Genome-Wide Identification and Expression Analysis of the MADS Gene Family in Tulips (Tulipa gesneriana)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Identification and Sequence Analysis of TgMADS Gene Family Members
2.3. Phylogenetic and Multiple Alignment Analysis of TgMADS Proteins
2.4. Distribution Analysis of TgMADS
2.5. Sequence Similarity Analysis of TgMADS Genes
2.6. Ka/Ks Analysis of TgMADS Genes
2.7. Gene Structure and Conserved Motif Analysis TgMADS Genes
2.8. Expression Analysis of TgMADS Genes Based on RNA-Seq
2.9. Expression Analysis of TgMADS Genes Based on qRT-PCR
3. Results
3.1. Identification of TgMADS Gene Family Members
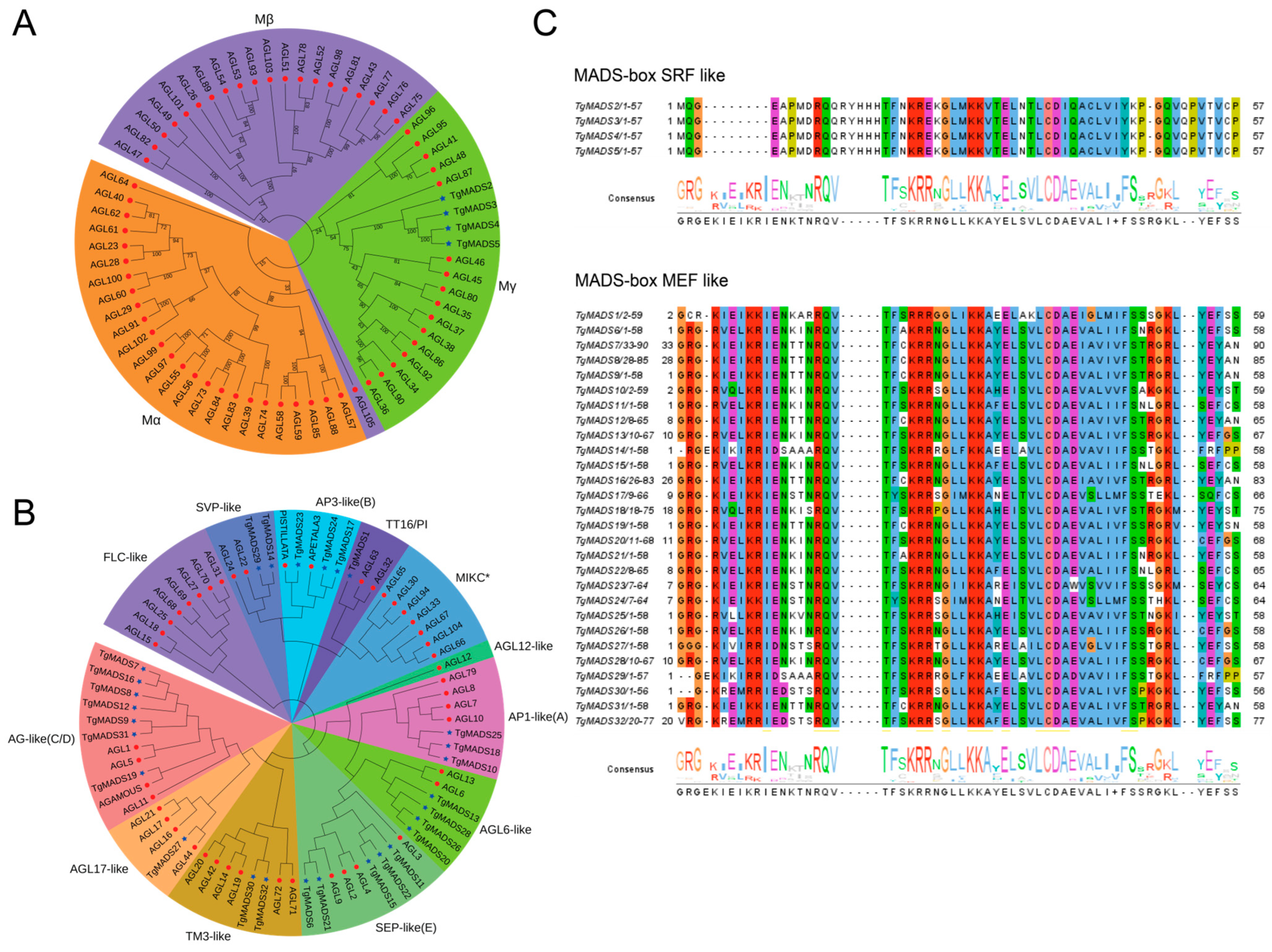
3.2. Phylogenetic Analysis of the TgMADS Gene Family
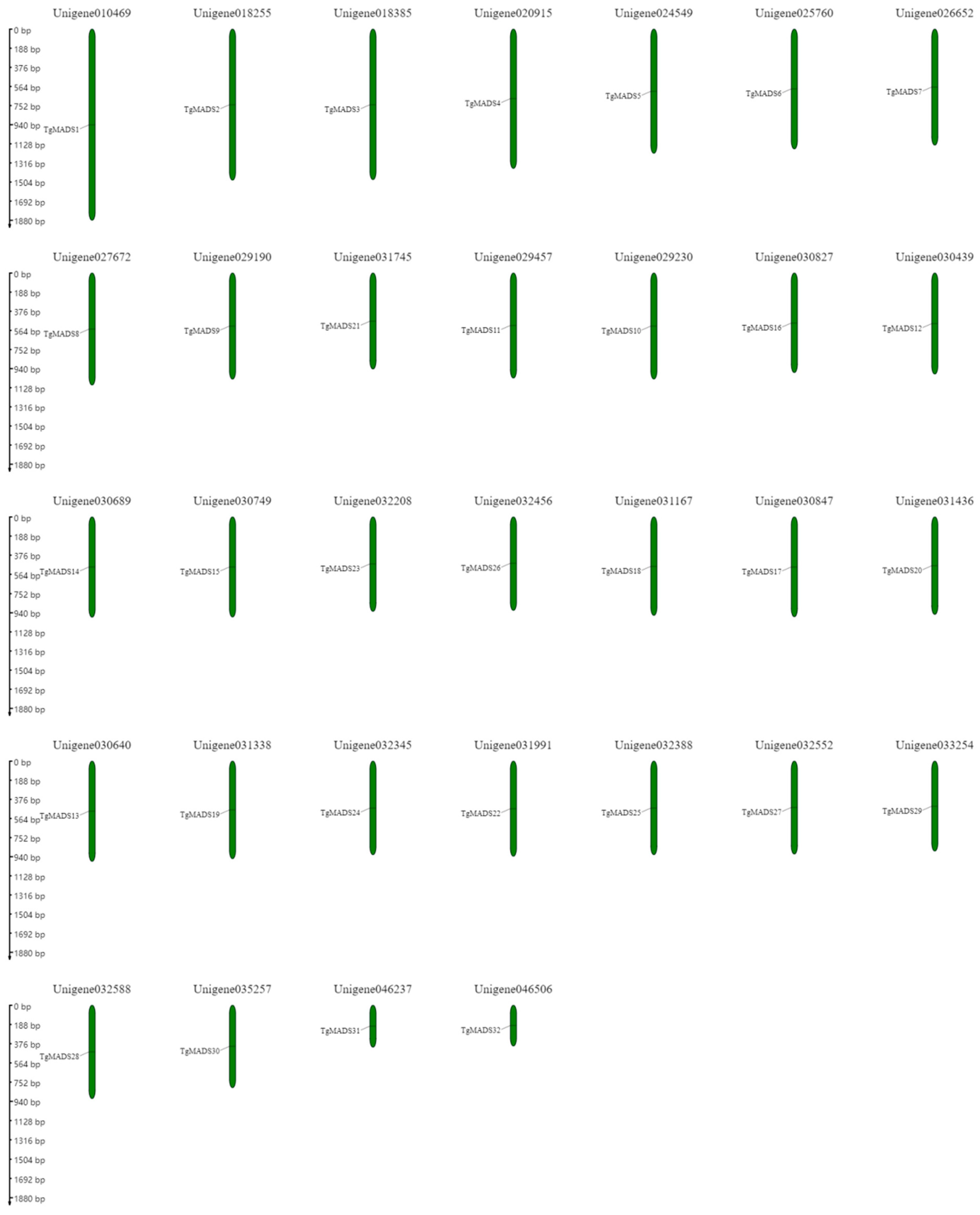
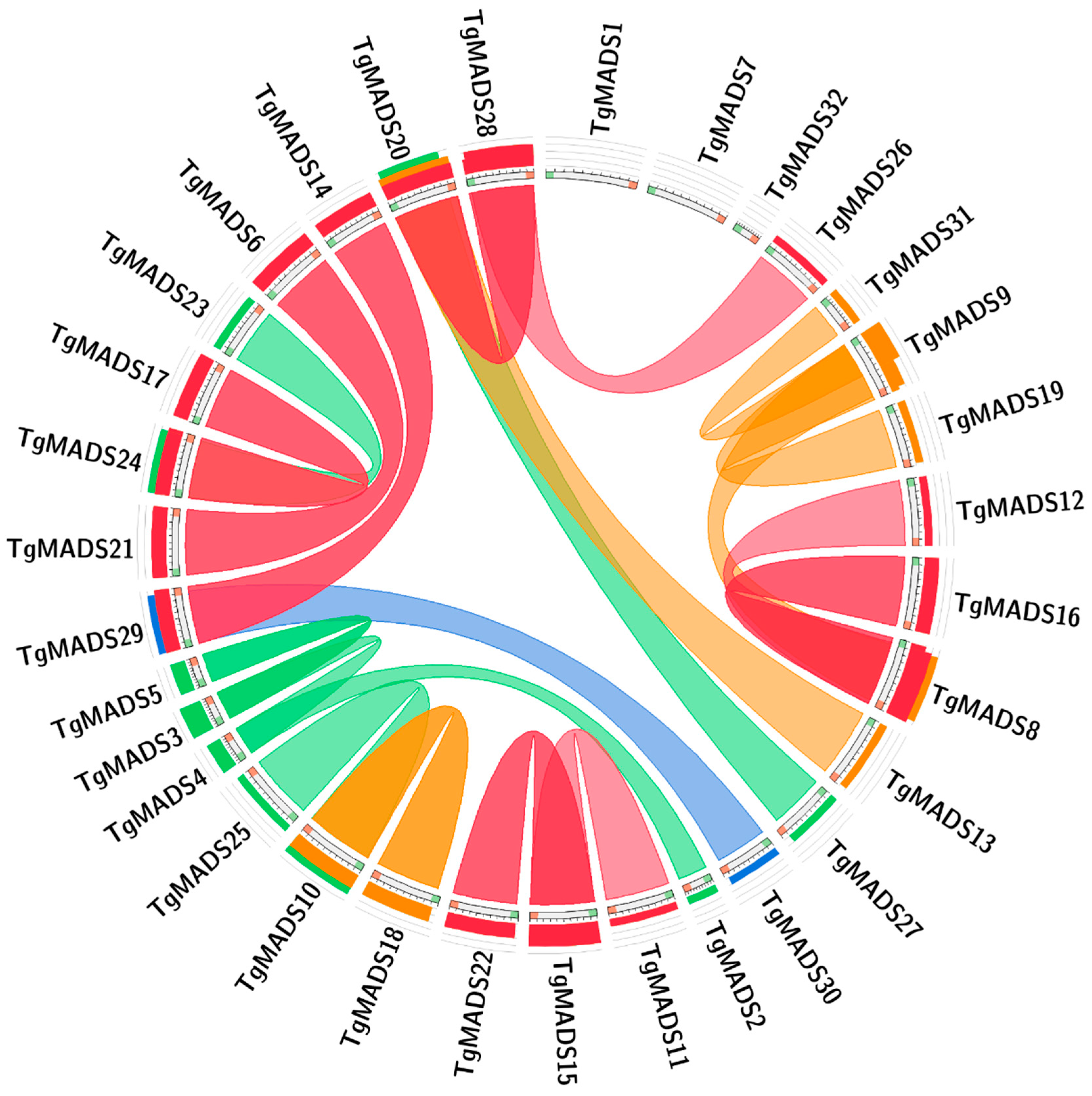
3.3. Localization and Collinearity Analysis of TgMADS Gene
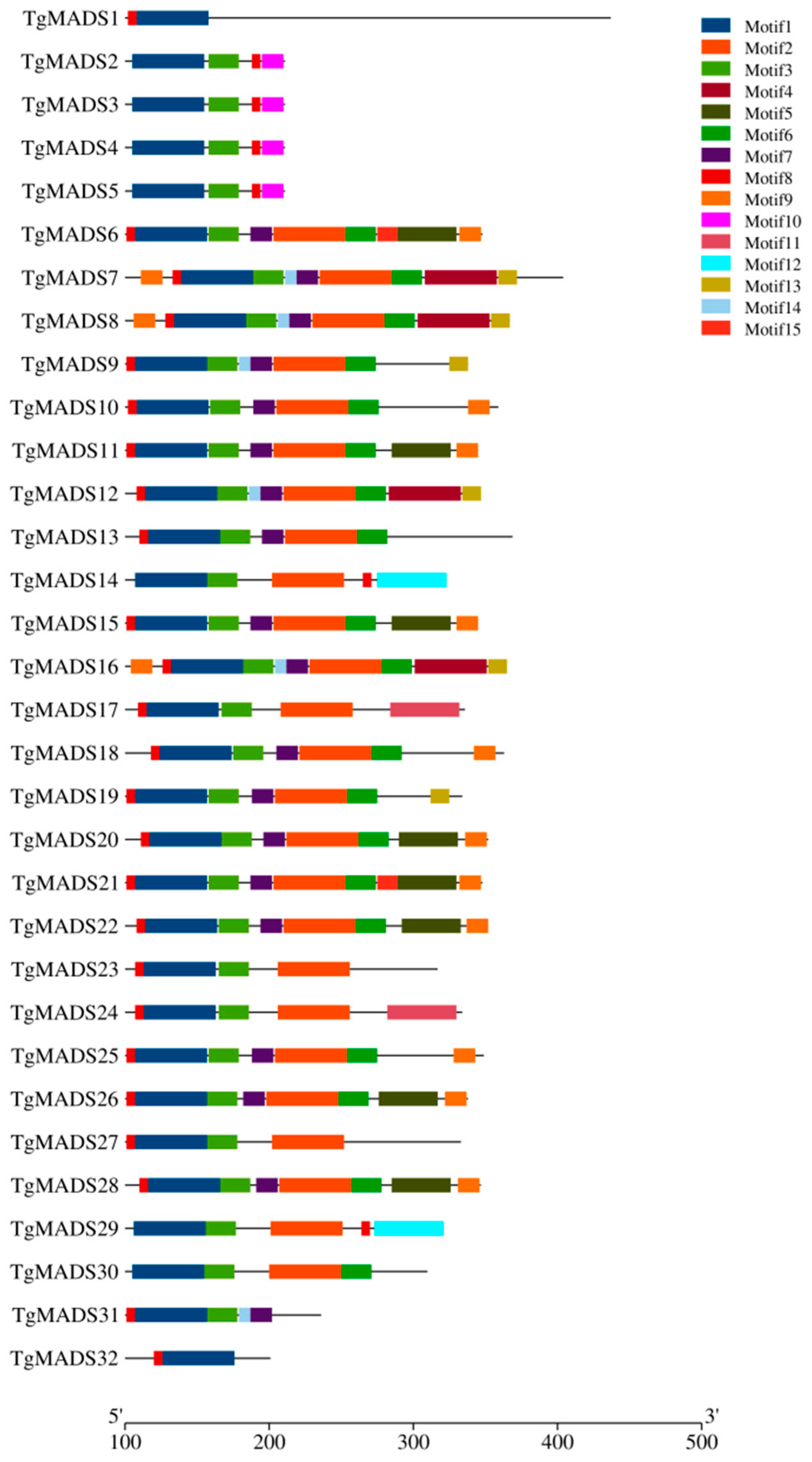
3.4. Analysis of TgMADS Gene Structure and Conserved Motifs
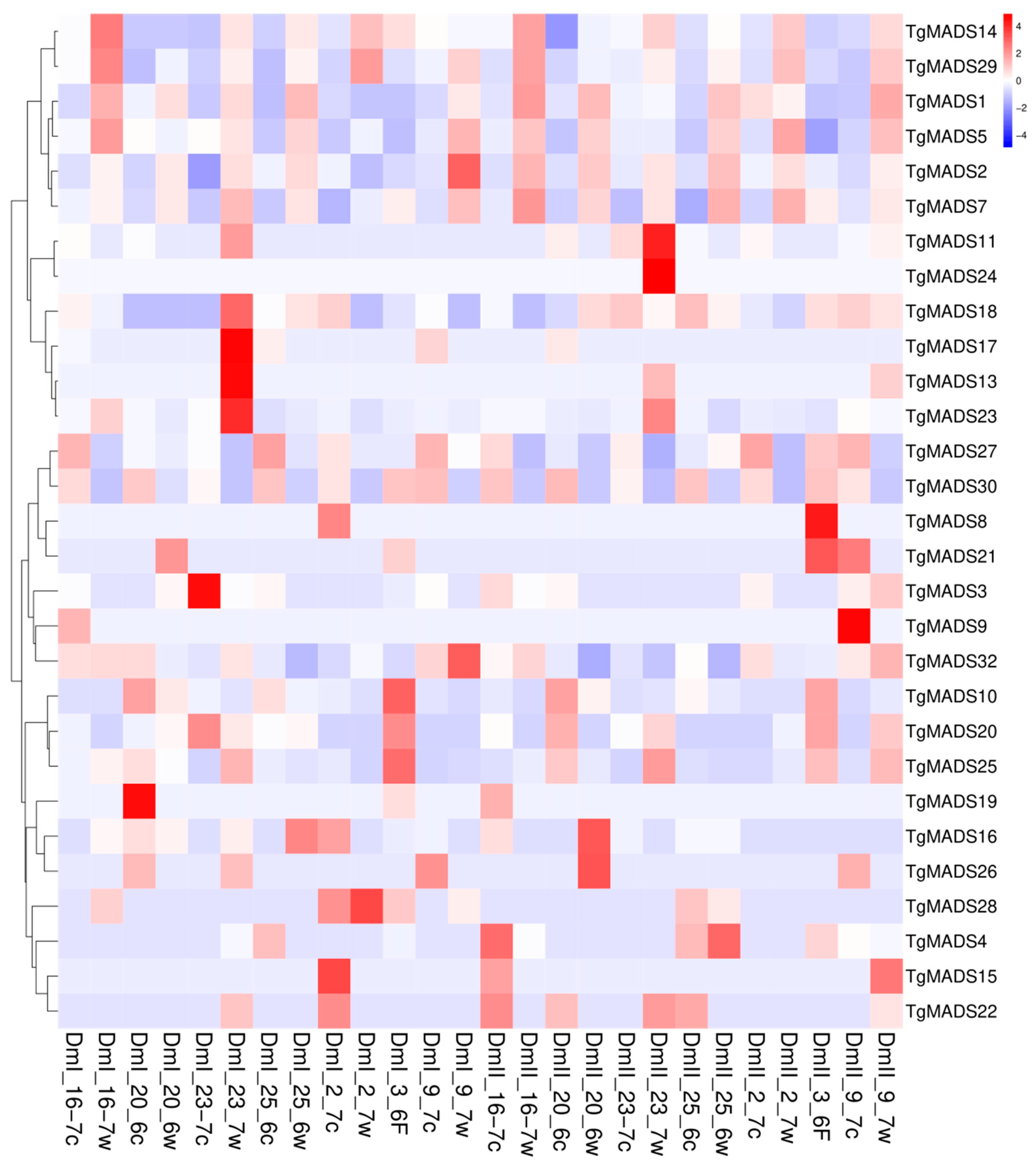
3.5. TgMADS Gene Expression Pattern Analysis
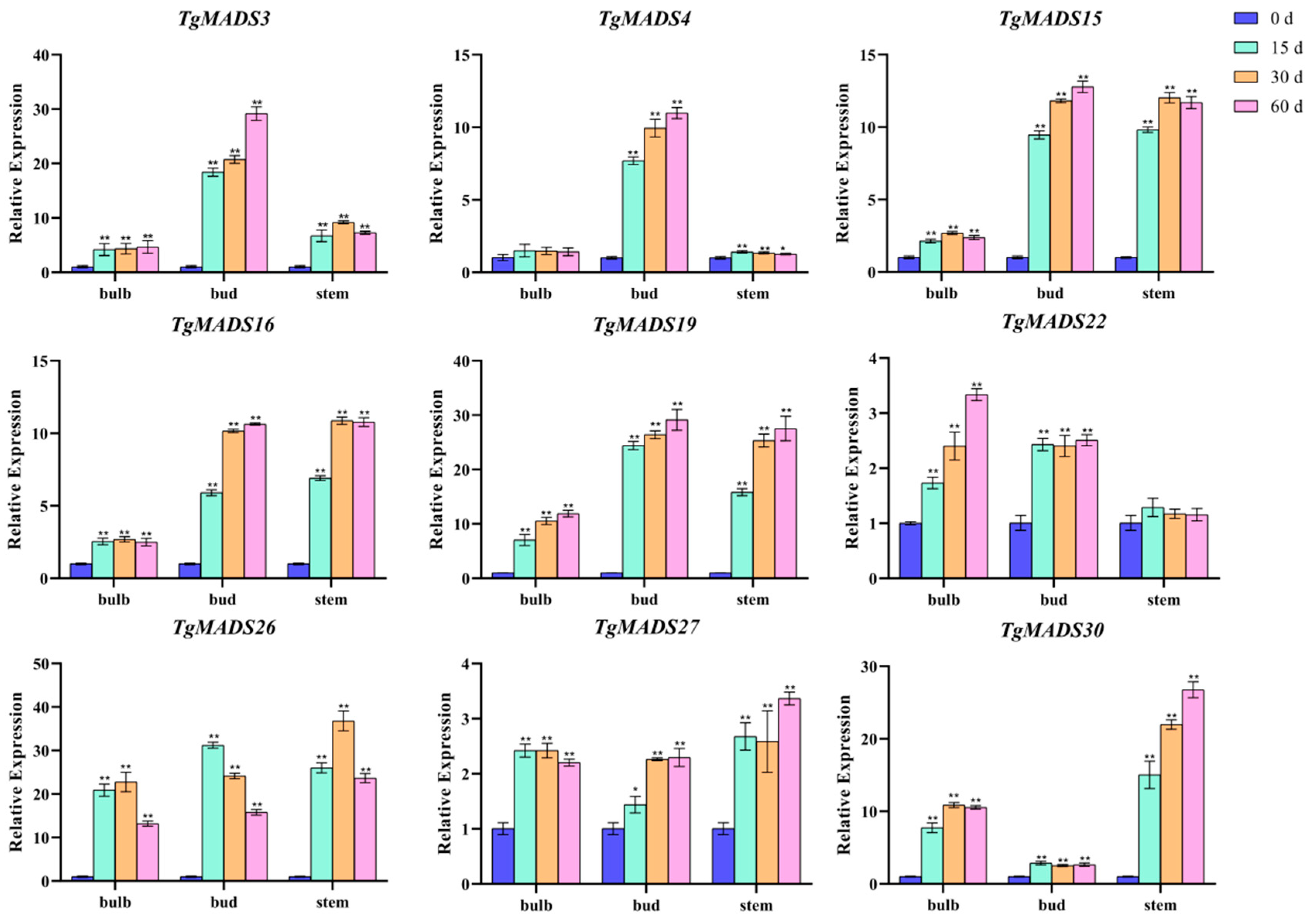
3.6. qRT-PCR Analysis of TgMADS Genes under Cold Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rietveld, P.L.; Wilkinson, C.; Franssen, H.M.; Balk, P.A.; van der Plas, L.H.; Weisbeek, P.J.; Douwe de Boer, A. Low temperature sensing in tulip (Tulipa gesneriana L.) is mediated through an increased response to auxin. J. Exp. Bot. 2000, 51, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Mondragón-Palomino, M. Perspectives on MADS-box expression during orchid flower evolution and development. Front. Plant Sci. 2013, 4, 377. [Google Scholar] [CrossRef] [PubMed]
- Saedler, H.; Becker, A.; Winter, K.-U.; Kirchner, C.; Theißen, G. MADS-box genes are involved in floral development and evolution. Acta Biochim. Pol. 2001, 48, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Yanofsky, M.F. Function and evolution of the plant MADS-box gene family. Nat. Rev. Genet. 2001, 2, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, J.L.; Meyerowitz, E.M. MADS domain proteins in plant development. Biol. Chem. 1997, 378, 1079–1102. [Google Scholar] [PubMed]
