serves as a novel diagnostic tool for ethnically diverse non-small-cell lung cancer patients. EBioMedicine 2015, 2, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Sulewska, A.; Pilz, L.; Manegold, C.; Ramlau, R.; Charkiewicz, R.; Niklinski, J. A Systematic Review of Progress toward Unlocking the Power of Epigenetics in NSCLC: Latest Updates and Perspectives. Cells 2023, 12, 905. [Google Scholar] [CrossRef]
- Osielska, M.A.; Jagodziński, P.P. Long non-coding RNA as potential biomarkers in non-small-cell lung cancer: What do we know so far? Biomed. Pharmacother. 2018, 101, 322–333. [Google Scholar] [CrossRef]
- Tang, Q.; Ni, Z.; Cheng, Z.; Xu, J.; Yu, H.; Yin, P. Three circulating long non-coding RNAs act as biomarkers for predicting NSCLC. Cell Physiol. Biochem. 2015, 37, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Bao, J.; Wang, Z.; Zhang, Z.; Gu, P.; Tao, F.; Cui, D.; Jiang, W. The plasma lncRNA acting as fingerprint in non-small-cell lung cancer. Tumor Biol. 2016, 37, 3497–3504. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Lv, T.; Shi, X.; Liu, H.; Zhu, Q.; Zeng, J.; Yang, W.; Yin, J.; Song, Y. Circulating long noncoding RNA GAS5 is a novel biomarker for the diagnosis of nonsmall cell lung cancer. Medicamento 2016, 95, e4608. [Google Scholar] [CrossRef] [PubMed]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy—New insights into old paradigms. Cancer Gene Ther. 2021, 28, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, R.; Yang, Z.; Li, W.; Yang, J.; Wang, Z.; Bai, H.; Cui, Y.; Tian, Y.; Wu, Z.; et al. Molecular profiling of human non-small cell lung cancer by single-cell RNA-seq. Genome Med. 2022, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Brockley, L.J.; Souza, V.G.P.; Forder, A.; Pewarchuk, M.E.; Erkan, M.; Telkar, N.; Benard, K.; Trejo, J.; Stewart, M.D.; Stewart, G.L.; et al. Sequence-Based Platforms for Discovering Biomarkers in Liquid Biopsy of Non-Small-Cell Lung Cancer. Cancers 2023, 15, 2275. [Google Scholar] [CrossRef] [PubMed]
- Wlosik, J.; Fattori, S.; Rochigneux, P.; Goncalves, A.; Olive, D.; Chretien, A.S. Immune biology of NSCLC revealed by single-cell technologies: Implications for the development of biomarkers in patients treated with immunotherapy. Semin. Immunopathol. 2023, 45, 29–41. [Google Scholar] [CrossRef]
- Datar, I.; Sanmamed, M.F.; Wang, J.; Henick, B.S.; Choi, J.; Badri, T.; Dong, W.; Mani, N.; Toki, M.; Mejías, L.D.; et al. Expression Analysis and Significance of PD-1, LAG-3, and TIM-3 in Human Non-Small Cell Lung Cancer Using Spatially Resolved and Multiparametric Single-Cell Analysis. Clin. Cancer Res. 2019, 25, 4663–4673. [Google Scholar] [CrossRef]
- Prazanowska, K.H.; Lim, S.B. An integrated single-cell transcriptomic dataset for non-small cell lung cancer. Sci. Data. 2023, 10, 167. [Google Scholar] [CrossRef]
