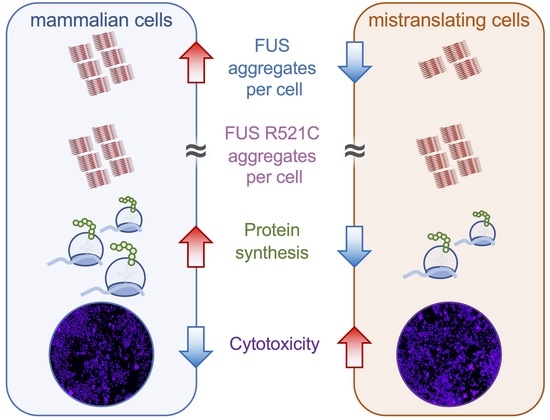
Genetic Interaction of tRNA-Dependent Mistranslation with Fused in Sarcoma Protein Aggregates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids and Strains
2.2. Cell Culture and Transfections
2.3. tRNA Sequencing
2.4. Fluorescence Microscopy
2.5. Semi-Denaturing Detergent Agarose Gel Electrophoresis (SDD-AGE)
3. Results
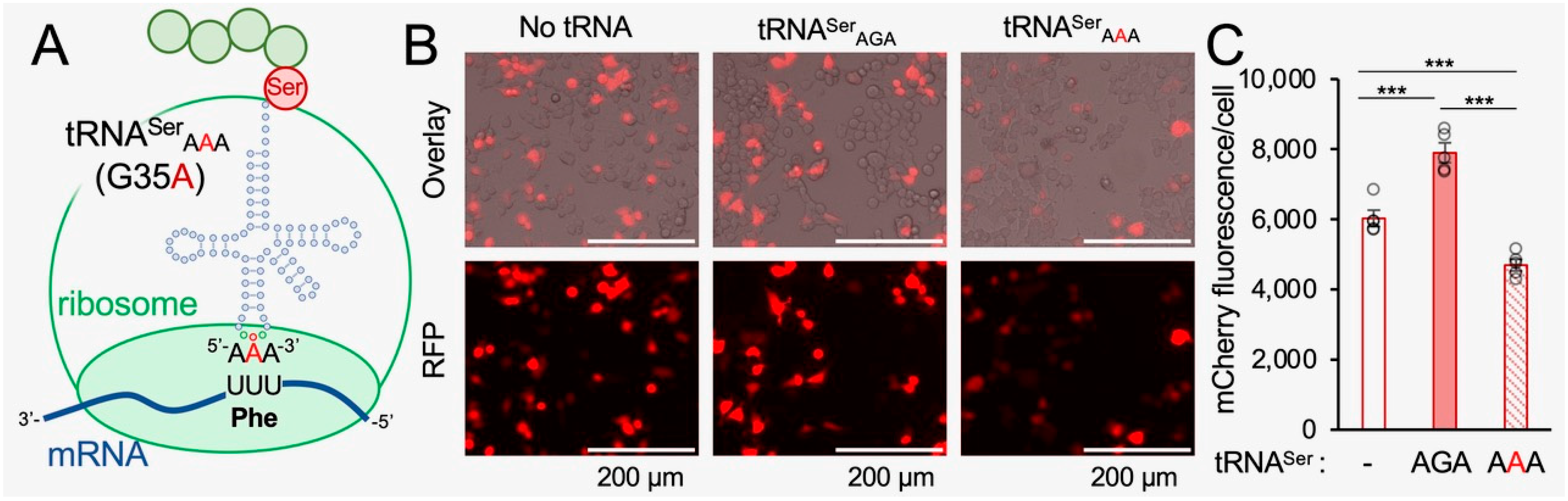
3.1. Protein Production in Mistranslating Cells
3.2. tRNA Sequencing Identifies and Quantitates tRNASerAAA Abundance in Mistranslating Cells
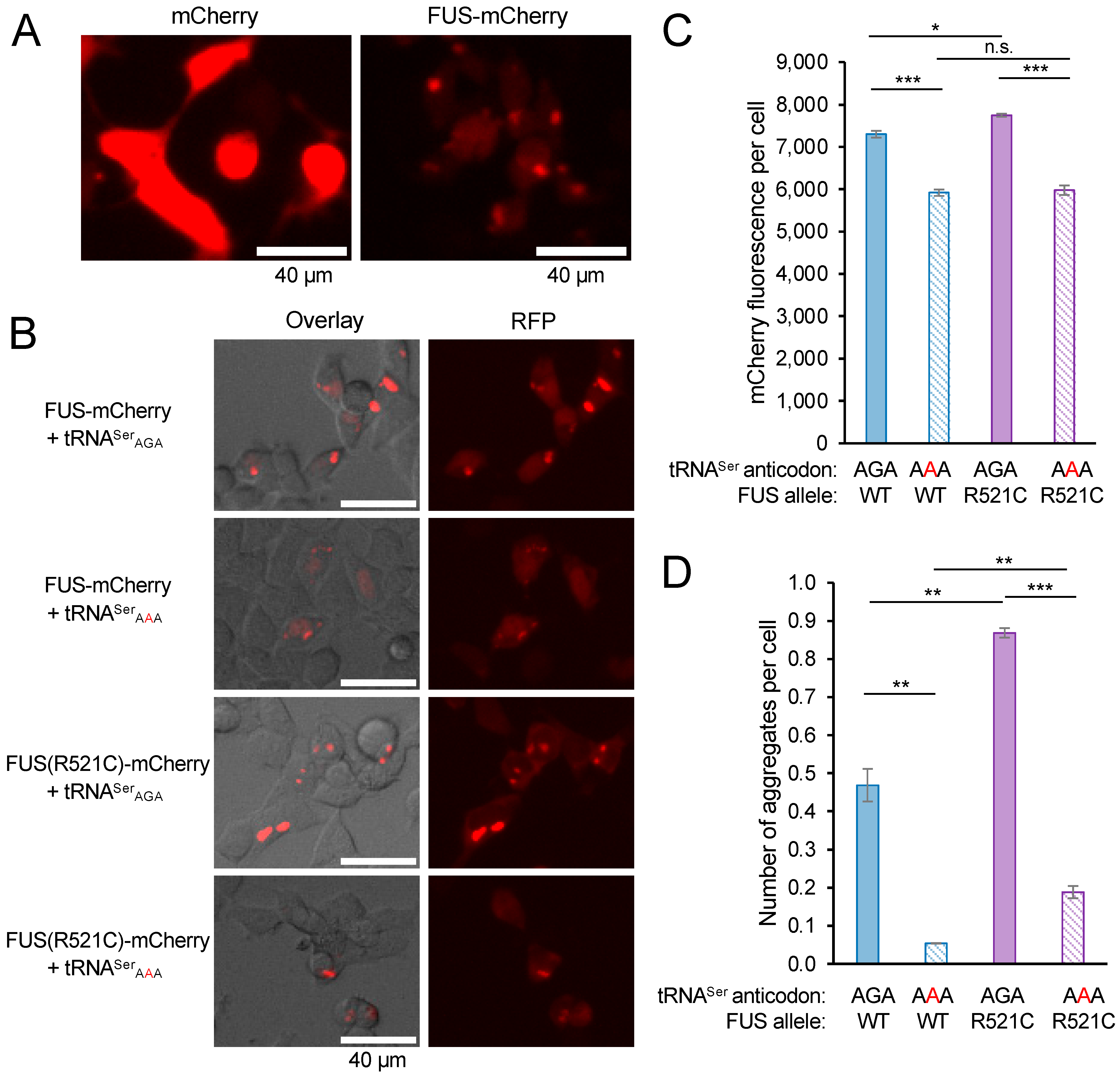
3.3. Aggregation of FUS Alleles in Normal and Mistranslating Cells
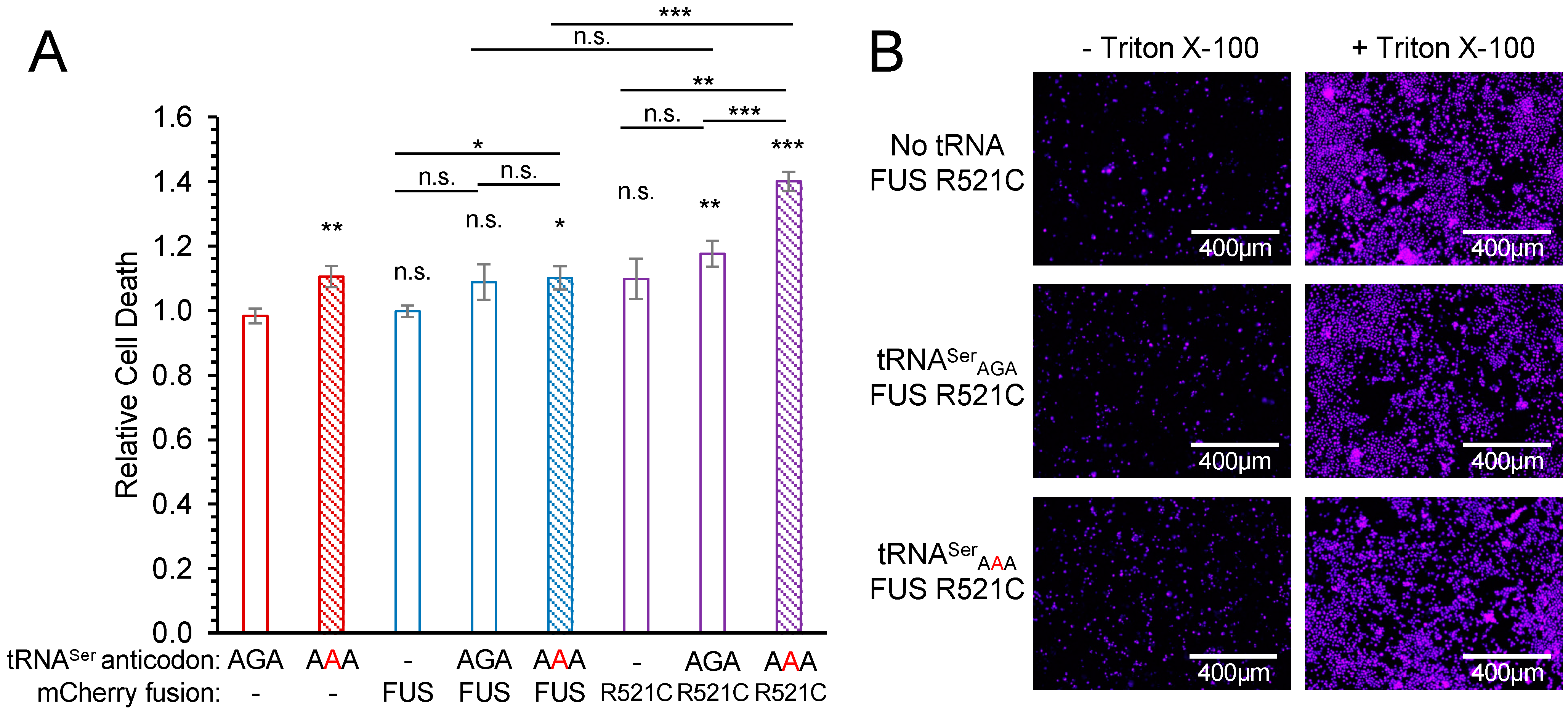
3.4. Toxicity of FUS Alleles in Mistranslating Cells
3.5. Kinetics of FUS Protein Production in Normal and Mistranslating Cells
3.6. FUS Aggregation Kinetics in Normal and Mistranslating Cells
3.7. FUS R521C Aggregation Kinetics in Normal and Mistranslating Cells
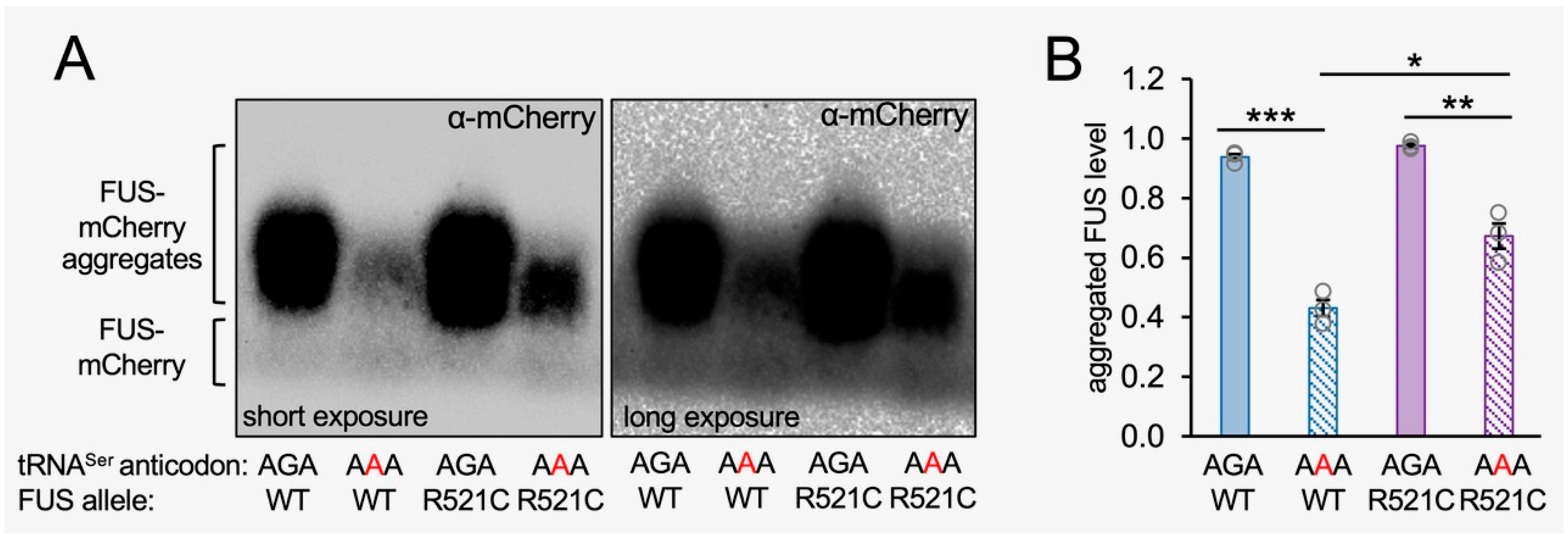
3.8. Measuring Total FUS Aggregate Levels in Normal and Mistranslating Cells
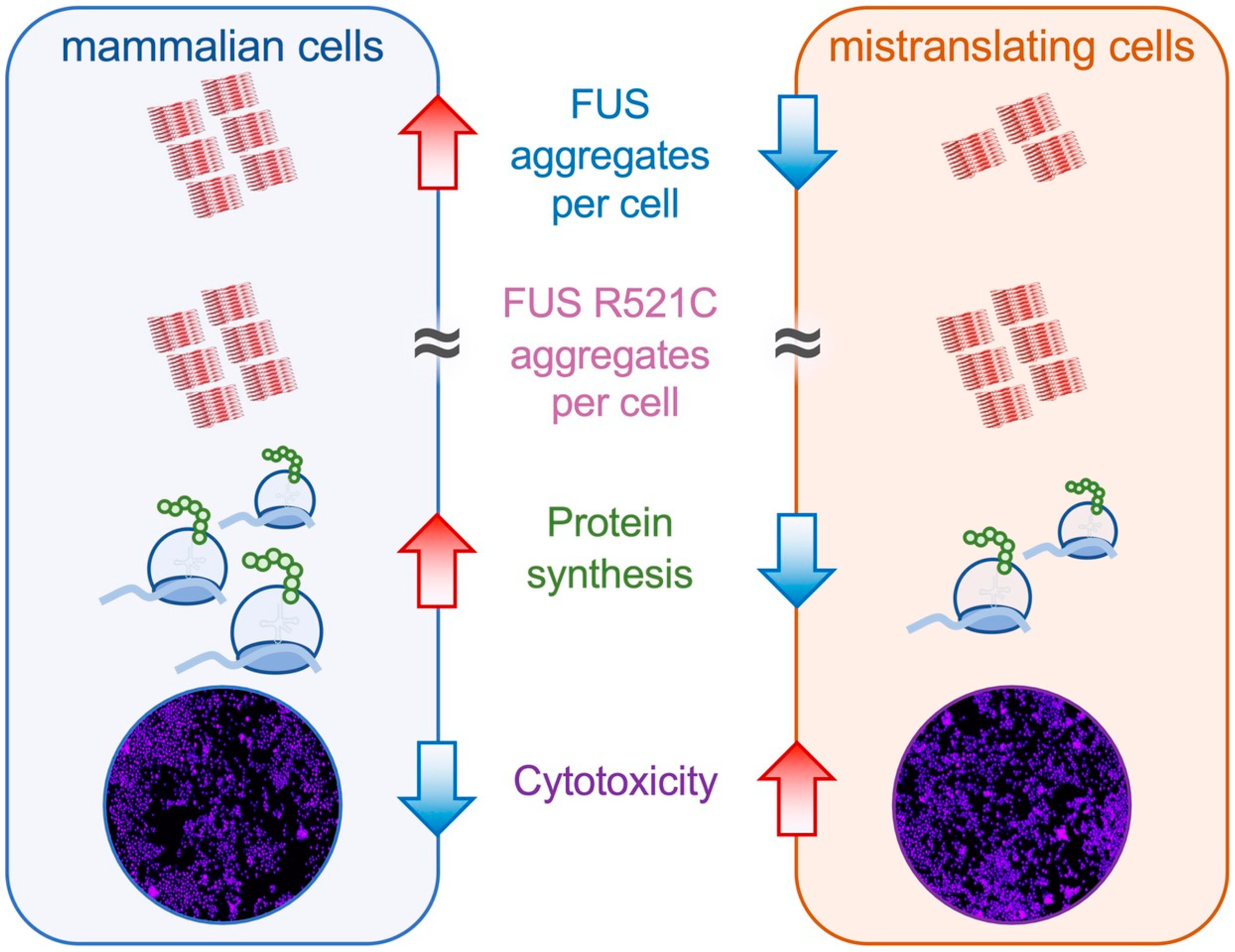
4. Discussion
4.1. De-Regulated Protein Homeostasis in ALS and in Cells Expressing Mutant or Wild-Type tRNAs
4.2. Considerations of Transfer RNA Variants as Therapeutics
4.3. Transfer RNA Variants as Modifiers of Neurodegenerative Diseases
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drummond, D.A.; Wilke, C.O. The evolutionary consequences of erroneous protein synthesis. Nat. Rev. Genet. 2009, 10, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Ruan, B.; Palioura, S.; Sabina, J.; Marvin-Guy, L.; Kochhar, S.; Larossa, R.A.; Soll, D. Quality control despite mistranslation caused by an ambiguous genetic code. Proc. Natl. Acad. Sci. USA 2008, 105, 16502–16507. [Google Scholar] [CrossRef] [Green Version]
- Lant, J.T.; Berg, M.D.; Sze, D.H.W.; Hoffman, K.S.; Akinpelu, I.C.; Turk, M.A.; Heinemann, I.U.; Duennwald, M.L.; Brandl, C.J.; O’Donoghue, P. Visualizing tRNA-dependent mistranslation in human cells. RNA Biol. 2018, 15, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Rozik, P.; Szabla, R.; Lant, J.T.; Kiri, R.; Wright, D.E.; Junop, M.; O’Donoghue, P. A novel fluorescent reporter sensitive to serine mis-incorporation. RNA Biol. 2022, 19, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc. Natl. Acad. Sci. USA 1963, 49, 517–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lant, J.T.; Berg, M.D.; Heinemann, I.U.; Brandl, C.J.; O’Donoghue, P. Pathways to disease from natural variations in human cytoplasmic tRNAs. J. Biol. Chem. 2019, 294, 5294–5308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Kenana, R.; Beharry, A.; Wilhelm, S.D.P.; Hsu, S.Y.; Siu, V.M.; Duennwald, M.; Heinemann, I.U. Histidine supplementation can escalate or rescue HARS deficiency in a Charcot Marie Tooth Disease model. Hum. Mol. Genet. 