Spinal Deformity in Ehlers–Danlos Syndrome: Focus on Musculocontractural Type
Abstract
:1. Introduction
1.1. The General Information of EDS Types
1.2. Frequency and Characteristics of Spinal Deformity in EDS
2. Materials and Methods
2.1. Musculocontractural EDS
2.2. Spinal Deformity in mcEDS
3. Results
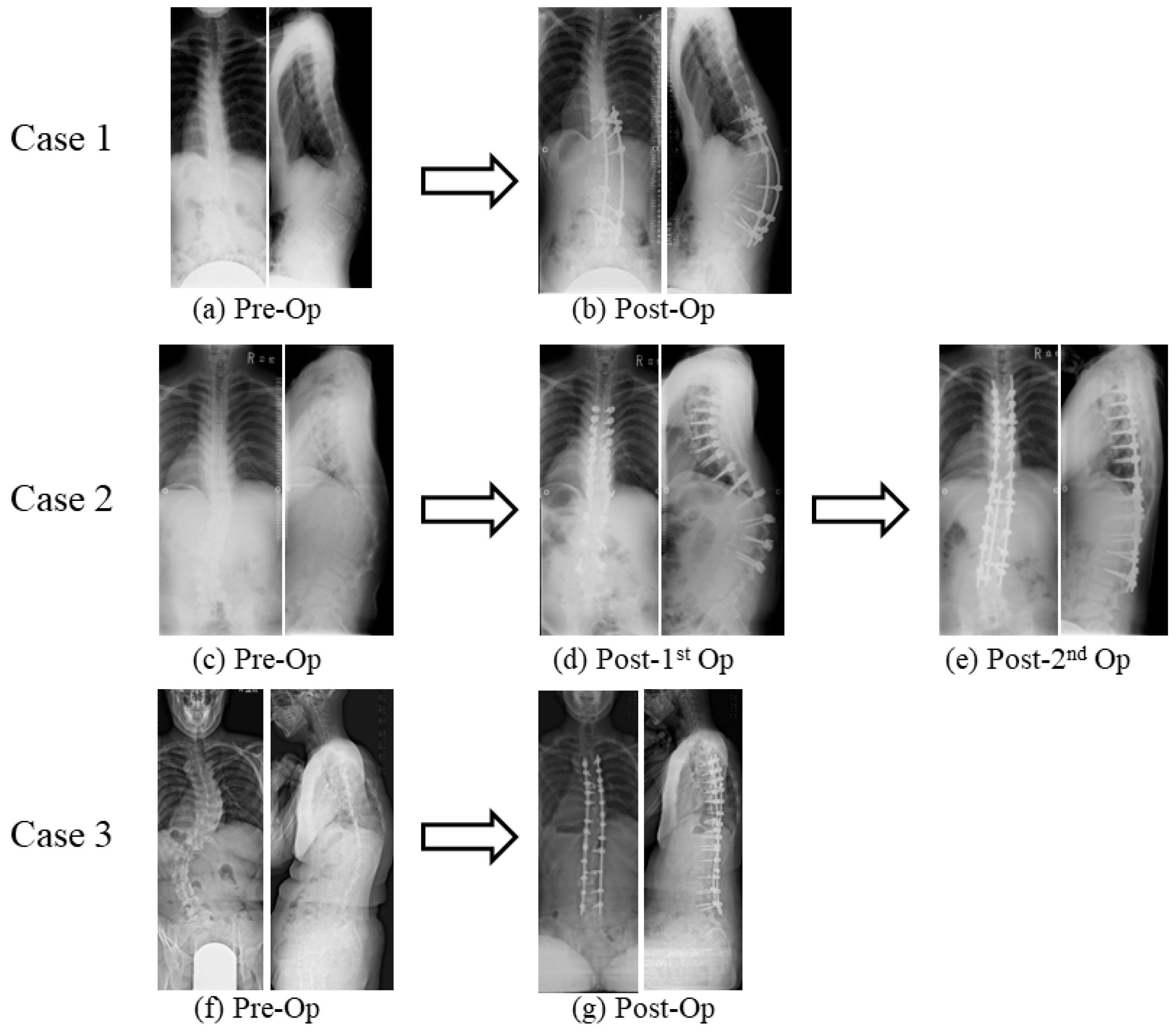
Surgical Case Presentations of Spinal Deformity in mcEDS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malfait, F.; Francomano, C.; Byers, P.; Belmont, J.; Berglund, B.; Black, J.; Bloom, L.; Bowen, J.M.; Brady, A.F.; Burrows, N.P.; et al. The 2017 international classification of the Ehlers-Danlos syndromes. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Malfait, F.; Castori, M.; Francomano, C.A.; Giunta, C.; Kosho, T.; Byers, P.H. The Ehlers-Danlos syndromes. Nat. Rev. Dis. Prim. 2020, 6, 64. [Google Scholar] [CrossRef]
- Natarajan, D.; Samartzis, D.; Wong, Y.W.; Luk, K.D.; Cheung, K.M. Natural history of spinal deformity in a patient with Ehlers-Danlos syndrome: Case report with 20-year follow-up. Spine J. 2011, 11, e1–e4. [Google Scholar] [CrossRef]
- Shirley, E.D.; Demaio, M.; Bodurtha, J. Ehlers-danlos syndrome in orthopaedics: Etiology, diagnosis, and treatment implications. Sport. Health 2012, 4, 394–403. [Google Scholar] [CrossRef]
- Glassman, S.D.; Bridwell, K.; Dimar, J.R.; Horton, W.; Berven, S.; Schwab, F. The impact of positive sagittal balance in adult spinal deformity. Spine 2005, 30, 2024–2029. [Google Scholar] [CrossRef]
- Pellisé, F.; Vila-Casademunt, A.; Ferrer, M.; Domingo-Sàbat, M.; Bagó, J.; Pérez-Grueso, F.J.; Alanay, A.; Mannion, A.F.; Acaroglu, E.; European Spine Study Group, ESSG. Impact on health related quality of life of adult spinal deformity (ASD) compared with other chronic conditions. Eur. Spine J. 2015, 24, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Ungar, B.; Blondel, B.; Buchowski, J.; Coe, J.; Deinlein, D.; DeWald, C.; Mehdian, H.; Shaffrey, C.; Tribus, C.; et al. Scoliosis Research Society-Schwab adult spinal deformity classification: A validation study. Spine 2012, 37, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, R.; Takeshita, K.; Takahashi, J.; Arai, Y.; Watanabe, K.; Yamato, Y.; Oba, H.; Matsumoto, M. The complication trends of adult spinal deformity surgery in Japan—The Japanese Scoliosis Society Morbidity and Mortality survey from 2012 to 2017. J. Orthop. Sci. 2021, 26, 533–537. [Google Scholar] [CrossRef]
- Konieczny, M.R.; Senyurt, H.; Krauspe, R. Epidemiology of adolescent idiopathic scoliosis. J. Child. Orthop. 2013, 7, 3–9. [Google Scholar] [CrossRef]