- Peng, H.; Wu, X.; Liu, S.; He, M.; Xie, C.; Zhong, R.; Liu, J.; Tang, C.; Li, C.; Xiong, S.; et al. Multiplex immunofluorescence and single-cell transcriptomic profiling reveal the spatial cell interaction networks in the non-small cell lung cancer microenvironment. Clin. Transl. Med. 2023, 13, e1155. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Yu, Q.; Chen, Y.; Yuan, H.; Sheng, M.; Tang, W. Integrating Single-cell RNA-seq to construct a Neutrophil prognostic model for predicting immune responses in non-small cell lung cancer. J. Transl. Med. 2022, 20, 531. [Google Scholar] [CrossRef]
- Lau, D.; Khare, S.; Stein, M.M.; Jain, P.; Gao, Y.; BenTaieb, A.; Rand, T.A.; Salahudeen, A.A.; Khan, A.A. Integration of tumor extrinsic and intrinsic features associates with immunotherapy response in non-small cell lung cancer. Nat. Commun. 2022, 13, 4053. [Google Scholar] [CrossRef] [PubMed]
- Sultana, A.; Alam, M.S.; Liu, X.; Sharma, R.; Singla, R.K.; Gundamaraju, R.; Shen, B. Single-cell RNA-seq analysis to identify potential biomarkers for diagnosis, and prognosis of non-small cell lung cancer by using comprehensive bioinformatics approaches. Transl. Oncol. 2023, 27, 101571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.C.; Feng, W.N.; Zhang, L.; Liu, X.Q.; Guo, W.B.; Deng, Y.M.; Zou, Q.F.; Yang, J.J.; Zhou, Q.; et al. Circulating tumor cells dynamics during chemotherapy predict survival and response in advanced non-small-cell lung cancer patients. Ther. Adv. Med. Oncol. 2023, 15, 17588359231167818. [Google Scholar] [CrossRef]
- Mirzayans, R.; Murray, D. What are the reasons for continuing failures in cancer therapy? Are misleading/inappropriate preclinical assays to be blamed? Might some modern therapies cause more harm than benefit? Int. J. Mol. Sci. 2022, 23, 13217. [Google Scholar] [CrossRef]
- Dörnen, J.; Sieler, M.; Weiler, J.; Keil, S.; Dittmar, T. Cell fusion-mediated tissue regeneration as an inducer of polyploidy and aneuploidy. Int. J. Mol. Sci. 2020, 21, 1811. [Google Scholar] [CrossRef] [PubMed]
- Mirzayans, R.; Murray, D. Do TUNEL and other apoptosis assays detect cell death in preclinical studies? Int. J. Mol. Sci. 2020, 21, 9090. [Google Scholar] [CrossRef]
- Mirzayans, R.; Murray, D. Intratumor heterogeneity and therapy resistance: Contributions of dormancy, apoptosis reversal (anastasis) and cell fusion to disease recurrence. Int. J. Mol. Sci. 2020, 21, 1308. [Google Scholar] [CrossRef]
- Mirzayans, R.; Murray, D. Intratumor Heterogeneity and Treatment Resistance of Solid Tumors with a Focus on Polyploid/Senescent Giant Cancer Cells (PGCCs). Int. J. Mol. Sci. 2023, 24, 11534. [Google Scholar] [CrossRef]
- Hass, R.; Von Der Ohe, J.; Dittmar, T. Cancer cell fusion and post-hybrid selection process (PHSP). Cancers 2021, 13, 4636. [Google Scholar] [CrossRef]
