Spinal Muscular Atrophy Treatment in Patients Identified by Newborn Screening—A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Selection of Studies
3. Results
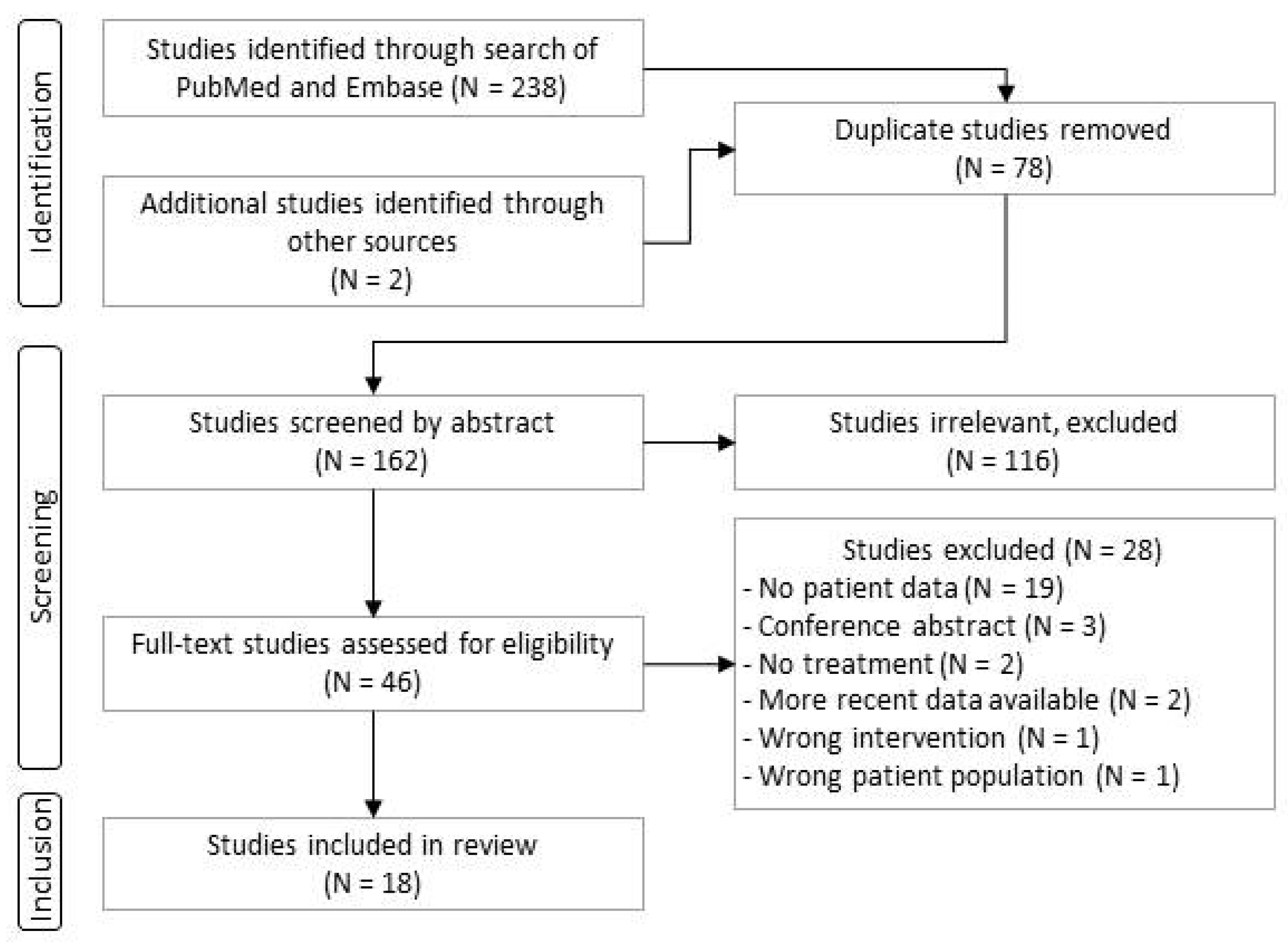
3.1. Study Selection Process
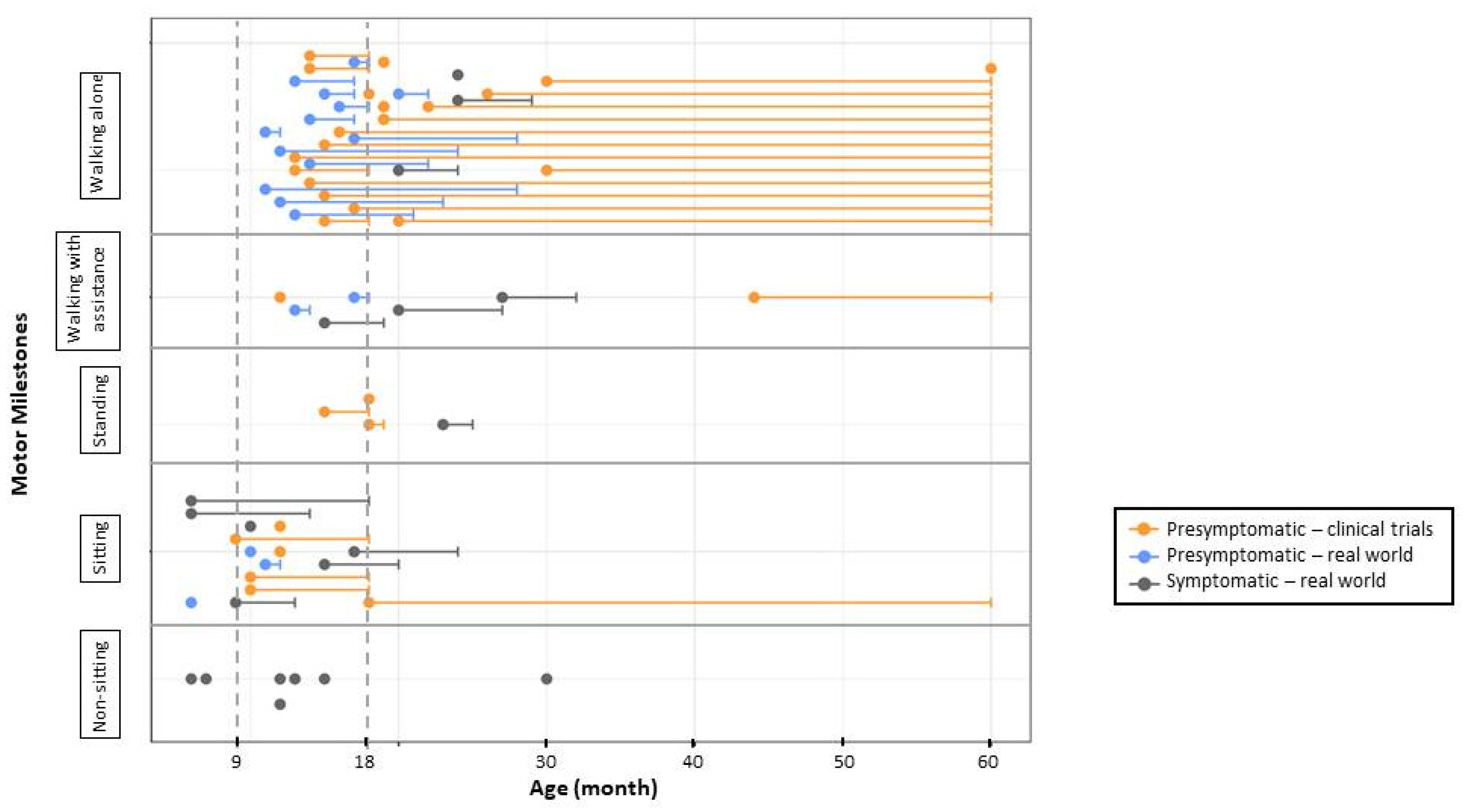
3.2. Results from Clinical Trials of Subjects Treated Presymptomatically