- Münster, T.; Wingen, L.U.; Faigl, W.; Werth, S.; Saedler, H.; Theißen, G. Characterization of three GLOBOSA-like MADS-box genes from maize: Evidence for ancient paralogy in one class of floral homeotic B-function genes of grasses. Gene 2001, 262, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gramzow, L.; Theißen, G. Phylogenomics of MADS-box genes in plants—Two opposing life styles in one gene family. Biology 2013, 2, 1150–1164. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Yu, D.; Yang, Z.; Li, C.; Qanmber, G.; Li, Y.; Li, J.; Liu, Z.; Lu, L.; Wang, L. Genome-wide identification of the MIKC-type MADS-box gene family in Gossypium hirsutum L. unravels their roles in flowering. Front. Plant Sci. 2017, 8, 384. [Google Scholar] [CrossRef]
- Becker, A.; Theißen, G. The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Mol. Phylogenet. Evol. 2003, 29, 464–489. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastava, H.; Das, A.; Tribhuvan, K.U.; Durgesh, K.; Joshi, R.; Sevanthi, A.M.; Jain, P.K.; Singh, N.K.; Gaikwad, K. Identification and characterization of MADS box gene family in pigeonpea for their role during floral transition. 3 Biotech 2021, 11, 108. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Chen, Y.; Xu, X.; Guang, X.; Zhang, Y. Genome-wide analysis of the MADS-box gene family in Watermelon. Comput. Biol. Chem. 2019, 80, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Dong, Q.; Ji, Z.; Chi, F.; Cong, P.; Zhou, Z. Genome-wide identification and analysis of the MADS-box gene family in apple. Gene 2015, 555, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.G.; Zhao, Z.Q.; Wang, J.S.; Yu, H.F.; Shen, Y.S.; Zeng, X.Y.; Gu, H.H. Genome wide analysis of MADS-box gene family in Brassica oleracea reveals conservation and variation in flower development. J. BMC Plant Biol. 2019, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yang, Y.; Luo, W.; Yang, C.; Ding, P.; Liu, Y.; Qiao, L.; Chang, Z.; Geng, H.; Wang, P. Genome-wide identification and analysis of the MADS-box gene family in bread wheat (Triticum aestivum L.). PLoS ONE 2017, 12, e0181443. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chen, Z.; Xu, L.; Zhang, L.; Zou, Q. Genome-Wide analysis of the MADS-Box gene family in maize: Gene structure, evolution, and relationships. Genes 2021, 12, 1956. [Google Scholar] [CrossRef] [PubMed]
