Mendelian Randomization and Transcriptome-Wide Association Analysis Identified Genes That Were Pleiotropically Associated with Intraocular Pressure
Abstract
:1. Introduction
2. Methods
2.1. Editorial Policies and Ethical Considerations
2.2. GWAS Data for IOP
2.3. eQTL Data
2.4. SMR Analysis
2.5. TWAS Analysis
3. Results
3.1. Basic Information of the Summarized Data
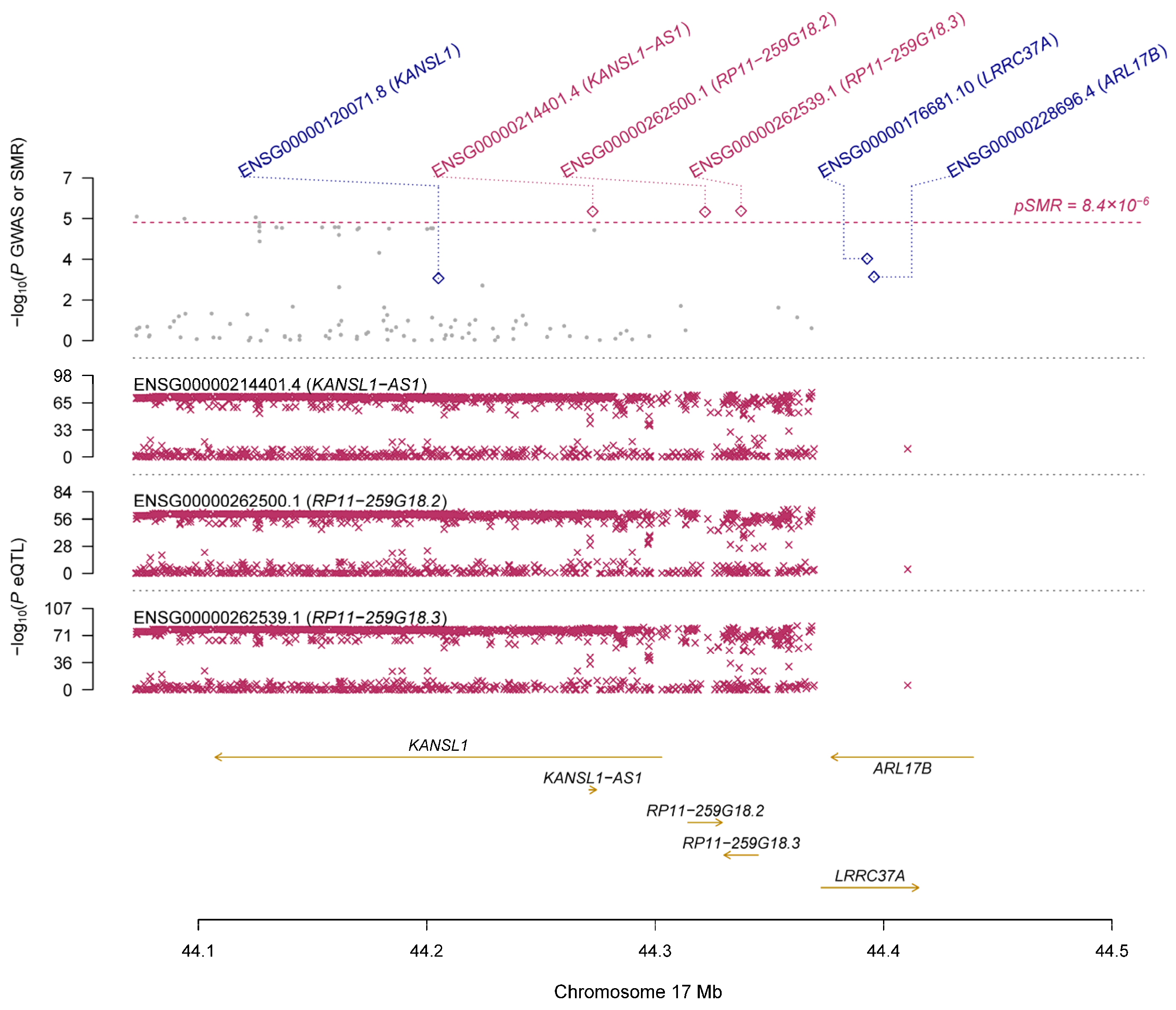
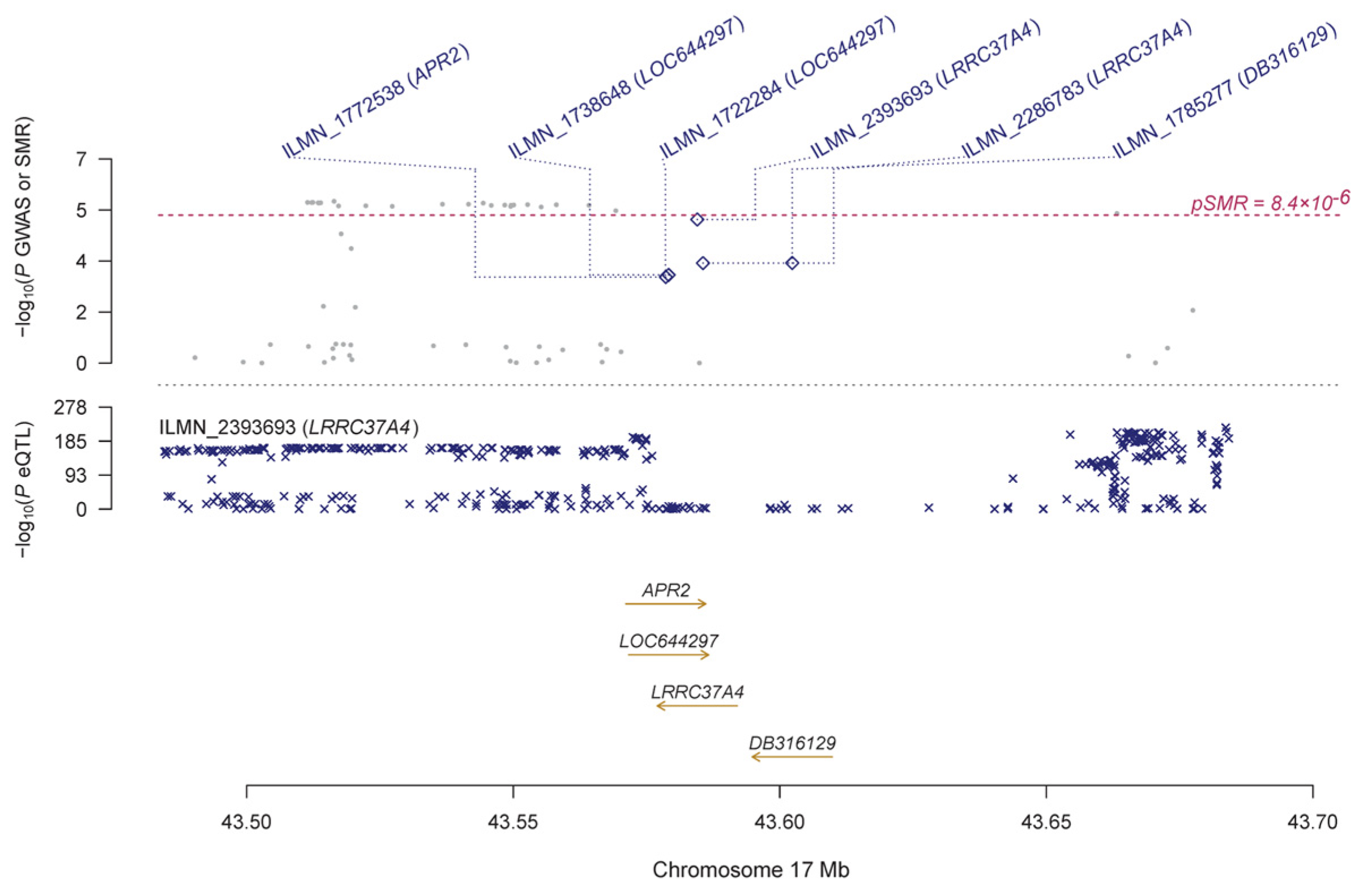
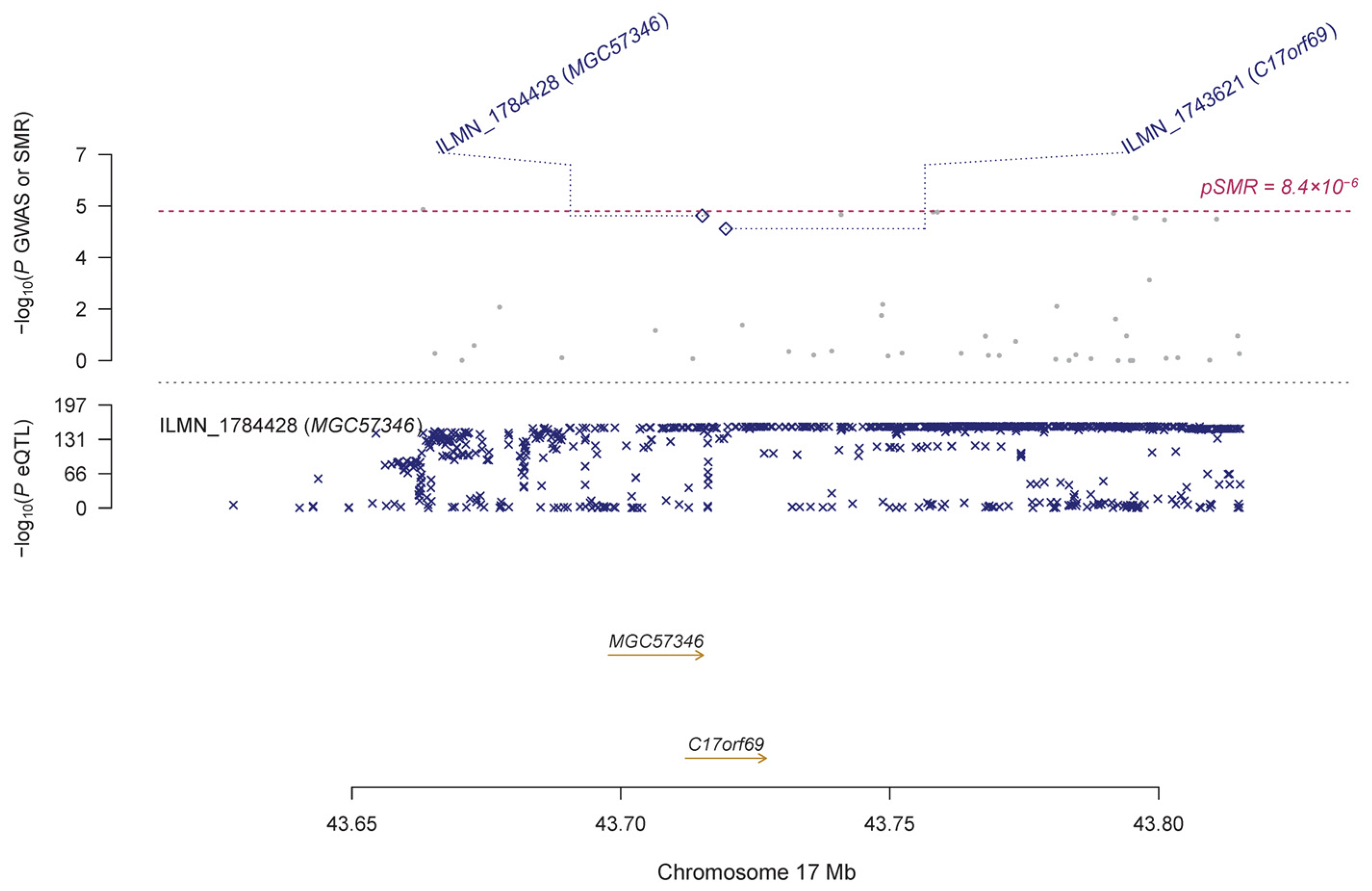
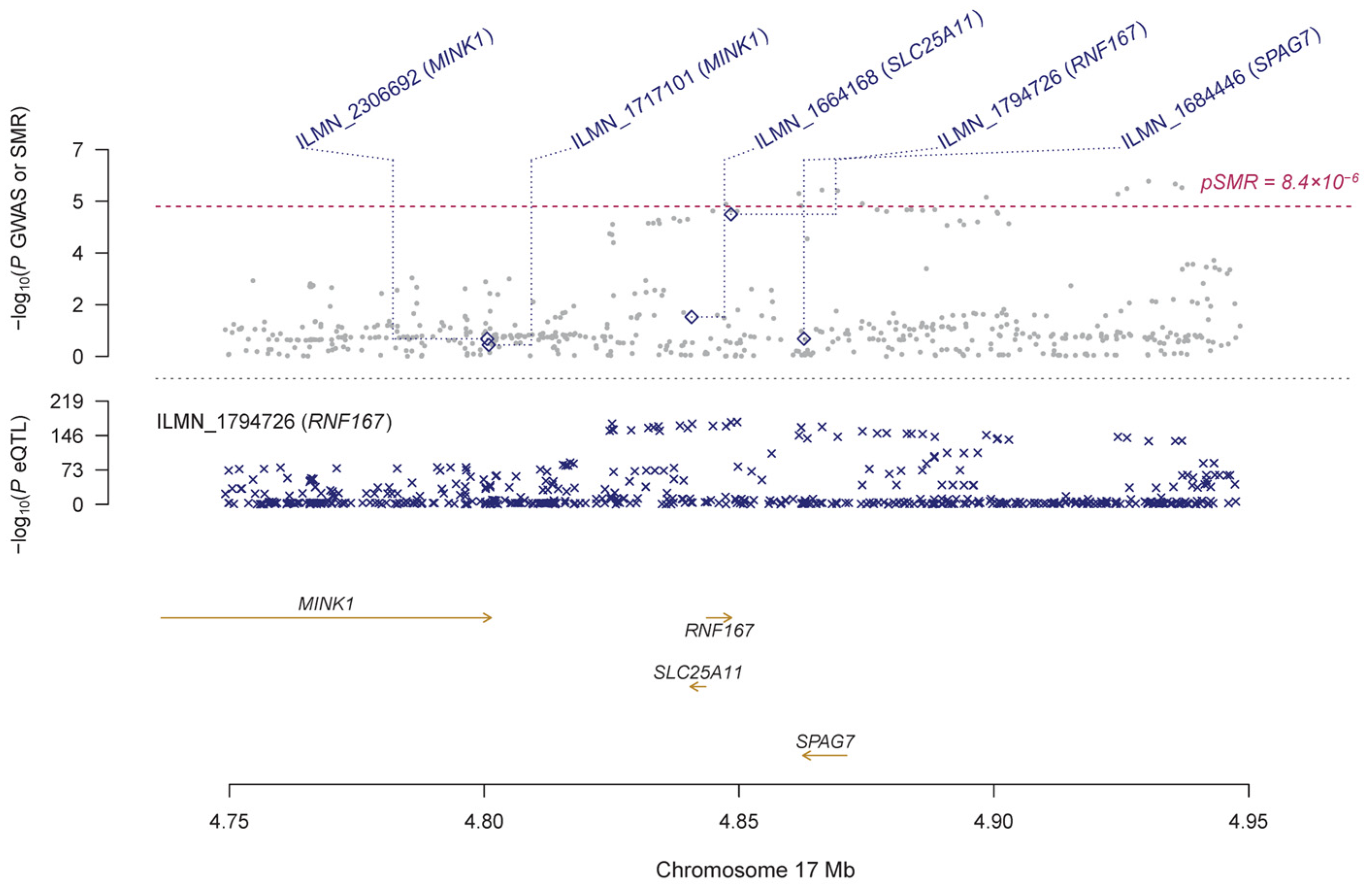
3.2. Pleiotropic Association with IOP
3.3. Cis-Regulated Gene Expression in Association with IOP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Armaly, M.F.; Krueger, D.E.; Maunder, L.; Becker, B.; Hetherington, J., Jr.; Kolker, A.E.; Levene, R.Z.; Maumenee, A.E.; Pollack, I.P.; Shaffer, R.N. Biostatistical analysis of the collaborative glaucoma study. I. Summary report of the risk factors for glaucomatous visual-field defects. Arch. Ophthalmol. 1980, 98, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Chamard, C.; Villain, M.; Bron, A.; Causse, A.; Bentaleb, Y.; Pelen, F.; Baudouin, C.; Daien, V. Prevalence of Unknown Ocular Hypertension, Glaucoma Suspects, and Glaucoma in Patients Seen in an Ophthalmology Center in France. Ophthalmic Res. 2020, 63, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Ying-Lai, M.; Francis, B.A.; Nguyen, B.B.; Deneen, J.; Wilson, M.R.; Azen, S.P.; Los Angeles Latino Eye Study Group. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: The Los Angeles Latino Eye Study. Ophthalmology 2004, 111, 1439–1448. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, Y.X.; Jonas, J.B.; Wang, Y.S.; Wang, S. Ocular hypertension and diabetes mellitus in the Beijing Eye Study. J. Glaucoma 2009, 18, 21–25. [Google Scholar] [CrossRef]
- Gedde, S.J.; Vinod, K.; Wright, M.M.; Muir, K.W.; Lind, J.T.; Chen, P.P.; Li, T.; Mansberger, S.L.; American Academy of Ophthalmology Preferred Practice Pattern Glaucoma Panel. Primary Open-Angle Glaucoma Preferred Practice Pattern®. Ophthalmology 2021, 128, P71–P150. [Google Scholar] [CrossRef]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713; discussion 730–829. [Google Scholar] [CrossRef] [Green Version]
- Heijl, A.; Leske, M.C.; Bengtsson, B.; Hyman, L.; Bengtsson, B.; Hussein, M.; Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: Results from the Early Manifest Glaucoma Trial. Arch. Ophthalmol. 2002, 120, 1268–1279. [Google Scholar] [CrossRef]