2022, 32, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Mohler, K.; Ibba, M. Translational fidelity and mistranslation in the cellular response to stress. Nat. Microbiol. 2017, 2, 17117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio Gomez, M.A.; Ibba, M. Aminoacyl-tRNA synthetases. RNA 2020, 26, 910–936. [Google Scholar] [CrossRef] [Green Version]
- Hoffer, E.D.; Maehigashi, T.; Fredrick, K.; Dunham, C.M. Ribosomal ambiguity (ram) mutations promote the open (off) to closed (on) transition and thereby increase miscoding. Nucleic Acids Res. 2019, 47, 1557–1563. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, D.; Gregory, S.T.; O’Connor, M. Error-prone and error-restrictive mutations affecting ribosomal protein S12. J. Mol. Biol. 2011, 410, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, N.M.; Lazazzera, B.A.; Ibba, M. Cellular mechanisms that control mistranslation. Nat. Rev. Microbiol. 2010, 8, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Giege, R.; Sissler, M.; Florentz, C. Universal rules and idiosyncratic features in tRNA identity. Nucleic Acids Res. 1998, 26, 5017–5035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, F.; Lant, J.T.; O’Donoghue, P. Perseverance of protein homeostasis despite mistranslation of glycine codons with alanine. Phil. Trans. R Soc. B 2023, 378, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.M.; Schimmel, P. A simple structural feature is a major determinant of the identity of a transfer RNA. Nature 1988, 333, 140–145. [Google Scholar] [CrossRef]
- Hoffman, K.S.; Berg, M.D.; Shilton, B.H.; Brandl, C.J.; O’Donoghue, P. Genetic selection for mistranslation rescues a defective co-chaperone in yeast. Nucleic Acids Res. 2017, 45, 3407–3421. [Google Scholar] [CrossRef] [Green Version]
- Lant, J.T.; Kiri, R.; Duennwald, M.L.; O’Donoghue, P. Formation and persistence of polyglutamine aggregates in mistranslating cells. Nucleic Acids Res. 2021, 49, 11883–11899. [Google Scholar] [CrossRef]
- Kisselev, L.L. The role of the anticodon in recognition of tRNA by aminoacyl-tRNA synthetases. Prog. Nucleic Acid Res. Mol. Biol. 1985, 32, 237–266. [Google Scholar] [CrossRef]
- Parisien, M.; Wang, X.; Pan, T. Diversity of human tRNA genes from the 1000-genomes project. RNA Biol. 2013, 10, 1853–1867. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.P.; Lowe, T.M. GtRNAdb 2.0: An expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 2016, 44, D184–D189. [Google Scholar] [CrossRef] [Green Version]