- Penha, P.J.; Ramos, N.L.J.P.; de Carvalho, B.K.G.; Andrade, R.M.; Schmitt, A.C.B.; João, S.M.A. Prevalence of Adolescent Idiopathic Scoliosis in the State of São Paulo, Brazil. Spine 2018, 43, 1710–1718. [Google Scholar] [CrossRef]
- Hengwei, F.; Zifang, H.; Qifei, W.; Weiqing, T.; Nali, D.; Ping, Y.; Junlin, Y. Prevalence of Idiopathic Scoliosis in Chinese Schoolchildren: A Large, Population-Based Study. Spine 2016, 41, 259–264. [Google Scholar] [CrossRef]
- Fong, D.Y.; Lee, C.F.; Cheung, K.M.; Cheng, J.C.; Ng, B.K.; Lam, T.P.; Mak, K.H.; Yip, P.S.; Luk, K.D. A meta-analysis of the clinical effectiveness of school scoliosis screening. Spine 2010, 35, 1061–1071. [Google Scholar] [CrossRef]
- Brady, A.F.; Demirdas, S.; Fournel-Gigleux, S.; Ghali, N.; Giunta, C.; Kapferer-Seebacher, I.; Kosho, T.; Mendoza-Londono, R.; Pope, M.F.; Rohrbach, M.; et al. The Ehlers-Danlos syndromes, rare types. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 70–115. [Google Scholar] [CrossRef] [PubMed]
- Ghali, N.; Sobey, G.; Burrows, N. Ehlers-Danlos syndromes. BMJ 2019, 366, l4966. [Google Scholar] [CrossRef] [PubMed]
- Damseh, N.; Dupuis, L.; O’Connor, C.; Oh, R.Y.; Wang, Y.W.; Stavropoulos, D.J.; Schwartz, S.B.; Mendoza-Londono, R. Diagnostic outcomes for molecular genetic testing in children with suspected Ehlers-Danlos syndrome. Am. J. Med. Genet. A 2022, 188, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Stanitski, D.F.; Nadjarian, R.; Stanitski, C.L.; Bawle, E.; Tsipouras, P. Orthopaedic manifestations of Ehlers-Danlos syndrome. Clin. Orthop. Relat. Res. 2000, 376, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Royce, P.M.; Steinmann, B.U. Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects, 2nd ed.; Wiley-Liss: New York, NY, USA, 2002. [Google Scholar]
- Tinkle, B.; Castori, M.; Berglund, B.; Cohen, H.; Grahame, R.; Kazkaz, H.; Levy, H. Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome Type III and Ehlers-Danlos syndrome hypermobility type): Clinical description and natural history. Am. J. Med. Genet. C Semin. Med. Genet. 2017, 175, 48–69. [Google Scholar] [CrossRef]
- Fuss, F.K.; Ahmad, A.; Tan, A.M.; Razman, R.; Weizman, Y. Pressure Sensor System for Customized Scoliosis Braces. Sensors 2021, 21, 1153. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, A.J.; Hasserius, R.; Ohlin, A.; Nachemson, A.L. Health-Related Quality of Life in Untreated Versus Brace-Treated Patients with Adolescent Idiopathic Scoliosis. Spine 2010, 35, 199–205. [Google Scholar] [CrossRef]
- Meng, Z.D.; Li, T.P.; Xie, X.H.; Luo, C.; Lian, X.Y.; Wang, Z.Y. Quality of life in adolescent patients with idiopathic scoliosis after brace treatment. Medicine 2017, 96, e6828. [Google Scholar] [CrossRef]