- Gordon, M.O.; Beiser, J.A.; Brandt, J.D.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; et al. The Ocular Hypertension Treatment Study: Baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 714–720; discussion 730–829. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Duggal, P.; Klein, A.P.; Lee, K.E.; Iyengar, S.K.; Klein, R.; Bailey-Wilson, J.E.; Klein, B.E. A genetic contribution to intraocular pressure: The beaver dam eye study. Investig. Ophthalmol. Vis. Sci. 2005, 46, 555–560. [Google Scholar] [CrossRef]
- Bonovas, S.; Filioussi, K.; Tsantes, A.; Peponis, V. Epidemiological association between cigarette smoking and primary open-angle glaucoma: A meta-analysis. Public Health 2004, 118, 256–261. [Google Scholar] [CrossRef]
- Kang, J.H.; Pasquale, L.R.; Rosner, B.A.; Willett, W.C.; Egan, K.M.; Faberowski, N.; Hankinson, S.E. Prospective study of cigarette smoking and the risk of primary open-angle glaucoma. Arch. Ophthalmol. 2003, 121, 1762–1768. [Google Scholar] [CrossRef] [Green Version]
- Renard, J.P.; Rouland, J.F.; Bron, A.; Sellem, E.; Nordmann, J.P.; Baudouin, C.; Denis, P.; Villain, M.; Chaine, G.; Colin, J.; et al. Nutritional, lifestyle and environmental factors in ocular hypertension and primary open-angle glaucoma: An exploratory case-control study. Acta Ophthalmol. 2013, 91, 505–513. [Google Scholar] [CrossRef]
- Sanfilippo, P.G.; Hewitt, A.W.; Hammond, C.J.; Mackey, D.A. The heritability of ocular traits. Surv. Ophthalmol. 2010, 55, 561–583. [Google Scholar] [CrossRef]
- Asefa, N.G.; Neustaeter, A.; Jansonius, N.M.; Snieder, H. Heritability of glaucoma and glaucoma-related endophenotypes: Systematic review and meta-analysis. Surv. Ophthalmol. 2019, 64, 835–851. [Google Scholar] [CrossRef]
- Johnson, A.T.; Drack, A.V.; Kwitek, A.E.; Cannon, R.L.; Stone, E.M.; Alward, W.L. Clinical features and linkage analysis of a family with autosomal dominant juvenile glaucoma. Ophthalmology 1993, 100, 524–529. [Google Scholar] [CrossRef]
- Sheffield, V.C.; Stone, E.M.; Alward, W.L.; Drack, A.V.; Johnson, A.T.; Streb, L.M.; Nichols, B.E. Genetic linkage of familial open angle glaucoma to chromosome 1q21-q31. Nat. Genet. 1993, 4, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Wirtz, M.K.; Samples, J.R.; Kramer, P.L.; Rust, K.; Topinka, J.R.; Yount, J.; Koler, R.D.; Acott, T.S. Mapping a gene for adult-onset primary open-angle glaucoma to chromosome 3q. Am. J. Hum. Genet. 1997, 60, 296–304. [Google Scholar]
- Wirtz, M.K.; Samples, J.R.; Rust, K.; Lie, J.; Nordling, L.; Schilling, K.; Acott, T.S.; Kramer, P.L. GLC1F, a new primary open-angle glaucoma locus, maps to 7q35-q36. Arch. Ophthalmol. 1999, 117, 237–241. [Google Scholar] [CrossRef]