- Berg, M.D.; Giguere, D.J.; Dron, J.S.; Lant, J.T.; Genereaux, J.; Liao, C.; Wang, J.; Robinson, J.F.; Gloor, G.B.; Hegele, R.A.; et al. Targeted sequencing reveals expanded genetic diversity of human transfer RNAs. RNA Biol. 2019, 16, 1574–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenhard, B.; Orellana, O.; Ibba, M.; Weygand-Durasevic, I. tRNA recognition and evolution of determinants in seryl-tRNA synthesis. Nucleic Acids Res. 1999, 27, 721–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsultan, A.A.; Waller, R.; Heath, P.R.; Kirby, J. The genetics of amyotrophic lateral sclerosis: Current insights. Degener Neurol. Neuromuscul Dis. 2016, 6, 49–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrone, L.; Drexler, H.C.A.; Wang, J.; Tripathi, P.; Distler, T.; Heisterkamp, P.; Anderson, E.N.; Kour, S.; Moraiti, A.; Maharana, S.; et al. FUS pathology in ALS is linked to alterations in multiple ALS-associated proteins and rescued by drugs stimulating autophagy. Acta Neuropathol. 2019, 138, 67–84. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Schwartz, J.C.; Cech, T.R. Nucleic acid-binding specificity of human FUS protein. Nucleic Acids Res. 2015, 43, 7535–7543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrado, L.; Del Bo, R.; Castellotti, B.; Ratti, A.; Cereda, C.; Penco, S.; Soraru, G.; Carlomagno, Y.; Ghezzi, S.; Pensato, V.; et al. Mutations of FUS gene in sporadic amyotrophic lateral sclerosis. J. Med. Genet. 2010, 47, 190–194. [Google Scholar] [CrossRef]
- Kwiatkowski, T.J., Jr.; Bosco, D.A.; Leclerc, A.L.; Tamrazian, E.; Vanderburg, C.R.; Russ, C.; Davis, A.; Gilchrist, J.; Kasarskis, E.J.; Munsat, T.; et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science 2009, 323, 1205–1208. [Google Scholar] [CrossRef] [Green Version]
- Vance, C.; Rogelj, B.; Hortobagyi, T.; De Vos, K.J.; Nishimura, A.L.; Sreedharan, J.; Hu, X.; Smith, B.; Ruddy, D.; Wright, P.; et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 2009, 323, 1208–1211. [Google Scholar] [CrossRef] [Green Version]
- Hou, L.; Jiao, B.; Xiao, T.; Zhou, L.; Zhou, Z.; Du, J.; Yan, X.; Wang, J.; Tang, B.; Shen, L. Screening of SOD1, FUS and TARDBP genes in patients with amyotrophic lateral sclerosis in central-southern China. Sci. Rep. 2016, 6, 32478. [Google Scholar] [CrossRef] [Green Version]
- Jia, W.; Kim, S.H.; Scalf, M.A.; Tonzi, P.; Millikin, R.J.; Guns, W.M.; Liu, L.; Mastrocola, A.S.; Smith, L.M.; Huang, T.T.; et al. Fused in sarcoma regulates DNA replication timing and kinetics. J. Biol. Chem. 2021, 297, 101049. [Google Scholar] [CrossRef]