- Demin, S.; Berdieva, M.; Goodkov, A. Cell-cell fusions and cell-in-cell phenomena in healthy cells and cancer: Lessons from protists and invertebrates. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022; pp. 96–105. [Google Scholar] [CrossRef]
- Alhaddad, L.; Osipov, A.N.; Leonov, S. The Molecular and Cellular Strategies of Glioblastoma and Non-Small-Cell Lung Cancer Cells Conferring Radioresistance. Int. J. Mol. Sci. 2022, 23, 13577. [Google Scholar] [CrossRef] [PubMed]
- Alhaddad, L.; Nofal, Z.; Pustovalova, M.; Osipov, A.N.; Leonov, S. Long-Term Cultured Human Glioblastoma Multiforme Cells Demonstrate Increased Radiosensitivity and Senescence-Associated Secretory Phenotype in Response to Irradiation. Int. J. Mol. Sci. 2023, 24, 2002. [Google Scholar] [CrossRef] [PubMed]
- Augustyn, A.; Adams, D.L.; He, J.; Qiao, Y.; Verma, V.; Liao, Z.; Tang, C.-M.; Heymach, J.V.; Tsao, A.S.; Lin, S.H. Giant circulating cancer-associated macrophage-like cells are associated with disease recurrence and survival in non–small-cell lung cancer treated with chemoradiation and atezolizumab. Clin. Lung Cancer 2021, 22, e451–e465. [Google Scholar] [CrossRef] [PubMed]
- Prakash, R.; Telleria, C.M. Cancer cell repopulation after therapy: Which is the mechanism? Oncoscience 2023, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, P.; Wei, J.; Zhang, X.; Guo, J.; Lin, Y. Recent progress in targeted therapy for non-small cell lung cancer. Front. Pharmacol. 2023, 14, 1125547. [Google Scholar] [CrossRef]
- Alves, L.N.R.; Meira, D.D.; Merigueti, L.P.; Casotti, M.C.; Ventorim, D.d.P.; Almeida, J.F.F.; Sousa, V.P.d.; Sant’Ana, M.C.; Cruz, R.G.C.d.; Louro, L.S.; et al. Biomarkers in Breast Cancer: An Old Story with a New End. Genes 2023, 14, 1364. [Google Scholar] [CrossRef] [PubMed]
- Bertran-Alamillo, J.; Cattan, V.; Schoumacher, M.; Codony-Servat, J.; Giménez-Capitán, A.; Cantero, F.; Burbridge, M.; Rodríguez, S.; Teixidó, C.; Roman, R.; et al. AURKB as a target in non-small cell lung cancer with acquired resistance to anti-EGFR therapy. Nat. Commun. 2019, 10, 1812. [Google Scholar] [CrossRef]
- Casotti, M.C.; Meira, D.D.; Zetum, A.S.S.; de Araújo, B.C.; da Silva, D.R.C.; Santos, E.d.V.W.d.; Garcia, F.M.; de Paula, F.; Santana, G.M.; Louro, L.S.; et al. Computational Biology Helps Understand How Polyploid Giant Cancer Cells Drive Tumor Success. Genes 2023, 14, 801. [Google Scholar] [CrossRef]
- Fukuoka, K.; Arioka, H.; Iwamoto, Y.; Fukumoto, H.; Kurokawa, H.; Ishida, T.; Tomonari, A.; Suzuki, T.; Usuda, J.; Kanzawa, F.; et al. Mechanism of the radiosensitization induced by vinorelbine in human non-small cell lung cancer cells. Lung Cancer 2001, 34, 451–460. [Google Scholar] [CrossRef]