- Won, S.Y.; Jung, J.-A.; Kim, J.S. Genome-wide analysis of the MADS-Box gene family in Chrysanthemum. Comput. Biol. Chem. 2021, 90, 107424. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, Z.; Lan, S. Genome sequencing reveals the role of MADS-box gene families in the floral morphology evolution of orchids. Hortic. Plant J. 2019, 5, 247–254. [Google Scholar] [CrossRef]
- Kanno, A.; Hirai, M.; Ochiai, T.; Simon, H.; Theissen, G. Reduced expression of DEFICIENS-like genes in the sepaloid tepals of viridiflora tulips. In Proceedings of the XXVII International Horticultural Congress-IHC2006: International Symposium on Structural and Functional Genomics of 763, Seoul, Korea, 13–19 August 2006; pp. 43–48. [Google Scholar]
- Hirai, M.; Ochiai, T.; Kanno, A. The expression of two DEFICIENS-like genes was reduced in the sepaloid tepals of viridiflora tulips. Breed. Sci. 2010, 60, 110–120. [Google Scholar] [CrossRef]
- Kanno, A.; Nakada, M.; Akita, Y.; Hirai, M. Class B gene expression and the modified ABC model in nongrass monocots. Sci. World J. 2007, 7, 268–279. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Xu, L.; Nie, S.; Chen, Y.; Liang, D.; Sun, X.; Karanja, B.K.; Luo, X.; Liu, L. Genome-wide characterization of the MADS-box gene family in radish (Raphanus sativus L.) and assessment of its roles in flowering and floral organogenesis. Front. Plant Sci. 2016, 7, 1390. [Google Scholar] [CrossRef]
- Theissen, G.; Melzer, R. Molecular mechanisms underlying origin and diversification of the angiosperm flower. Ann. Bot. 2007, 100, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.S.; Puranik, S.; Round, A.; Brennich, M.; Jourdain, A.; Parcy, F.; Hugouvieux, V.; Zubieta, C. Evolution of the plant reproduction master regulators LFY and the MADS transcription factors: The role of protein structure in the evolutionary development of the flower. Front. Plant Sci. 2016, 6, 1193. [Google Scholar] [CrossRef] [PubMed]
- Zahn, L.M.; Feng, B.; Ma, H. Beyond the ABC-model: Regulation of floral homeotic genes. Adv. Bot. Res. 2006, 44, 163–207. [Google Scholar]
- Arora, R.; Agarwal, P.; Ray, S.; Singh, A.K.; Singh, V.P.; Tyagi, A.K.; Kapoor, S. MADS-box gene family in rice: Genome-wide identification, organization and expression profiling during reproductive development and stress. BMC Genom. 2007, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- van Tunen, A.J.; Eikelboom, W.; Angenent, G.C. Floral organogenesis in Tulipa. Flower. Newsl. 1993, 16, 33–38. [Google Scholar]