- Tan, A.Y.; Manley, J.L. The TET family of proteins: Functions and roles in disease. J. Mol. Cell Biol. 2009, 1, 82–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, Y.; Huang, E.J. Mechanisms of FUS mutations in familial amyotrophic lateral sclerosis. Brain Res. 2016, 1647, 65–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerga, A.; Hallier, M.; Delva, L.; Orvain, C.; Gallais, I.; Marie, J.; Moreau-Gachelin, F. Identification of an RNA binding specificity for the potential splicing factor TLS. J. Biol. Chem. 2001, 276, 6807–6816. [Google Scholar] [CrossRef] [Green Version]
- Ishigaki, S.; Masuda, A.; Fujioka, Y.; Iguchi, Y.; Katsuno, M.; Shibata, A.; Urano, F.; Sobue, G.; Ohno, K. Position-dependent FUS-RNA interactions regulate alternative splicing events and transcriptions. Sci. Rep. 2012, 2, 529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shelkovnikova, T.A.; Robinson, H.K.; Southcombe, J.A.; Ninkina, N.; Buchman, V.L. Multistep process of FUS aggregation in the cell cytoplasm involves RNA-dependent and RNA-independent mechanisms. Hum. Mol. Genet. 2014, 23, 5211–5226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naumann, M.; Pal, A.; Goswami, A.; Lojewski, X.; Japtok, J.; Vehlow, A.; Naujock, M.; Gunther, R.; Jin, M.; Stanslowsky, N.; et al. Impaired DNA damage response signaling by FUS-NLS mutations leads to neurodegeneration and FUS aggregate formation. Nat. Commun. 2018, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Lenzi, J.; De Santis, R.; de Turris, V.; Morlando, M.; Laneve, P.; Calvo, A.; Caliendo, V.; Chio, A.; Rosa, A.; Bozzoni, I. ALS mutant FUS proteins are recruited into stress granules in induced pluripotent stem cell-derived motoneurons. Dis. Model Mech. 2015, 8, 755–766. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Lee, H.O.; Jawerth, L.; Maharana, S.; Jahnel, M.; Hein, M.Y.; Stoynov, S.; Mahamid, J.; Saha, S.; Franzmann, T.M.; et al. A Liquid-to-Solid Phase Transition of the ALS Protein FUS Accelerated by Disease Mutation. Cell 2015, 162, 1066–1077. [Google Scholar] [CrossRef] [Green Version]
- Schlachetzki, J.C.; Saliba, S.W.; Oliveira, A.C. Studying neurodegenerative diseases in culture models. Braz. J. Psychiatry 2013, 35 (Suppl. 2), S92–S100. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.C.; Kordala, A.J.; Strack, R.; Wang, X.; Geslain, R.; Delaney, K.; Clark, W.C.; Keenan, R.; Pan, T. A dual fluorescent reporter for the investigation of methionine mistranslation in live cells. RNA 2016, 22, 467–476. [Google Scholar] [CrossRef] [Green Version]
- Halfmann, R.; Lindquist, S. Screening for amyloid aggregation by Semi-Denaturing Detergent-Agarose Gel Electrophoresis. J. Vis. Exp. 2008, 17, 838. [Google Scholar] [CrossRef] [Green Version]