- Klimaszewska-Wiśniewska, A.; Hałas-Wiśniewska, M.; Izdebska, M.; Gagat, M.; Grzanka, A.; Grzanka, D. Antiproliferative and antimetastatic action of quercetin on A549 non-small cell lung cancer cells through its effect on the cytoskeleton. Acta Histochem. 2017, 119, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Lee, Y.R.; Liao, J.D.; Lin, C.Y.; Chen, Y.Y.; Chen, P.T.; Tseng, Y.S. Reversine induced multinucleated cells, cell apoptosis and autophagy in human non-small cell lung cancer cells. PLoS ONE 2016, 11, e0158587. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Jiang, D.; Shen, J.; Liu, W.; Tan, X.; Cao, G. Potential Role of the Fragile Histidine Triad in Cancer Evo-Dev. Cancers 2023, 15, 1144. [Google Scholar] [CrossRef]
- Pustovalova, M.V.; Yashkina, E.I.; Alhaddad, L.; Osipov, A.N.; Chen, Y.; Leonov, S.V. Phenotypic Characteristics of Dormant Human Non-Small Cell Lung Cancer Cells Surviving Multifraction X-Ray Irradiation. Bull. Exp. Biol. Med. 2022, 174, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Pustovalova, M.V.; Blokhina, T.; Alhaddad, L.; Chigasova, A.; Chuprov-Netochin, R.; Veviorskiy, A.; Filkov, G.; Osipov, A.N.; Leonov, S. CD44+ and CD133+ non-small cell lung cancer cells exhibit DNA damage response pathways and dormant polyploid giant cancer cell enrichment relating to their p53 status. Int. J. Mol. Sci. 2022, 23, 4922. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Blokhina, T.; Alhaddad, L.; Chigasova, A.; Chuprov-Netochin, R.; Veviorskiy, A.; Filkov, G.; Osipov, A.N.; Leonov, S. Targeting tumor cell senescence and polyploidy as potential therapeutic strategies. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2022; Volume 81, pp. 37–47. [Google Scholar] [CrossRef]
- Tang, W.F.; Fan, X.J.; Bao, H.; Fu, R.; Liang, Y.; Wu, M.; Zhang, C.; Su, J.; Wu, Y.L.; Zhong, W.Z. Acquired DNA damage repairs deficiency-driven immune evolution and involved immune factors of local versus distant metastases in non-small cell lung cancer. OncoImmunology 2023, 12, 2215112. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ouyang, M.; Zhao, S.; Song, S.; Liu, S.; Yu, H. Study on the proliferation and apoptosis characteristics of polyploid non-small cell lung cancer A549 cells induced by docetaxel. Cancer Res. Clinic. 2020, 6, 606–612. [Google Scholar] [CrossRef]
- White-Gilbertson, S.; Lu, P.; Jones, C.M.; Chiodini, S.; Hurley, D.; Das, A.; Delaney, J.R.; Norris, J.S.; Voelkel-Johnson, C. Tamoxifen is a candidate first-in-class inhibitor of acid ceramidase that reduces amitotic division in polyploid giant cancer cells-Unrecognized players in tumorigenesis. Cancer Med. 2020, 9, 3142–3152. [Google Scholar] [CrossRef]
- Marrocco, I.; Giri, S.; Simoni-Nieves, A.; Gupta, N.; Rudnitsky, A.; Haga, Y.; Romaniello, D.; Sekar, A.; Zerbib, M.; Oren, R.; et al. L858R emerges as a potential biomarker predicting response of lung cancer models to anti-EGFR antibodies: Comparison of osimertinib vs. cetuximab. Cell Rep. Med. 2023, 4, 101142. [Google Scholar] [CrossRef]