- Suriyapperuma, S.P.; Child, A.; Desai, T.; Brice, G.; Kerr, A.; Crick, R.P.; Sarfarazi, M. A new locus (GLC1H) for adult-onset primary open-angle glaucoma maps to the 2p15-p16 region. Arch. Ophthalmol. 2007, 125, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Stone, E.M.; Fingert, J.H.; Alward, W.L.; Nguyen, T.D.; Polansky, J.R.; Sunden, S.L.; Nishimura, D.; Clark, A.F.; Nystuen, A.; Nichols, B.E.; et al. Identification of a gene that causes primary open angle glaucoma. Science 1997, 275, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Duggal, P.; Klein, A.P.; Lee, K.E.; Klein, R.; Klein, B.E.; Bailey-Wilson, J.E. Identification of novel genetic loci for intraocular pressure: A genomewide scan of the Beaver Dam Eye Study. Arch. Ophthalmol. 2007, 125, 74–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.K.; Woo, S.J.; Kim, J.I.; Cho, S.I.; Kim, H.; Sung, J.; Seo, J.S.; Kim, D.M. Replication of a glaucoma candidate gene on 5q22.1 for intraocular pressure in mongolian populations: The GENDISCAN Project. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1335–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotimi, C.N.; Chen, G.; Adeyemo, A.A.; Jones, L.S.; Agyenim-Boateng, K.; Eghan, B.A., Jr.; Zhou, J.; Doumatey, A.; Lashley, K.; Huang, H.; et al. Genomewide scan and fine mapping of quantitative trait loci for intraocular pressure on 5q and 14q in West Africans. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3262–3267. [Google Scholar] [CrossRef]
- Choquet, H.; Thai, K.K.; Yin, J.; Hoffmann, T.J.; Kvale, M.N.; Banda, Y.; Schaefer, C.; Risch, N.; Nair, K.S.; Melles, R.; et al. A large multi-ethnic genome-wide association study identifies novel genetic loci for intraocular pressure. Nat. Commun. 2017, 8, 2108. [Google Scholar] [CrossRef] [Green Version]
- Nag, A.; Venturini, C.; Small, K.S.; International Glaucoma Genetics, C.; Young, T.L.; Viswanathan, A.C.; Mackey, D.A.; Hysi, P.G.; Hammond, C. A genome-wide association study of intra-ocular pressure suggests a novel association in the gene FAM125B in the TwinsUK cohort. Hum. Mol. Genet. 2014, 23, 3343–3348. [Google Scholar] [CrossRef]
- Van Koolwijk, L.M.; Ramdas, W.D.; Ikram, M.K.; Jansonius, N.M.; Pasutto, F.; Hysi, P.G.; Macgregor, S.; Janssen, S.F.; Hewitt, A.W.; Viswanathan, A.C.; et al. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS Genet. 2012, 8, e1002611. [Google Scholar] [CrossRef] [Green Version]
- Bonnemaijer, P.W.M.; Leeuwen, E.M.V.; Iglesias, A.I.; Gharahkhani, P.; Vitart, V.; Khawaja, A.P.; Simcoe, M.; Hohn, R.; Cree, A.J.; Igo, R.P.; et al. Multi-trait genome-wide association study identifies new loci associated with optic disc parameters. Commun. Biol. 2019, 2, 435. [Google Scholar] [CrossRef] [Green Version]
- GTEx Consortium. Genetic effects on gene expression across human tissues. Nature 2017, 550, 204–213. [Google Scholar] [CrossRef] [Green Version]