- Van Bosse, H.J.P.; Butler, M.G. Clinical Observations and Treatment Approaches for Scoliosis in Prader-Willi Syndrome. Genes 2020, 11, 260. [Google Scholar] [CrossRef]
- Foy, M.; de Mazancourt, P.; Bremond Gignac, D.; Gillas, F.; Trigui, N.; Mekki, A.; Carlier, R.; Benistan, K. Classical Ehlers-Danlos syndrome with severe kyphoscoliosis due to a novel pathogenic variant of COL5A2. Clin. Case Rep. 2022, 10, e06338. [Google Scholar] [CrossRef]
- LoPresti, M.A.; Athukuri, P.; Khan, A.B.; Prablek, M.; Patel, R.; Mayer, R.; Bauer, D.F.; Gerow, F.T.; Morris, S.A.; Lam, S.; et al. Thoracolumbar Scoliosis in Pediatric Patients with Loeys-Dietz Syndrome: A Case Series. Cureus 2023, 15, e36372. [Google Scholar] [CrossRef] [PubMed]
- Bressner, J.A.; MacCarrick, G.L.; Dietz, H.C.; Sponseller, P.D. Management of Scoliosis in Patients with Loeys-Dietz Syndrome. J. Pediatr. Orthop. 2017, 37, e492–e499. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, S.; Gogus, A.; Talu, U.; Hamzaoglu, A.; Dikici, F. Surgical management of the spinal deformity in Ehlers-Danlos syndrome type VI. Eur. Spine J. 2003, 12, 135–140. [Google Scholar] [CrossRef]
- Vogel, L.C.; Lubicky, J.P. Neurologic and vascular complications of scoliosis surgery in patients with Ehlers-Danlos syndrome. A case report. Spine 1996, 21, 2508–2514. [Google Scholar] [CrossRef]
- Yang, J.S.; Sponseller, P.D.; Yazici, M.; Johnston, C.E., II. Vascular complications from anterior spine surgery in three patients with Ehlers-Danlos syndrome. Spine 2009, 34, E153–E157. [Google Scholar] [CrossRef] [PubMed]
- Jasiewicz, B.; Potaczek, T.; Tesiorowski, M.; Lokas, K. Spine deformities in patients with Ehlers-Danlos syndrome, type IV—Late results of surgical treatment. Scoliosis 2010, 5, 26. [Google Scholar] [CrossRef]
- Mazziotti, G.; Dordoni, C.; Doga, M.; Galderisi, F.; Venturini, M.; Calzabara-Pinton, P.; Maroldi, R.; Giustina, A.; Colombi, M. High prevalence of radiological vertebral fractures in adult patients with Ehlers-Danlos syndrome. Bone 2016, 84, 88–92. [Google Scholar] [CrossRef]
- Uyama, T.; Kitagawa, H.; Tamura, J.J.; Sugahara, K. Molecular cloning and expression of human chondroitin N-acetylgalactosaminyltransferase: The key enzyme for chain initiation and elongation of chondroitin/dermatan sulfate on the protein linkage region tetrasaccharide shared by heparin/heparan sulfate. J. Biol. Chem. 2002, 277, 8841–8846. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, M.; Sato, T.; Akashima, T.; Iwasaki, H.; Kameyama, A.; Mochizuki, H.; Yada, T.; Inaba, N.; Zhang, Y.; Kikuchi, N.; et al. Enzymatic synthesis of chondroitin with a novel chondroitin sulfate N-acetylgalactosaminyltransferase that transfers N-acetylgalactosamine to glucuronic acid in initiation and elongation of chondroitin sulfate synthesis. J. Biol. Chem. 2002, 277, 38189–38196. [Google Scholar] [CrossRef] [PubMed]