- Michaels, S.D.; Amasino, R.M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 1999, 11, 949–956. [Google Scholar] [CrossRef]
- Hartmann, U.; Höhmann, S.; Nettesheim, K.; Wisman, E.; Saedler, H.; Huijser, P. Molecular cloning of SVP: A negative regulator of the floral transition in Arabidopsis. Plant J. 2000, 21, 351–360. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Ditta, G.S.; Eshed, Y.; Savidge, B.; Bowman, J.L.; Yanofsky, M.F. SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 2000, 404, 766–770. [Google Scholar] [CrossRef]
- Lawton-Rauh, A.L.; Alvarez-Buylla, E.R.; Purugganan, M.D. Molecular evolution of flower development. Trends Ecol. Evol. 2000, 15, 144–149. [Google Scholar] [CrossRef]
- Henschel, K.; Kofuji, R.; Hasebe, M.; Saedler, H.; Münster, T.; Theißen, G. Two ancient classes of MIKC-type MADS-box genes are present in the moss Physcomitrella patens. Mol. Biol. Evol. 2002, 19, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Krogan, N.; Ashton, N. Ancestry of plant MADS-box genes revealed by bryophyte (Physcomitrella patens) homologues. New Phytol. 2000, 147, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Winter, K.-U.; Becker, A.; Münster, T.; Kim, J.T.; Saedler, H.; Theissen, G. MADS-box genes reveal that gnetophytes are more closely related to conifers than to flowering plants. Proc. Natl. Acad. Sci. USA 1999, 96, 7342–7347. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. A new generation of homology search tools based on probabilistic inference. In Genome Informatics 2009: Genome Informatics Series Vol. 23; World Scientific: Singapore, 2009; pp. 205–211. [Google Scholar]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.; Potter, S.C.; Finn, R.D. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef] [PubMed]
- Mistry, J.; Bateman, A.; Finn, R.D. Predicting active site residue annotations in the Pfam database. BMC Bioinform. 2007, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Sonnhammer, E.L.; Eddy, S.R.; Durbin, R. Pfam: A comprehensive database of protein domain families based on seed alignments. Proteins Struct. Funct. Bioinform. 1997, 28, 405–420. [Google Scholar] [CrossRef]