- Lloyd-Jones, L.R.; Holloway, A.; McRae, A.; Yang, J.; Small, K.; Zhao, J.; Zeng, B.; Bakshi, A.; Metspalu, A.; Dermitzakis, M.; et al. The Genetic Architecture of Gene Expression in Peripheral Blood. Am. J. Hum. Genet. 2017, 100, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Zhang, F.; Hu, H.; Bakshi, A.; Robinson, M.R.; Powell, J.E.; Montgomery, G.W.; Goddard, M.E.; Wray, N.R.; Visscher, P.M.; et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 2016, 48, 481–487. [Google Scholar] [CrossRef]
- Lawlor, D.A.; Harbord, R.M.; Sterne, J.A.; Timpson, N.; Davey Smith, G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 2008, 27, 1133–1163. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, J.; Liu, D.; Yu, W. Mendelian randomization analysis identified genes pleiotropically associated with central corneal thickness. BMC Genom. 2021, 22, 517. [Google Scholar] [CrossRef]
- Gusev, A.; Ko, A.; Shi, H.; Bhatia, G.; Chung, W.; Penninx, B.W.; Jansen, R.; de Geus, E.J.; Boomsma, D.I.; Wright, F.A.; et al. Integrative approaches for large-scale transcriptome-wide association studies. Nat. Genet. 2016, 48, 245–252. [Google Scholar] [CrossRef] [Green Version]
- Hartwig, F.P.; Davies, N.M.; Hemani, G.; Davey Smith, G. Two-sample Mendelian randomization: Avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int. J. Epidemiol. 2016, 45, 1717–1726. [Google Scholar] [CrossRef] [Green Version]
- Pierce, B.L.; Burgess, S. Efficient design for Mendelian randomization studies: Subsample and 2-sample instrumental variable estimators. Am. J. Epidemiol. 2013, 178, 1177–1184. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Solon, G. Two-Sample Instrumental Variables Estimators. Rev. Econ. Stat. 2010, 92, 557–561. Available online: https://www.jstor.org/stable/27867559 (accessed on 27 April 2023). [CrossRef]
- Stefansson, H.; Helgason, A.; Thorleifsson, G.; Steinthorsdottir, V.; Masson, G.; Barnard, J.; Baker, A.; Jonasdottir, A.; Ingason, A.; Gudnadottir, V.G.; et al. A common inversion under selection in Europeans. Nat. Genet. 2005, 37, 129–137. [Google Scholar] [CrossRef]
- Cruts, M.; Rademakers, R.; Gijselinck, I.; van der Zee, J.; Dermaut, B.; de Pooter, T.; de Rijk, P.; Del-Favero, J.; van Broeckhoven, C. Genomic architecture of human 17q21 linked to frontotemporal dementia uncovers a highly homologous family of low-copy repeats in the tau region. Hum. Mol. Genet. 2005, 14, 1753–1762. [Google Scholar] [CrossRef]
- Gijselinck, I.; Bogaerts, V.; Rademakers, R.; van der Zee, J.; van Broeckhoven, C.; Cruts, M. Visualization of MAPT inversion on stretched chromosomes of tau-negative frontotemporal dementia patients. Hum. Mutat. 2006, 27, 1057–1059. [Google Scholar] [CrossRef]