- Mirzayans, R.; Andrais, B.; Murray, D. Roles of polyploid/multinucleated giant cancer cells in metastasis and disease relapse following anticancer treatment. Cancers 2018, 10, 118. [Google Scholar] [CrossRef]


| Drug Name | Class | Study/NCT * Name |
|---|---|---|
| Nivolumab | Anti PD-1 | CheckMate-012 (NCT01454102) CheckMate 227 (NCT02477826) |
| Pembrolizumab | Anti PD-1 | KEYNOTE-001 (NCT01295827) |
| Atezolizumab | Anti PD-1 | POPLAR (NCT01903993) OAK (NCT02008227) |
| Durvalumab | Anti PD-1 | PACIFIC (NCT02125461) |
| Sacituzumab Govitecan | Anti-TROP2 | TROPION-PanTumor01 (NCT03401385) |
| DS-102 | Anti-TROP2 | TROPION-PanTumor01 (NCT03401385) |
| Erlotinib | TKI EGFR inhibitor | EUTARC (NCT00446225) |
| Dacomitinib | TKI, ErbB/HER inhibitor | ARCHER1050 (NCT01774721) |
| Osimertinib | TKI, EGFR mutation inhibitor | FLAURA (NCT02296125) |
| Crizotinib | ALK inhibitor | NCT01154140 |
| Alectinib | ALK inhibitor | ALEX (NCT02075840) |
| Brigatinib | ALK inhibitor | NCT02737501 |
| Certinib | ALK inhibitor | NCT01828112 |
| Lorlatinib | ALK inhibitor | NCT01970865 |
| Trametinib | MEK inhibitor | NCT01336634 |
| Selumetinib | MEK inhibitor | SELECT-2 (NCT01750281) |
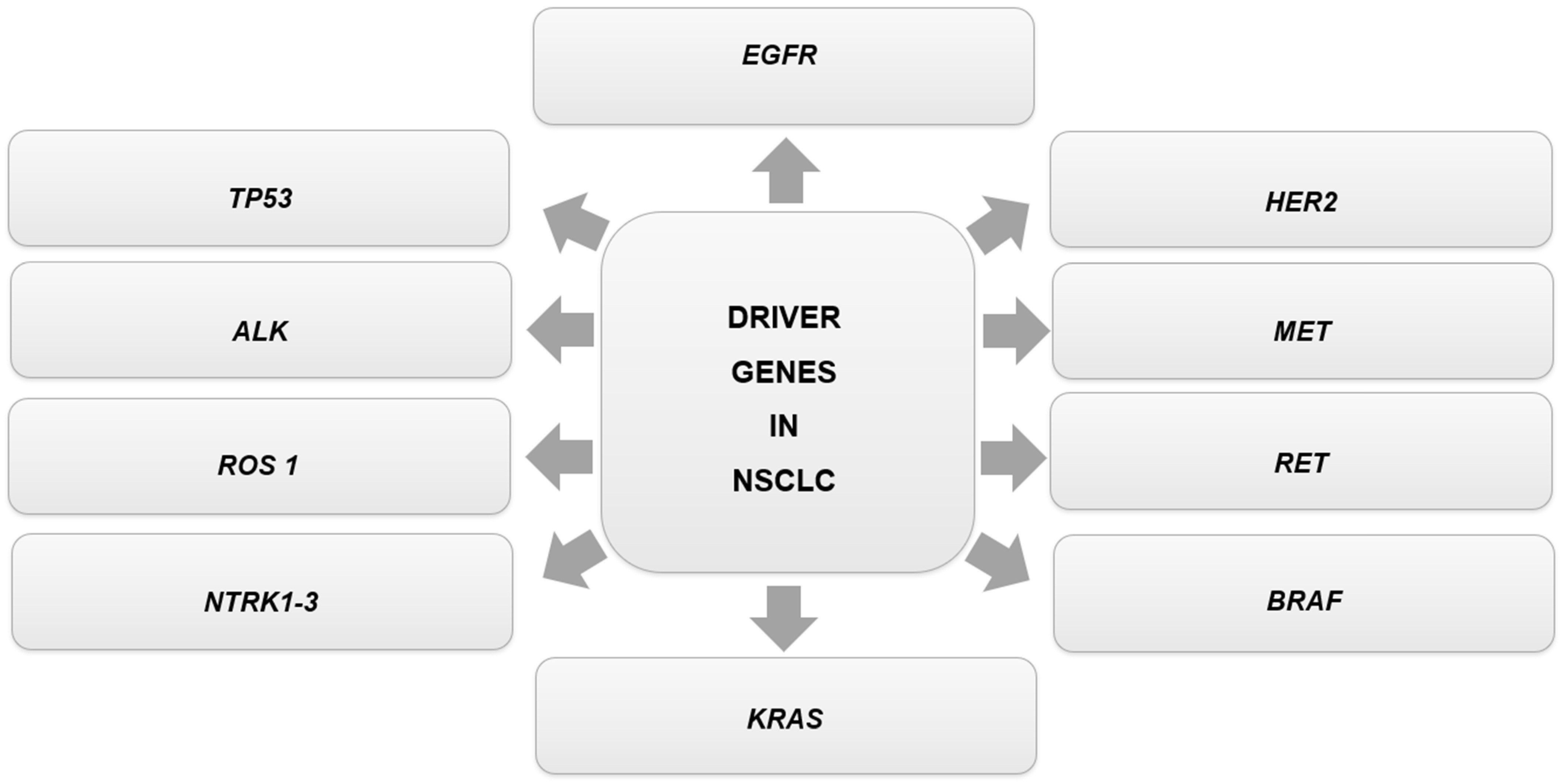
| Oncogenic Driver Genes | Comments | Genetic Alteration | Targeted Therapies for the Genetic Alteration in NSCLC According to NCCN Guidelines Version 3.2023 | References |
|---|---|---|---|---|
| EGFR | The alterations are related to the PIK3/AKT/mTOR and RAS/RAF/MEK pathways, which induce anti-apoptotic activity, cell proliferation, and promote tumor growth and survival. These abnormalities consist of gene mutation, gene amplification, and protein overexpression. | EGFR Exon 19 Deletion or L858R | First-line therapy: afatinib; erlotinib; dacomitinib; gefitinib; osimertinib; erlotinib + ramucirumab; erlotinib + bevacizumab (nonsquamous). Subsequent therapy: osimertinib. | [4,42,94] |
| EGFR S768I, L861Q and/or G719X | First-line therapy: afatinib; erlotinib; dacomitinib; gefitinib; osimertinib. Subsequent therapy: osimertinib. | |||
| EGFR Exon 20 Insertion Mutation | Subsequent therapy: amivantamab-vmjw; mobocertinib. | |||
| ALK | Most ALK-positive patients carry the ALK-fusion gene 4 (EML4)-ALK rearrangement. This fusion gene encodes a protein related to cell proliferation, differentiation, and inhibition of apoptosis. | ALK Rearrangement Positive | First-line therapy: alectinib; brigatinib; ceritinib; crizotinib; lorlatinib. Subsequent therapy: alectinib; brigatinib; ceritinib; lorlatinib. | [4,42,94] |