- Hallgren, J.; Tsirigos, K.D.; Pedersen, M.D.; Almagro Armenteros, J.J.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts α and β transmembrane proteins using deep neural networks. BioRxiv 2022. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment are version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kumar, S.; Tamura, K.; Nei, M. MEGA: Molecular evolutionary genetics analysis software for microcomputers. Bioinformatics 1994, 10, 189–191. [Google Scholar] [CrossRef]
- Waterhouse, A.; Procter, J.; Martin, D.A.; Barton, G.J. Jalview: Visualization and analysis of molecular sequences, alignments, and structures. BMC Bioinform. 2005, 6, P28. [Google Scholar] [CrossRef]
- Chao, J.; Li, Z.; Sun, Y.; Aluko, O.O.; Wu, X.; Wang, Q.; Liu, G. MG2C: A user-friendly online tool for drawing genetic maps. Mol. Hortic. 2021, 1, 16. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Zhang, Z.; Zhu, J.; Yu, J. KaKs_Calculator 2.0: A toolkit incorporating gamma-series methods and sliding window strategies. Genom. Proteom. Bioinform. 2010, 8, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.-Y.; Zhu, Q.-H.; Chen, X.; Luo, J.-C. GSDS: A gene structure display server. Yi Chuan = Hereditas 2007, 29, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef]
- Mo, F.; Xue, X.; Meng, L.; Zhang, Y.; Cui, Y.; Liu, J.; Cheng, M.; Wang, P.; Lv, R.; Meng, F. Genome-wide identification and expression analysis of SLAC1 gene family in tomato (Solanum lycopersicum) and the function of SlSLAC1–6 under cold stress. Sci. Hortic. 2023, 313, 111904. [Google Scholar] [CrossRef]
- Leeggangers, H.A.; Nijveen, H.; Bigas, J.N.; Hilhorst, H.W.; Immink, R.G. Molecular regulation of temperature-dependent floral induction in Tulipa gesneriana. Plant Physiol. 2017, 173, 1904–1919. [Google Scholar] [CrossRef]
- Lalitha, S. Primer premier 5. Biotech Softw. Internet Rep. Comput. Softw. J. Sci. 2000, 1, 270–272. [Google Scholar] [CrossRef]