- De Jong, S.; Chepelev, I.; Janson, E.; Strengman, E.; van den Berg, L.H.; Veldink, J.H.; Ophoff, R.A. Common inversion polymorphism at 17q21.31 affects expression of multiple genes in tissue-specific manner. BMC Genom. 2012, 13, 458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharahkhani, P.; Jorgenson, E.; Hysi, P.; Khawaja, A.P.; Pendergrass, S.; Han, X.; Ong, J.S.; Hewitt, A.W.; Segre, A.V.; Rouhana, J.M.; et al. Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat. Commun. 2021, 12, 1258. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.R.; Huang, H.; Kim, H. Genome-wide association analyses identify 139 loci associated with macular thickness in the UK Biobank cohort. Hum. Mol. Genet. 2019, 28, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Strunz, T.; Kiel, C.; Grassmann, F.; Ratnapriya, R.; Kwicklis, M.; Karlstetter, M.; Fauser, S.; Arend, N.; Swaroop, A.; Langmann, T.; et al. A mega-analysis of expression quantitative trait loci in retinal tissue. PLoS Genet. 2020, 16, e1008934. [Google Scholar] [CrossRef]
- Kominato, Y.; Sano, R.; Takahashi, Y.; Hayakawa, A.; Ogasawara, K. Human ABO gene transcriptional regulation. Transfusion 2020, 60, 860–869. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, F.; McNeill, P.D.; Hakomori, S. Genomic organization of human histo-blood group ABO genes. Glycobiology 1995, 5, 51–58. [Google Scholar] [CrossRef]
- Bennett, E.P.; Steffensen, R.; Clausen, H.; Weghuis, D.O.; Geurts van Kessel, A. Genomic cloning of the human histo-blood group ABO locus. Biochem. Biophys. Res. Commun. 1995, 211, 347. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Schooling, C.M. A phenome-wide association study of ABO blood groups. BMC Med. 2020, 18, 334. [Google Scholar] [CrossRef]
- Yamamoto, F.; Cid, E.; Yamamoto, M.; Blancher, A. ABO research in the modern era of genomics. Transfus. Med. Rev. 2012, 26, 103–118. [Google Scholar] [CrossRef]
- Hysi, P.G.; Cheng, C.Y.; Springelkamp, H.; Macgregor, S.; Bailey, J.N.C.; Wojciechowski, R.; Vitart, V.; Nag, A.; Hewitt, A.W.; Hohn, R.; et al. Genome-wide analysis of multi-ancestry cohorts identifies new loci influencing intraocular pressure and susceptibility to glaucoma. Nat. Genet. 2014, 46, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Springelkamp, H.; Iglesias, A.I.; Mishra, A.; Hohn, R.; Wojciechowski, R.; Khawaja, A.P.; Nag, A.; Wang, Y.X.; Wang, J.J.; Cuellar-Partida, G.; et al. New insights into the genetics of primary open-angle glaucoma based on meta-analyses of intraocular pressure and optic disc characteristics. Hum. Mol. Genet. 2017, 26, 438–453. [Google Scholar] [CrossRef]