- Geslain, R.; Cubells, L.; Bori-Sanz, T.; Alvarez-Medina, R.; Rossell, D.; Marti, E.; Ribas de Pouplana, L. Chimeric tRNAs as tools to induce proteome damage and identify components of stress responses. Nucleic Acids Res. 2010, 38, e30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNicoll, F.; Muller-McNicoll, M. A Quantitative Heterokaryon Assay to Measure the Nucleocytoplasmic Shuttling of Proteins. Bio Protoc. 2018, 8, e2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bracha, D.; Walls, M.T.; Wei, M.T.; Zhu, L.; Kurian, M.; Avalos, J.L.; Toettcher, J.E.; Brangwynne, C.P. Mapping Local and Global Liquid Phase Behavior in Living Cells Using Photo-Oligomerizable Seeds. Cell 2018, 175, 1467–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogami, M.; Sano, O.; Adachi-Tominari, K.; Hayakawa-Yano, Y.; Furukawa, T.; Iwata, H.; Ogi, K.; Okano, H.; Yano, M. DNA damage stress-induced translocation of mutant FUS proteins into cytosolic granules and screening for translocation inhibitors. Front. Mol. Neurosci. 2022, 15, 953365. [Google Scholar] [CrossRef]
- Renger, R.; Morin, J.A.; Lemaitre, R.; Ruer-Gruss, M.; Julicher, F.; Hermann, A.; Grill, S.W. Co-condensation of proteins with single- and double-stranded DNA. Proc. Natl. Acad. Sci. USA 2022, 119, e2107871119. [Google Scholar] [CrossRef]
- Hamad, N.; Mashima, T.; Yamaoki, Y.; Kondo, K.; Yoneda, R.; Oyoshi, T.; Kurokawa, R.; Nagata, T.; Katahira, M. RNA sequence and length contribute to RNA-induced conformational change of TLS/FUS. Sci. Rep. 2020, 10, 2629. [Google Scholar] [CrossRef] [Green Version]
- Kamelgarn, M.; Chen, J.; Kuang, L.; Jin, H.; Kasarskis, E.J.; Zhu, H. ALS mutations of FUS suppress protein translation and disrupt the regulation of nonsense-mediated decay. Proc. Natl. Acad. Sci. USA 2018, 115, E11904–E11913. [Google Scholar] [CrossRef] [Green Version]
- Klickstein, J.A.; Mukkavalli, S.; Raman, M. AggreCount: An unbiased image analysis tool for identifying and quantifying cellular aggregates in a spatially defined manner. J. Biol. Chem. 2020, 295, 17672–17683. [Google Scholar] [CrossRef]
- Chiaraviglio, L.; Kirby, J.E. Evaluation of impermeant, DNA-binding dye fluorescence as a real-time readout of eukaryotic cell toxicity in a high throughput screening format. Assay Drug Dev. Technol. 2014, 12, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Chan, L.L.; Wilkinson, A.R.; Paradis, B.D.; Lai, N. Rapid image-based cytometry for comparison of fluorescent viability staining methods. J. Fluoresc. 