- Crevillen, P.; Dean, C. Regulation of the floral repressor gene FLC: The complexity of transcription in a chromatin context. Curr. Opin. Plant Biol. 2011, 14, 38–44. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Z.; Dong, W.; Wang, Z.; Zhang, L. Expansion and functional divergence of the SHORT VEGETATIVE PHASE (SVP) genes in eudicots. Genome Biol. Evol. 2018, 10, 3026–3037. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, K.; Bhide, A.S.; Tekleyohans, D.G.; Wittkop, B.; Snowdon, R.J.; Becker, A. The MADS box genes ABS, SHP1, and SHP2 are essential for the coordination of cell divisions in ovule and seed coat development and for endosperm formation in Arabidopsis thaliana. PLoS ONE 2016, 11, e0165075. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, S.; Wu, F.; Yan, S.; Lin, X.; Du, X.; Chong, K.; Schilling, S.; Theißen, G.; Meng, Z. Functional conservation of MIKC*-Type MADS box genes in Arabidopsis and rice pollen maturation. Plant Cell 2013, 25, 1288–1303. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liang, W.; Yin, C.; Ji, S.; Wang, H.; Su, X.; Guo, C.; Kong, H.; Xue, H.; Zhang, D. The SEPALLATA-like gene OsMADS34 is required for rice inflorescence and spikelet development. Plant Physiol. 2010, 153, 728–740. [Google Scholar] [CrossRef]
- Fornara, F.; Parenicová, L.; Falasca, G.; Pelucchi, N.; Masiero, S.; Ciannamea, S.; Lopez-Dee, Z.; Altamura, M.M.; Colombo, L.; Kater, M.M. Functional characterization of OsMADS18, a member of the AP1/SQUA subfamily of MADS box genes. Plant Physiol. 2004, 135, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Zhao, S.; Shan, H.; Kong, H.; Lu, W.; Theissen, G.; Chen, Z.; Meng, Z. The MIK region rather than the C-terminal domain of AP3-like class B floral homeotic proteins determines functional specificity in the development and evolution of petals. New Phytol. 2008, 178, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, K.; Melzer, R.; Theißen, G. MIKC-type MADS-domain proteins: Structural modularity, protein interactions and network evolution in land plants. Gene 2005, 347, 183–198. [Google Scholar] [CrossRef]
- Shih, M.-C.; Chou, M.-L.; Yue, J.-J.; Hsu, C.-T.; Chang, W.-J.; Ko, S.-S.; Liao, D.-C.; Huang, Y.-T.; Chen, J.J.; Yuan, J.-L. BeMADS1 is a key to delivery MADSs into nucleus in reproductive tissues-De novo characterization of Bambusa edulis transcriptome and study of MADS genes in bamboo floral development. BMC Plant Biol. 2014, 14, 179. [Google Scholar] [CrossRef]