- Qian, Y.; Baisden, J.M.; Cherezova, L.; Summy, J.M.; Guappone-Koay, A.; Shi, X.; Mast, T.; Pustula, J.; Zot, H.G.; Mazloum, N.; et al. PC phosphorylation increases the ability of AFAP-110 to cross-link actin filaments. Mol. Biol. Cell 2002, 13, 2311–2322. [Google Scholar] [CrossRef]
- Qian, Y.; Baisden, J.M.; Zot, H.G.; Van Winkle, W.B.; Flynn, D.C. The carboxy terminus of AFAP-110 modulates direct interactions with actin filaments and regulates its ability to alter actin filament integrity and induce lamellipodia formation. Exp. Cell. Res. 2000, 255, 102–113. [Google Scholar] [CrossRef]
- Inoue, T.; Tanihara, H. Rho-associated kinase inhibitors: A novel glaucoma therapy. Prog. Retin. Eye Res. 2013, 37, 1–12. [Google Scholar] [CrossRef]
- Junglas, B.; Kuespert, S.; Seleem, A.A.; Struller, T.; Ullmann, S.; Bosl, M.; Bosserhoff, A.; Kostler, J.; Wagner, R.; Tamm, E.R.; et al. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am. J. Pathol. 2012, 180, 2386–2403. [Google Scholar] [CrossRef]
- Bailey, J.N.; Loomis, S.J.; Kang, J.H.; Allingham, R.R.; Gharahkhani, P.; Khor, C.C.; Burdon, K.P.; Aschard, H.; Chasman, D.I.; Igo, R.P., Jr.; et al. Genome-wide association analysis identifies TXNRD2, ATXN2 and FOXC1 as susceptibility loci for primary open-angle glaucoma. Nat. Genet. 2016, 48, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Gharahkhani, P.; Burdon, K.P.; Fogarty, R.; Sharma, S.; Hewitt, A.W.; Martin, S.; Law, M.H.; Cremin, K.; Bailey, J.N.C.; Loomis, S.J.; et al. Common variants near ABCA1, AFAP1 and GMDS confer risk of primary open-angle glaucoma. Nat. Genet. 2014, 46, 1120–1125. [Google Scholar] [CrossRef]
- Gao, X.R.; Huang, H.; Nannini, D.R.; Fan, F.; Kim, H. Genome-wide association analyses identify new loci influencing intraocular pressure. Hum. Mol. Genet. 2018, 27, 2205–2213. [Google Scholar] [CrossRef]
- Liou, S.T.; Wang, C. Small glutamine-rich tetratricopeptide repeat-containing protein is composed of three structural units with distinct functions. Arch. Biochem. Biophys. 2005, 435, 253–263. [Google Scholar] [CrossRef]
- Winnefeld, M.; Rommelaere, J.; Cziepluch, C. The human small glutamine-rich TPR-containing protein is required for progress through cell division. Exp. Cell Res. 2004, 293, 43–57. [Google Scholar] [CrossRef]