2012, 22, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, M.; Bourdeau Julien, I.; Venkatasubramani, J.P.; Hui, J.B.; Dutchak, P.A.; Sephton, C.F. FUS contributes to mTOR-dependent inhibition of translation. J. Biol. Chem. 2020, 295, 18459–18473. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Erauskin, J.; Tadokoro, T.; Baughn, M.W.; Myers, B.; McAlonis-Downes, M.; Chillon-Marinas, C.; Asiaban, J.N.; Artates, J.; Bui, A.T.; Vetto, A.P.; et al. ALS/FTD-Linked Mutation in FUS Suppresses Intra-axonal Protein Synthesis and Drives Disease Without Nuclear Loss-of-Function of FUS. Neuron 2018, 100, 816–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Gal, J.; Chen, J.; Zhu, H. Self-assembled FUS binds active chromatin and regulates gene transcription. Proc. Natl. Acad. Sci. USA 2014, 111, 17809–17814. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Liu, S.; Ozturk, A.; Hicks, G.G. FUS-regulated RNA metabolism and DNA damage repair: Implications for amyotrophic lateral sclerosis and frontotemporal dementia pathogenesis. Rare Dis. 2014, 2, e29515. [Google Scholar] [CrossRef] [Green Version]
- Varanda, A.S.; Santos, M.; Soares, A.R.; Vitorino, R.; Oliveira, P.; Oliveira, C.; Santos, M.A.S. Human cells adapt to translational errors by modulating protein synthesis rate and protein turnover. RNA Biol. 2020, 17, 135–149. [Google Scholar] [CrossRef]
- Ishimura, R.; Nagy, G.; Dotu, I.; Zhou, H.; Yang, X.L.; Schimmel, P.; Senju, S.; Nishimura, Y.; Chuang, J.H.; Ackerman, S.L. RNA function. Ribosome stalling induced by mutation of a CNS-specific tRNA causes neurodegeneration. Science 2014, 345, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Meyer-Schuman, R.; Antonellis, A. Emerging mechanisms of aminoacyl-tRNA synthetase mutations in recessive and dominant human disease. Hum. Mol. Genet 2017, 26, R114–R127. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Diaz, Z.; Fang, X.; Hart, M.P.; Chesi, A.; Shorter, J.; Gitler, A.D. Molecular determinants and genetic modifiers of aggregation and toxicity for the ALS disease protein FUS/TLS. PLoS Biol. 2011, 9, e1000614. [Google Scholar] [CrossRef]
- Bosco, D.A.; Lemay, N.; Ko, H.K.; Zhou, H.; Burke, C.; Kwiatkowski, T.J., Jr.; Sapp, P.; McKenna-Yasek, D.; Brown, R.H., Jr.; Hayward, L.J. Mutant FUS proteins that cause amyotrophic lateral sclerosis incorporate into stress granules. Hum. Mol. Genet. 2010, 19, 4160–4175. [Google Scholar] [CrossRef] [Green Version]