| ROS1 | The ROS1 kinase domain can fuse with differentiation cluster 74 (CD74), leading to its kinase activity. The changes are related to rearrangements in the ROS1 gene. | ROS1 Rearrangement Positive | First-line therapy: ceritinib; crizotinib; entrectinib. Subsequent therapy: lorlatinib; entrectinib. | [4,42] |
| NTRK1-3 | The NTRK1, NTRK2, and NTRK3 genes encode proteins that act as growth factor receptors in the nervous system during normal physiology. The alterations are related to oncogenic fusions of the NTRK genes. | NTRK1/2/3 Gene Fusion Positive | Larotrectinib; entrectinib. | [4,42,94] |
| MET | The alterations relate to amino acid substitutions in Y1003 or mutations/deletions in METex14 (mutation of MET exon 14) or its lateral introns. | MET Exon 14 Skipping Mutation | Capmatinib; crizotinib; tepotinib. | [4,42] |
| RET | The alterations are related to gene rearrangements involving RET, resulting in dysregulation and inappropriate signaling through the RET kinase domain. | RET Rearrangement Positive | Selpercatinib; pralsetinib; cabozantinib. | [4,42,94] |
| HER2 | A total of 96% of the alterations are kinase-activating exon 20 insertion mutations. | HER2 Mutation Positive | Subsequent therapy: fam-trastuzumab deruxtecan-nxki; ado-trastuzumab emtansine. | [4,42] |
| Epigenetic Modification | Biomarker | Regulation in NSCLC | References |
|---|---|---|---|
| DNA methylation | DNMT1, MGMT, DAPK, RASSF1a, CDKN2A, APC, CHD13, KLK10, DLEC1, AGTR1, GALR1, SLC5A8, NTSR1, SULF2. | Hypermethylation | [109,113,114,116,117,118,119] |
| ZMYND10 | Hypomethylation | [109,118] | |
| PITX2, SHOX2 | Methylation | [120] | |
| Histone Modification | H4K5/H4K8 | Hyperacetylation | [109,118] |
| H4K12/H4K16 | Hypoacetylation | ||
| H4K20me3 | Loss trimethylation | ||
| miRNAs | let-7c, miR-138, miR-145, miR-183, miR-29, miR-34a, miR-34c-3p, miR-101-3p, miR-129, miR-200b, miR-212, miR-218, miR-449a e miR-451 | Downregulated | [121,122,123,124,125] |
| miR-126, miR-21, miRs-34a miR-19, mi-150 e miR-141, miR-124, miR-132, miR-155, miR-331-5p e miR-483-5p | Upregulated | [111,122,126,127,128] | |
| miR-30a, miR-107, miR-138, miR-204, miR-32, miR-148b, miR-145, miR-224, miR-200c, miR-125b e miR-375, miR-23b-3p | Downregulated | [106,126,129] | |
| miR-21/155, miR-25, miR-31, miR-221/222, miR-224, miR-191, miR-494, miR-19a and miR-346, miR-10b-5p | Upregulated | [122,129,130] | |
| lncRNA | MCM3AP-AS1, TP53TG1 | Downregulated | [131,132] |
| RP11-397D12.4, AC007403.1, ERICH1-AS1, SPRY4-IT1, ANRIL and NEAT1 | Upregulated | [133,134] | |
| GAS5; HAGLR, ADAMTS9-AS2, TP73-AS1, LINC00261 e LINC00312 | Downregulated | [131,135] | |
| TBILA, AGAP2-AS1, LINC00673, LOC730101 | Upregulated | [131,136] |
| Potential Biomarkers/Factors | Description | References |
|---|---|---|
| p16INK4A, p53, p21, CDK1 and survivin | • High expressions of specific genes are observed after radiotherapy in NSCLC; | [155,156,157,158] |