- Wang, H.; Shen, H.; Wang, Y.; Li, Z.; Yin, H.; Zong, H.; Jiang, J.; Gu, J. Overexpression of small glutamine-rich TPR-containing protein promotes apoptosis in 7721 cells. FEBS Lett. 2005, 579, 1279–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuong, T.A.; Lee, S.J.; Leem, Y.E.; Lee, J.R.; Bae, G.U.; Kang, J.S. SGTb regulates a surface localization of a guidance receptor BOC to promote neurite outgrowth. Cell. Signal. 2019, 55, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, A.I.; Mishra, A.; Vitart, V.; Bykhovskaya, Y.; Hohn, R.; Springelkamp, H.; Cuellar-Partida, G.; Gharahkhani, P.; Bailey, J.N.C.; Willoughby, C.E.; et al. Cross-ancestry genome-wide association analysis of corneal thickness strengthens link between complex and Mendelian eye diseases. Nat. Commun. 2018, 9, 1864. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, A.P.; Cooke Bailey, J.N.; Wareham, N.J.; Scott, R.A.; Simcoe, M.; Igo, R.P., Jr.; Song, Y.E.; Wojciechowski, R.; Cheng, C.Y.; Khaw, P.T.; et al. Genome-wide analyses identify 68 new loci associated with intraocular pressure and improve risk prediction for primary open-angle glaucoma. Nat. Genet. 2018, 50, 778–782. [Google Scholar] [CrossRef]
- Khatib, Z.A.; Inaba, T.; Valentine, M.; Look, A.T. Chromosomal localization and cDNA cloning of the human DBP and TEF genes. Genomics 1994, 23, 344–351. [Google Scholar] [CrossRef]
- Hysi, P.G.; Choquet, H.; Khawaja, A.P.; Wojciechowski, R.; Tedja, M.S.; Yin, J.; Simcoe, M.J.; Patasova, K.; Mahroo, O.A.; Thai, K.K.; et al. Meta-analysis of 542,934 subjects of European ancestry identifies new genes and mechanisms predisposing to refractive error and myopia. Nat. Genet. 2020, 52, 401–407. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
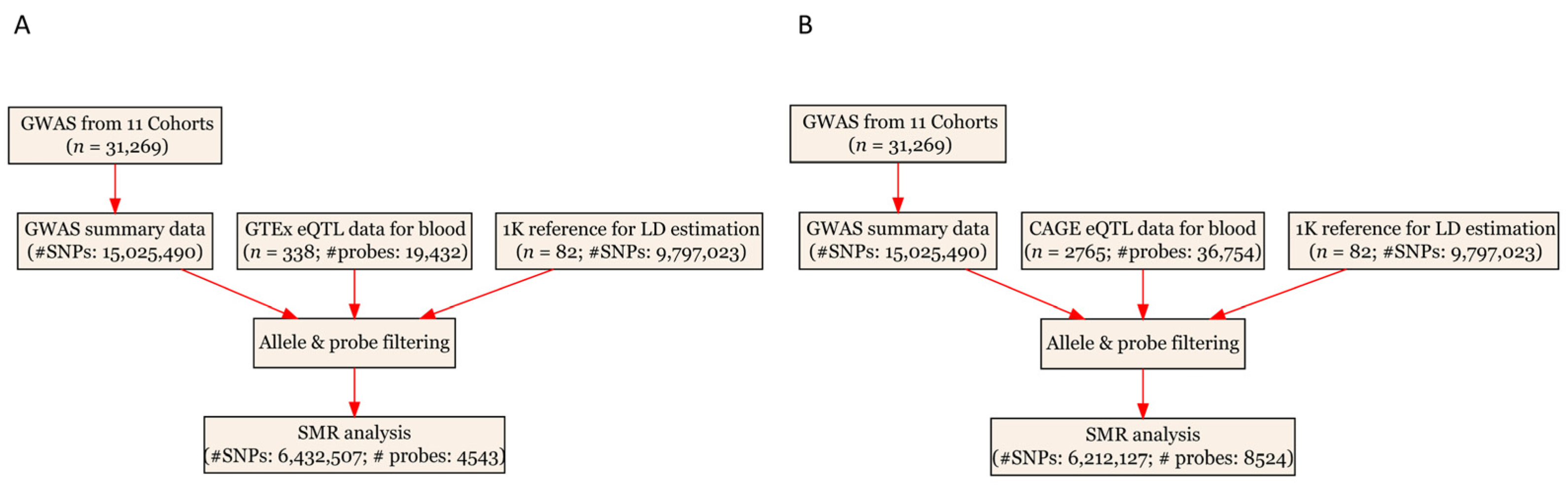

| Data Source | Total Number of Participants | Number of Eligible Genetic Variants or Probes |
|---|---|---|
| SMR using GTEx eQTL data | ||
| eQTL | 338 | 4543 |
| GWAS | 31,269 | 6,432,507 |
| SMR using CAGE eQTL data | ||
| eQTL | 2765 | 8524 |
| GWAS | 31,269 | 6,212,127 |
| eQTL Data | Probe | Gene | CHR | Top SNP | PeQTL | PGWAS | Beta | SE | PSMR | PHEIDI | Q Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| GTEx | ENSG00000214401.4 | KANSL1-AS1 | 17 | rs199534 | 2.42 × 10−76 | 1.24 × 10−6 | 0.125 | 0.027 | 2.78 × 10−6 | 0.022 | 1.84 × 10−5 |
| ENSG00000262500.1 | RP11-259G18.2 | 17 | rs199451 | 2.19 × 10−66 | 1.19 × 10−6 | 0.141 | 0.030 | 2.91 × 10−6 | 0.049 | 1.84 × 10−5 | |
| ENSG00000262539.1 | RP11-259G18.3 | 17 | rs199534 | 5.96 × 10−81 | 1.24 × 10−6 | 0.120 | 0.026 | 2.66 × 10−6 | 0.046 | 1.84 × 10−5 | |
| ENSG00000175164.9 | ABO | 9 | rs12216891 | 1.20 × 10−22 | 3.70 × 10−7 | 0.208 | 0.046 | 6.40 × 10−6 | 0.412 | 3.04 × 10−5 | |
| ENSG00000264070.1 | DND1P1 | 17 | rs112578465 | 1.20 × 10−60 | 5.00 × 10−6 | 0.120 | 0.027 | 1.07 × 10−5 | 0.370 | 4.08 × 10−5 | |
| ENSG00000214425.2 | LRRC37A4P | 17 | rs79501144 | 1.11 × 10−102 | 1.04 × 10−5 | −0.115 | 0.027 | 1.57 × 10−5 | 0.526 | 4.50 × 10−5 | |