- Pavon-Eternod, M.; Gomes, S.; Rosner, M.R.; Pan, T. Overexpression of initiator methionine tRNA leads to global reprogramming of tRNA expression and increased proliferation in human epithelial cells. RNA 2013, 19, 461–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, N.H.; Lee, M.R.; Kong, J.; Park, S.K.; Hwang, B.J.; Kim, B.G.; Lee, E.S.; Moon, H.G.; Kim, S. Transfer-RNA-mediated enhancement of ribosomal proteins S6 kinases signaling for cell proliferation. RNA Biol. 2018, 15, 635–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Ye, Y.; Gong, J.; Ruan, H.; Liu, C.J.; Xiang, Y.; Cai, C.; Guo, A.Y.; Ling, J.; Diao, L.; et al. Global analysis of tRNA and translation factor expression reveals a dynamic landscape of translational regulation in human cancers. Commun. Biol. 2018, 1, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshraghi, M.; Karunadharma, P.P.; Blin, J.; Shahani, N.; Ricci, E.P.; Michel, A.; Urban, N.T.; Galli, N.; Sharma, M.; Ramirez-Jarquin, U.N.; et al. Mutant Huntingtin stalls ribosomes and represses protein synthesis in a cellular model of Huntington disease. Nat. Commun. 2021, 12, 1461. [Google Scholar] [CrossRef]
- Dolgin, E. tRNA therapeutics burst onto startup scene. Nat. Biotechnol. 2022, 40, 283–286. [Google Scholar] [CrossRef]
- Zuko, A.; Mallik, M.; Thompson, R.; Spaulding, E.L.; Wienand, A.R.; Been, M.; Tadenev, A.L.D.; van Bakel, N.; Sijlmans, C.; Santos, L.A.; et al. tRNA overexpression rescues peripheral neuropathy caused by mutations in tRNA synthetase. Science 2021, 373, 1161–1166. [Google Scholar] [CrossRef]
- Wilhelm, S.D.P.; Rosa, K.; Qiu, Y.; O’Donoghue, P.; Heinemann, I.U. Towards a Cure for HARS Disease. Genes 2023, 14, 254. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Y.; Mendonca, C.A.; Yukselen, O.; Muneeruddin, K.; Ren, L.; Liang, J.; Zhou, C.; Xie, J.; Li, J.; et al. AAV-delivered suppressor tRNA overcomes a nonsense mutation in mice. Nature 2022, 604, 343–348. [Google Scholar] [CrossRef]
- Porter, J.J.; Heil, C.S.; Lueck, J.D. Therapeutic promise of engineered nonsense suppressor tRNAs. Wiley Interdiscip. Rev. RNA 2021, 12, e1641. [Google Scholar] [CrossRef]
- Albers, S.; Beckert, B.; Matthies, M.C.; Mandava, C.S.; Schuster, R.; Seuring, C.; Riedner, M.; Sanyal, S.; Torda, A.E.; Wilson, D.N.; et al. Repurposing tRNAs for nonsense suppression. Nat. Commun. 2021, 12, 3850. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Beebe, K.; Nangle, L.A.; Jang, J.; Longo-Guess, C.M.; Cook, S.A.; Davisson, M.T.; Sundberg, J.P.; Schimmel, P.; Ackerman, S.L. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature 2006, 443, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Kapur, M.; Monaghan, C.E.; Ackerman, S.L. Regulation of mRNA Translation in Neurons-A Matter of Life and Death. Neuron 2017, 96, 616–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapur, M.; Ganguly, A.; Nagy, G.; Adamson, S.I.; Chuang, J.H.; Frankel, W.N.; Ackerman, S.L. Expression of the Neuronal tRNA n-Tr20 Regulates Synaptic Transmission and Seizure Susceptibility. Neuron 2020, 108, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Brown, R.H., Jr. Genetics of Amyotrophic Lateral Sclerosis. Cold Spring Harb. Perspect Med. 2018, 8, a024125. [Google Scholar] [CrossRef] [PubMed]