| • Lung cancer cells lacking p53 have the ability to evade chemotherapy-induced senescence; | ||
| • CDK1 is a potential biomarker for the transition between senescence and the repopulation of cancer cells from giant polyploid cancer cells; | ||
| • Phenotypic characteristics of NSCLC lineages, A549 (wild-type TP53) and H1299 (TP53 deficient), as well as their surviving descendants after multifractionated X-ray irradiation, exhibit a strong association with p53 as a biomarker for the formation of multinucleated giant cancer cells. | ||
| Giant CAMLs | • Giant CAMLs as a potential peripheral blood biomarker for NSCLC progression due to their relationship with metastatic disease and worse survival, despite the use of maintenance immunotherapy. | [159] |
| CDH1 | • CDH1 as a potential new drug target, and its hypermethylation can be reversed through demethylation, being used in lung cancer, which may present a possible relationship with the stress response mechanisms in NSCLC. | [160] |
| Aurora kinase A and B, JAK2, SRC, and histone H3 | • Resistance to EGFR TKIs in NSCLC is frequently associated with activation of AURKB and increased levels of histone H3 phosphorylation; | [161,162] |
| • Reversine as an anticancer agent in human NSCLC and influences potential cell cycle biomarkers associated with polyploidy such as Aurora kinase A and B, JAK2, and SRC. | ||
| Staurosporine | • Association between staurosporine (potential biomarker) and PGCCs (giant cancer cells) and features of polyploid and multinucleated growth in lung cancer cell lines. | [163] |
| Mitotic microtubule polymerization | • Mitotic microtubule polymerization as a critical hallmark of NSCLC polyploidization after vinorelbine treatment, inducing prolonged accumulation in the G2/M phase after radiotherapy. | [164] |
| Vimentin and N-cadherin | • Inhibition of vimentin and N-cadherin in the face of the stressful effect of quercetin acts on the main elements of the cytoskeleton (microfilaments, microtubules, and intermediate filaments) but also associates with mitotic catastrophe, cytokinesis failure, induced G2/M arrest, polyploidy, increased cell size, and multinucleation in NSCLC. | [165] |
| FHIT | • In over 90% of lung tumors, FHIT exhibits loss of heterozygosity, and in advanced cases, it shows promoter methylation; | [166] |
| • FHIT stands out as a key biomarker for understanding the bridge between macroevolution and microevolution in lung cancer; | ||
| • Abnormal FHIT expression promotes genomic instability, leading to increased aneuploid chromosomes, single-stranded DNA, and induced genetic mutations driving microevolution. | ||