| ENSG00000263503.1 | RP11-707O23.5 | 17 | rs111273167 | 1.88 × 10−75 | 1.08 × 10−5 | 0.118 | 0.028 | 1.90 × 10−5 | 0.530 | 4.50 × 10−5 | |
| ENSG00000204650.9 | CRHR1-IT1 | 17 | rs1724390 | 1.21 × 10−53 | 7.53 × 10−6 | 0.251 | 0.058 | 1.66 × 10−5 | 0.893 | 4.50 × 10−5 | |
| ENSG00000238083.3 | LRRC37A2 | 17 | rs17426174 | 6.85 × 10−38 | 1.63 × 10−5 | 0.161 | 0.039 | 4.28 × 10−5 | 0.002 | 9.04 × 10−5 | |
| ENSG00000196526.6 | AFAP1 | 4 | rs62290601 | 7.45 × 10−80 | 8.11 × 10−5 | −0.096 | 0.025 | 1.12 × 10−4 | 0.030 | 2.12 × 10−4 | |
| CAGE | ILMN_1794726 | RNF167 | 17 | rs238243 | 2.22 × 10−175 | 1.23 × 10−5 | 0.147 | 0.034 | 1.53 × 10−5 | 0.627 | 1.48 × 10−4 |
| ILMN_2393693 | LRRC37A4 | 17 | rs113661667 | 5.03 × 10−228 | 9.86 × 10−6 | −0.124 | 0.028 | 1.19 × 10−5 | 0.003 | 1.48 × 10−4 | |
| ILMN_1784428 | MGC57346 | 17 | rs62057067 | 2.43 × 10−157 | 8.99 × 10−6 | 0.146 | 0.033 | 1.19 × 10−5 | 0.010 | 1.48 × 10−4 | |
| ILMN_1680353 | NSF | 17 | rs199442 | 5.94 × 10−15 | 3.84 × 10−7 | 0.612 | 0.144 | 2.11 × 10−5 | 0.108 | 1.53 × 10−4 | |
| ILMN_2109343 | SGTB | 5 | rs42884 | 4.56 × 10−137 | 2.68 × 10−5 | −0.159 | 0.038 | 3.45 × 10−5 | 0.015 | 1.67 × 10−4 | |
| ILMN_1743621 | C17orf69 | 17 | rs113029914 | 8.60 × 10−34 | 1.01 × 10−5 | 0.334 | 0.081 | 3.32 × 10−5 | 0.005 | 1.67 × 10−4 | |
| ILMN_1701998 | AFAP1 | 4 | rs62290601 | 1.26 × 10−179 | 8.11 × 10−5 | −0.127 | 0.033 | 9.23 × 10−5 | 0.009 | 3.35 × 10−4 | |
| ILMN_2330845 | NSF | 17 | rs199446 | 1.03 × 10−10 | 7.35 × 10−7 | 0.736 | 0.187 | 8.50 × 10−5 | 0.257 | 3.35 × 10−4 | |
| ILMN_1706511 | TEF | 22 | rs4822025 | 1.02 × 10−27 | 5.19 × 10−5 | 0.324 | 0.085 | 1.49 × 10−4 | 0.381 | 4.80 × 10−4 | |
| ILMN_1737195 | CENPK | 5 | rs154940 | 3.27 × 10−299 | 1.58 × 10−4 | −0.100 | 0.027 | 1.75 × 10−4 | 0.002 | 5.09 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Zhang, Z.; Zhu, Y.; Yuan, G.; Yang, J.; Yu, W. Mendelian Randomization and Transcriptome-Wide Association Analysis Identified Genes That Were Pleiotropically Associated with Intraocular Pressure. Genes 2023, 14, 1027. https://doi.org/10.3390/genes14051027
Yang Z, Zhang Z, Zhu Y, Yuan G, Yang J, Yu W. Mendelian Randomization and Transcriptome-Wide Association Analysis Identified Genes That Were Pleiotropically Associated with Intraocular Pressure. Genes. 2023; 14(5):1027. https://doi.org/10.3390/genes14051027
Chicago/Turabian StyleYang, Zhikun, Zhewei Zhang, Yining Zhu, Guangwei Yuan, Jingyun Yang, and Weihong Yu. 2023. "Mendelian Randomization and Transcriptome-Wide Association Analysis Identified Genes That Were Pleiotropically Associated with Intraocular Pressure" Genes 14, no. 5: 1027. https://doi.org/10.3390/genes14051027