- Ida-Yonemochi, H.; Morita, W.; Sugiura, N.; Kawakami, R.; Morioka, Y.; Takeuchi, Y.; Sato, T.; Shibata, S.; Watanabe, H.; Imamura, T.; et al. Craniofacial abnormality with skeletal dysplasia in mice lacking chondroitin sulfate N-acetylgalactosaminyltransferase-1. Sci. Rep. 2018, 8, 17134. [Google Scholar] [CrossRef]
- Desai, J.; Shannon, M.E.; Johnson, M.D.; Ruff, D.W.; Hughes, L.A.; Kerley, M.K.; Carpenter, D.A.; Johnson, D.K.; Rinchik, E.M.; Culiat, C.T. Nell1-deficient mice have reduced expression of extracellular matrix proteins causing cranial and vertebral defects. Hum. Mol. Genet. 2006, 15, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.S.; Ovchinnikov, D.A.; Yoshii, I.; Mishina, Y.; Behringer, R.R.; Lyons, K.M. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 5062–5067. [Google Scholar] [CrossRef] [PubMed]
- Cundy, T.; Hegde, M.; Naot, D.; Chong, B.; King, A.; Wallace, R.; Mulley, J.; Love, D.R.; Seidel, J.; Fawkner, M.; et al. A mutation in the gene TNFRSF11B encoding osteoprotegerin causes an idiopathic hyperphosphatasia phenotype. Hum. Mol. Genet. 2002, 11, 2119–2127. [Google Scholar] [CrossRef] [PubMed]
- Beighton, P.; De Paepe, A.; Steinmann, B.; Tsipouras, P.; Wenstrup, R.J. Ehlers-Danlos syndromes: Revised nosology, Villefranche, 1997. Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK). Am. J. Med. Genet. 1998, 77, 31–37. [Google Scholar] [CrossRef]
- Kosho, T.; Takahashi, J.; Ohashi, H.; Nishimura, G.; Kato, H.; Fukushima, Y. Ehlers–Danlos syndrome type VIB with characteristic facies, decreased curvatures of the spinal column, and joint contractures in two unrelated girls. Am. J. Med. Genet. A 2005, 138A, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Kosho, T.; Miyake, N.; Hatamochi, A.; Takahashi, J.; Kato, H.; Miyahara, T.; Igawa, Y.; Yasui, H.; Ishida, T.; Ono, K.; et al. A new Ehlers–Danlos syndrome with craniofacial characteristics, multiple congenital contractures, progressive joint and skin laxity, and multisystem fragility-related manifestations. Am. J. Med. Genet. A 2010, 152A, 1333–1346. [Google Scholar] [CrossRef]
- Miyake, N.; Kosho, T.; Mizumoto, S.; Furuichi, T.; Hatamochi, A.; Nagashima, Y.; Arai, E.; Takahashi, K.; Kawamura, R.; Wakui, K.; et al. Loss-of-function mutations of CHST14 in a new type of Ehles–Danlos syndrome. Hum. Mutat. 2010, 31, 966–974. [Google Scholar] [CrossRef]