- Connolly, O.; Le Gall, L.; McCluskey, G.; Donaghy, C.G.; Duddy, W.J.; Duguez, S. A Systematic Review of Genotype-Phenotype Correlation across Cohorts Having Causal Mutations of Different Genes in ALS. J. Pers Med. 2020, 10, 58. [Google Scholar] [CrossRef]
- Naumann, M.; Peikert, K.; Gunther, R.; van der Kooi, A.J.; Aronica, E.; Hubers, A.; Danel, V.; Corcia, P.; Pan-Montojo, F.; Cirak, S.; et al. Phenotypes and malignancy risk of different FUS mutations in genetic amyotrophic lateral sclerosis. Ann. Clin. Transl. Neurol. 2019, 6, 2384–2394. [Google Scholar] [CrossRef] [Green Version]
- Fairley, S.; Lowy-Gallego, E.; Perry, E.; Flicek, P. The International Genome Sample Resource (IGSR) collection of open human genomic variation resources. Nucleic Acids Res. 2020, 48, D941–D947. [Google Scholar] [CrossRef]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alfoldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef]
- Project MinE: Study design and pilot analyses of a large-scale whole-genome sequencing study in amyotrophic lateral sclerosis. Eur. J. Hum. Genet. 2018, 26, 1537–1546. [CrossRef] [Green Version]
- Gogakos, T.; Brown, M.; Garzia, A.; Meyer, C.; Hafner, M.; Tuschl, T. Characterizing Expression and Processing of Precursor and Mature Human tRNAs by Hydro-tRNAseq and PAR-CLIP. Cell Rep. 2017, 20, 1463–1475. [Google Scholar] [CrossRef] [Green Version]
- Martin, M. Cutadapt remoes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Juhling, F.; Morl, M.; Hartmann, R.K.; Sprinzl, M.; Stadler, P.F.; Putz, J. tRNAdb 2009: Compilation of tRNA sequences and tRNA genes. Nucleic Acids Res 2009, 37, D159–D162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lant, J.T.; Hasan, F.; Briggs, J.; Heinemann, I.U.; O’Donoghue, P. Genetic Interaction of tRNA-Dependent Mistranslation with Fused in Sarcoma Protein Aggregates. Genes 2023, 14, 518. https://doi.org/10.3390/genes14020518
Lant JT, Hasan F, Briggs J, Heinemann IU, O’Donoghue P. Genetic Interaction of tRNA-Dependent Mistranslation with Fused in Sarcoma Protein Aggregates. Genes. 2023; 14(2):518. https://doi.org/10.3390/genes14020518
Chicago/Turabian StyleLant, Jeremy T., Farah Hasan, Julia Briggs, Ilka U. Heinemann, and Patrick O’Donoghue. 2023. "Genetic Interaction of tRNA-Dependent Mistranslation with Fused in Sarcoma Protein Aggregates" Genes 14, no. 2: 518. https://doi.org/10.3390/genes14020518