- Zobell, O.; Faigl, W.; Saedler, H.; Münster, T. MIKC* MADS-box proteins: Conserved regulators of the gametophytic generation of land plants. Mol. Biol. Evol. 2010, 27, 1201–1211. [Google Scholar] [CrossRef]
- Urbanus, S.L.; de Folter, S.; Shchennikova, A.V.; Kaufmann, K.; Immink, R.G.; Angenent, G.C. In planta localisation patterns of MADS domain proteins during floral development in Arabidopsis thaliana. BMC Plant Biol. 2009, 9, 5. [Google Scholar] [CrossRef]
- Lin, Z.; Cao, D.; Damaris, R.N.; Yang, P. Genome-wide identification of MADS-box gene family in sacred lotus (Nelumbo nucifera) identifies a SEPALLATA homolog gene involved in floral development. BMC Plant Biol. 2020, 20, 497. [Google Scholar] [CrossRef]
- Gabaldón, T.; Koonin, E.V. Functional and evolutionary implications of gene orthology. Nat. Rev. Genet. 2013, 14, 360–366. [Google Scholar] [CrossRef]
- Hurst, L.D. The Ka/Ks ratio: Diagnosing the form of sequence evolution. Trends Genet. 2002, 18, 486–487. [Google Scholar] [CrossRef]
- Saha, G.; Park, J.-I.; Jung, H.-J.; Ahmed, N.U.; Kayum, M.A.; Chung, M.-Y.; Hur, Y.; Cho, Y.-G.; Watanabe, M.; Nou, I.-S. Genome-wide identification and characterization of MADS-box family genes related to organ development and stress resistance in Brassica rapa. BMC Genom. 2015, 16, 178. [Google Scholar] [CrossRef]

| Gene Name | Subcellular Localization | Protein Length (aa) | Molecular Weight (Da) | Isoelectric Point | Stability Index | Liposolubility Index | GRAVY |
|---|---|---|---|---|---|---|---|
| TgMADS1 | Intercellular space | 336 | 37,396.38 | 7.54 | 53.86 | 81.34 | −0.625 |
| TgMADS2 | nucleus | 110 | 12,828.82 | 9.35 | 50.10 | 77.91 | −0.642 |
| TgMADS3 | nucleus | 110 | 12,828.82 | 9.35 | 50.10 | 77.91 | −0.642 |
| TgMADS4 | nucleus | 110 | 12,828.82 | 9.35 | 50.10 | 77.91 | −0.642 |
| TgMADS5 | nucleus | 110 | 12,828.82 | 9.35 | 50.10 | 77.91 | −0.642 |
| TgMADS6 | nucleus | 247 | 27,919.65 | 9.16 | 52.28 | 78.70 | −0.655 |
| TgMADS7 | nucleus | 303 | 35,098.54 | 8.94 | 59.48 | 70.86 | −0.889 |
| TgMADS8 | nucleus | 266 | 30,840.78 | 9.15 | 56.99 | 73.38 | −0.878 |
| TgMADS9 | nucleus | 237 | 27,825.35 | 9.33 | 61.48 | 68.73 | −1.029 |
| TgMADS10 | nucleus | 258 | 29,553.34 | 8.95 | 57.47 | 73.80 | −0.909 |
| TgMADS11 | nucleus | 244 | 27,871.45 | 7.02 | 52.36 | 79.14 | −0.72 |