- Minatogawa, M.; Unzaki, A.; Morisaki, H.; Syx, D.; Sonoda, T.; Janecke, A.R.; Slavotinek, A.; Voermans, N.C.; Lacassie, Y.; Mendoza-Londono, R.; et al. Clinical and molecular features of 66 patients with musculocontractural Ehlers-Danlos syndrome caused by pathogenic variants in CHST14 (mcEDS-CHST14). J. Med. Genet. 2022, 59, 865–877. [Google Scholar] [CrossRef]
- Uehara, M.; Kosho, T.; Yamamoto, N.; Takahashi, H.E.; Shimakura, T.; Nakayama, J.; Kato, H.; Takahashi, J. Spinal manifestations in 12 patients with musculocontractural Ehlers-Danlos syndrome caused by CHST14/D4ST1 deficiency (mcEDS-CHST14). Am. J. Med. Genet. A 2018, 176, 2331–2341. [Google Scholar] [CrossRef]
- Uehara, M.; Oba, H.; Hatakenaka, T.; Ikegami, S.; Kuraishi, S.; Takizawa, T.; Munakata, R.; Mimura, T.; Yamaguchi, T.; Kosho, T.; et al. Posterior Spinal Fusion for Severe Spinal Deformities in Musculocontractural Ehlers-Danlos Syndrome: Detailed Observation of a Novel Case and Review of 2 Reported Cases. World Neurosurg. 2020, 143, 454–461. [Google Scholar] [CrossRef]
- Li, Q.Y.; Zhong, G.B.; Liu, Z.D.; Lao, L.F. Effect of Asymmetric Tension on Biomechanics and Metabolism of Vertebral Epiphyseal Plate in a Rodent Model of Scoliosis. Orthop. Surg. 2017, 9, 311–318. [Google Scholar] [CrossRef]
- Evers, M.R.; Xia, G.; Kang, H.G.; Schachner, M.; Baenziger, J.U. Molecular cloning and characterization of a dermatan-specific N-acetylgalactosamine 4-O-sulfotransferase. J. Biol. Chem. 2001, 276, 36344–36353. [Google Scholar] [CrossRef]
- Mikami, T.; Mizumoto, S.; Kago, N.; Kitagawa, H.; Sugahara, K. Specificities of three distinct human chondroitin/dermatan N-acetylgalactosamine 4-O-sulfotransferases demonstrated using partially desulfated dermatan sulfate as an acceptor: Implication of differential roles in dermatan sulfate biosynthesis. J. Biol. Chem. 2003, 278, 36115–36127. [Google Scholar] [CrossRef]
- Masamoto, K.; Otsuki, B.; Fujibayashi, S.; Shima, K.; Ito, H.; Furu, M.; Hashimoto, M.; Tanaka, M.; Lyman, S.; Yoshitomi, H.; et al. Factors influencing spinal sagittal balance, bone mineral density, and Oswestry Disability Index outcome measures in patients with rheumatoid arthritis. Eur. Spine J. 2018, 27, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Katsu, M.; Ohba, T.; Ebata, S.; Oba, H.; Koyama, K.; Haro, H. Potential Role of Paraspinal Musculature in the Maintenance of Spinopelvic Alignment in Patients with Adult Spinal Deformities. Clin. Spine Surg. 2020, 33, E76–E80. [Google Scholar] [CrossRef]