| Cyclin b1 e CDC2 | • Proliferation and apoptosis characteristics of the docetaxel-induced polyploid NSCLC cellular model, as well as the potential role of polyploid tumor cells in chemotherapy resistance and tumor recurrence. Increased expression of anti-apoptotic proteins (such as bcl-2, pbcl-2, and bcl-xl) and survival proteins (such as survivin), along with the inhibition of cyclin B1/cdc2 complex activity in A549 cells, leading to a G2/M cell cycle arrest, and generation of polyploid tumor cells. | [167,168] |
| CD8+ and SBS3 | • Evolution of the immune profile from primary tumors to distant and local metastases in NSCLC revealed that the level of CD8+ T cells was lower in polyploid samples than in diploid samples. Furthermore, the SBS3 signature, closely associated with genomic instability, exhibited a significantly higher proportion in polyploid metastases, highlighting it as a potential biomarker of tumor evolution. | [169] |
| ASAH1 and L858R | • Inhibition of acid sphingolipid enzyme ceramidase (ASAH1) activity as a complementary action in combination with cisplatin against NSCLC, favoring the reduction in aneuploid offspring resulting from the depolyploidization process of PGCCs; | [170,171,172,173] |
| • Unlike other EGFR mutations, L858R needs dimerization that is inhibited by cetuximab, reducing the viability of cells expressing L858R-EGFR and blocking the FOXM1-aurora survival pathway, whereas other mutants show no responses; | ||
| • Cetuximab completely prevents relapses of L858R+ tumors, differently to TKI-treated patient-derived xenografts, which relapse post osimertinib treatment. Osimertinib’s lower efficacy is associated with the induction of mutagenic reactive oxygen species, while cetuximab downregulates adaptive survival pathways (HER2). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meira, D.D.; de Castro e Caetano, M.C.; Casotti, M.C.; Zetum, A.S.S.; Gonçalves, A.F.M.; Moreira, A.R.; de Oliveira, A.H.; Pesente, F.; Santana, G.M.; de Almeida Duque, D.; et al. Prognostic Factors and Markers in Non-Small Cell Lung Cancer: Recent Progress and Future Challenges. Genes 2023, 14, 1906. https://doi.org/10.3390/genes14101906
Meira DD, de Castro e Caetano MC, Casotti MC, Zetum ASS, Gonçalves AFM, Moreira AR, de Oliveira AH, Pesente F, Santana GM, de Almeida Duque D, et al. Prognostic Factors and Markers in Non-Small Cell Lung Cancer: Recent Progress and Future Challenges. Genes. 2023; 14(10):1906. https://doi.org/10.3390/genes14101906
Chicago/Turabian StyleMeira, Débora Dummer, Maria Clara de Castro e Caetano, Matheus Correia Casotti, Aléxia Stefani Siqueira Zetum, André Felipe Monteiro Gonçalves, André Rodrigues Moreira, Augusto Henrique de Oliveira, Fellipe Pesente, Gabriel Mendonça Santana, Daniel de Almeida Duque, and et al. 2023. "Prognostic Factors and Markers in Non-Small Cell Lung Cancer: Recent Progress and Future Challenges" Genes 14, no. 10: 1906. https://doi.org/10.3390/genes14101906