| TgMADS12 | nucleus | 246 | 28,678.39 | 9.06 | 55.32 | 75.37 | −0.855 |
| TgMADS13 | nucleus | 268 | 30,635.63 | 9.07 | 48.64 | 73.88 | −0.799 |
| TgMADS14 | nucleus | 222 | 25,059.44 | 6.43 | 59.46 | 88.74 | −0.609 |
| TgMADS15 | nucleus | 244 | 27,881.48 | 7.07 | 49.34 | 79.14 | −0.721 |
| TgMADS16 | nucleus | 264 | 30,754.68 | 9.16 | 56.43 | 73.94 | −0.884 |
| TgMADS17 | nucleus | 235 | 26,910.46 | 9.18 | 35.68 | 75.49 | −0.784 |
| TgMADS18 | nucleus | 262 | 30,382.42 | 8.48 | 69.94 | 79.27 | −0.767 |
| TgMADS19 | nucleus | 233 | 26,757.12 | 9.28 | 45.40 | 79.61 | −0.713 |
| TgMADS20 | nucleus | 251 | 28,962.96 | 9.14 | 47.27 | 75.78 | −0.757 |
| TgMADS21 | nucleus | 247 | 27,919.65 | 9.16 | 52.28 | 78.70 | −0.655 |
| TgMADS22 | nucleus | 251 | 28,627.34 | 7.74 | 50.81 | 76.93 | −0.747 |
| TgMADS23 | nucleus | 216 | 25,461.11 | 9.05 | 65.98 | 73.10 | −0.965 |
| TgMADS24 | nucleus | 233 | 26,880.42 | 8.69 | 42.52 | 76.14 | −0.791 |
| TgMADS25 | nucleus | 248 | 28,396.23 | 6.38 | 60.33 | 80.20 | −0.698 |
| TgMADS26 | nucleus | 237 | 27,159.91 | 8.93 | 47.53 | 80.25 | −0.662 |
| TgMADS27 | nucleus | 232 | 26,620.5 | 8.58 | 51.10 | 102.03 | −0.484 |
| TgMADS28 | nucleus | 246 | 28,341.24 | 9.08 | 46.61 | 77.32 | −0.744 |
| TgMADS29 | nucleus | 220 | 24,846.2 | 6.06 | 59.73 | 89.55 | −0.592 |
| TgMADS30 | nucleus | 209 | 24,012.38 | 6.20 | 75.65 | 78.42 | −0.765 |
| TgMADS31 | nucleus | 136 | 15,554.62 | 9.80 | 45.92 | 77.50 | −0.857 |
| TgMADS32 | nucleus | 100 | 11,462.43 | 11.16 | 65.23 | 74.10 | −0.673 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Qu, L.; Xing, G.; Liu, Z.; Lu, X.; Han, X. Genome-Wide Identification and Expression Analysis of the MADS Gene Family in Tulips (Tulipa gesneriana). Genes 2023, 14, 1974. https://doi.org/10.3390/genes14101974
Lu J, Qu L, Xing G, Liu Z, Lu X, Han X. Genome-Wide Identification and Expression Analysis of the MADS Gene Family in Tulips (Tulipa gesneriana). Genes. 2023; 14(10):1974. https://doi.org/10.3390/genes14101974
Chicago/Turabian StyleLu, Jiaojiao, Lianwei Qu, Guimei Xing, Zhenlei Liu, Xiaochun Lu, and Xiaori Han. 2023. "Genome-Wide Identification and Expression Analysis of the MADS Gene Family in Tulips (Tulipa gesneriana)" Genes 14, no. 10: 1974. https://doi.org/10.3390/genes14101974