- Yoshizawa, T.; Mizumoto, S.; Takahashi, Y.; Shimada, S.; Sugahara, K.; Nakayama, J.; Takeda, S.; Nomura, Y.; Nitahara-Kasahara, Y.; Okada, T.; et al. Vascular abnormalities in the placenta of Chst14-/- fetuses: Implications in the pathophysiology of perinatal lethality of the murine model and vascular lesions in human CHST14/D4ST1 deficiency. Glycobiology 2018, 28, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Yoshizawa, T.; Takahashi, Y.; Nitahara-Kasahara, Y.; Okada, T.; Nomura, Y.; Yamanaka, H.; Kosho, T.; Matsumoto, K. Backcrossing to an appropriate genetic background improves the birth rate of carbohydrate sulfotransferase 14 gene-deleted mice. Exp. Anim. 2020, 69, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Nitahara-Kasahara, Y.; Mizumoto, S.; Inoue, Y.U.; Saka, S.; Posadas-Herrera, G.; Nakamura-Takahashi, A.; Takahashi, Y.; Hashimoto, A.; Konishi, K.; Miyata, S.; et al. A new mouse model of Ehlers-Danlos syndrome generated using CRISPR/Cas9-mediated genomic editing. Dis. Model. Mech. 2021, 14, dmm048963. [Google Scholar] [CrossRef]
- Uehara, M.; Takahashi, J.; Kuraishi, S.; Shimizu, M.; Ikegami, S.; Futatsugi, T.; Oba, H.; Kato, H. Computer-assisted skip pedicle screw fixation for adolescent idiopathic scoliosis. J. Orthop. Sci. 2017, 22, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Rabenhorst, B.M.; Garg, S.; Herring, J.A. Posterior spinal fusion in patients with Ehlers-Danlos syndrome: A report of six cases. J. Child. Orthop. 2012, 6, 131–136. [Google Scholar] [CrossRef] [PubMed]
- McMaster, M.J. Spinal deformity in Ehlers-Danlos syndrome. Five patients treated by spinal fusion. J. Bone Joint Surg. Br. 1994, 76, 773–777. [Google Scholar] [CrossRef] [PubMed]

| Clinical Type | Genetic Pattern | Causative Gene(s) |
|---|---|---|
| Kyphoscoliotic (Type VI) | AR | PLOD1 FKBP14 |
| Hypermobile (Type III) | AD | Unknown |
| Arthrochalasia (Type VIIA, VIIB) | AD | COL1A1 COL1A2 |
| Brittle cornea syndrome | AR | ZNF469 PRDM5 |
| Spondylodysplastic | AR | B4GALT7 B4GALT6 SLC39A13 |
| Myopathic | AD, AR | COL12A1 |
| Musculocontractural | AR | CHST14 DSE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uehara, M.; Takahashi, J.; Kosho, T. Spinal Deformity in Ehlers–Danlos Syndrome: Focus on Musculocontractural Type. Genes 2023, 14, 1173. https://doi.org/10.3390/genes14061173
Uehara M, Takahashi J, Kosho T. Spinal Deformity in Ehlers–Danlos Syndrome: Focus on Musculocontractural Type. Genes. 2023; 14(6):1173. https://doi.org/10.3390/genes14061173
Chicago/Turabian StyleUehara, Masashi, Jun Takahashi, and Tomoki Kosho. 2023. "Spinal Deformity in Ehlers–Danlos Syndrome: Focus on Musculocontractural Type" Genes 14, no. 6: 1173. https://doi.org/10.3390/genes14061173