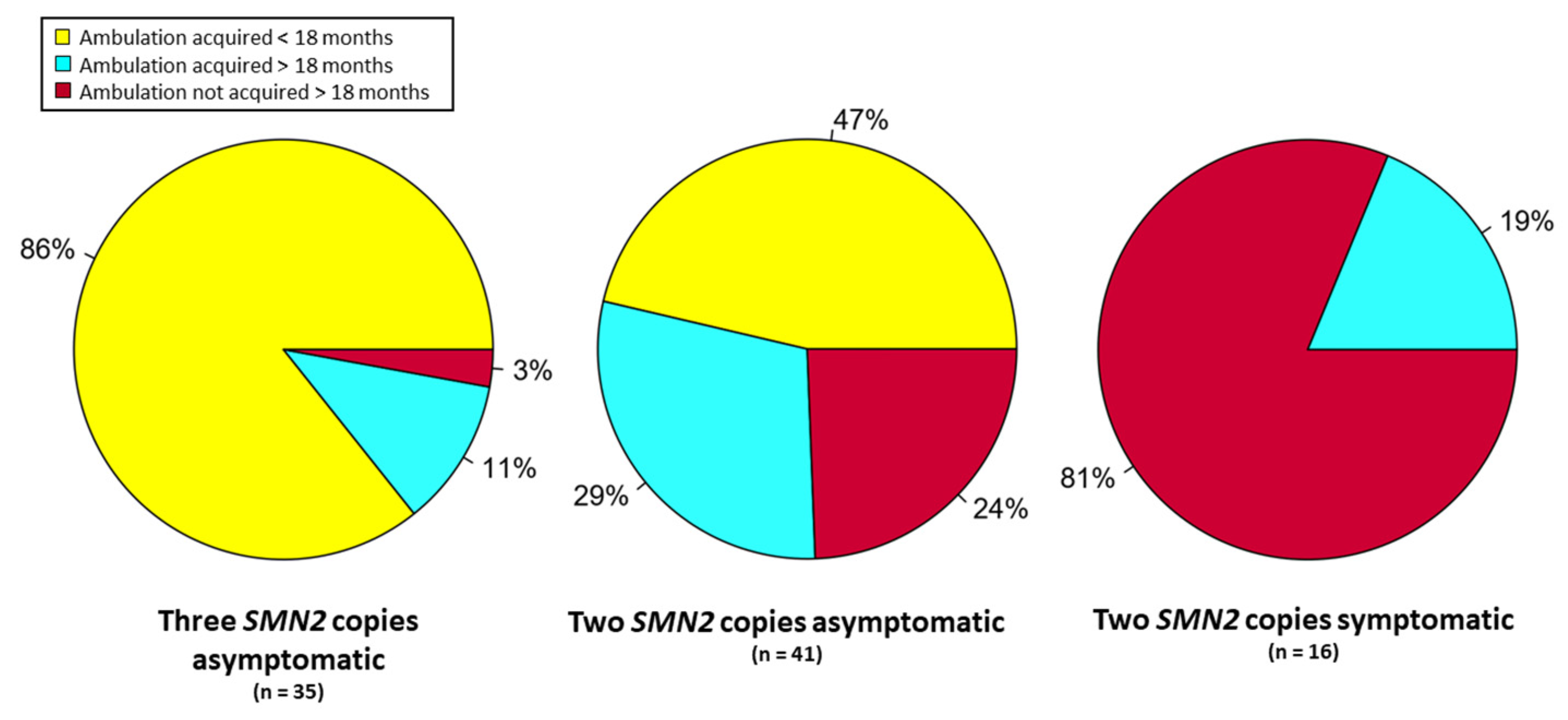
3.3. Results from Subjects Identified by NBS
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vill, K.; Schwartz, O.; Blaschek, A.; Gläser, D.; Nennstiel, U.; Wirth, B.; Burggraf, S.; Röschinger, W.; Becker, M.; Czibere, L.; et al. Newborn screening for spinal muscular atrophy in Germany: Clinical results after 2 years. Orphanet J. Rare Dis. 2021, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Kimizu, T.; Ida, S.; Okamoto, K.; Awano, H.; Niba, E.T.E.; Wijaya, Y.O.S.; Okazaki, S.; Shimomura, H.; Lee, T.; Tominaga, K.; et al. Spinal Muscular Atrophy: Diagnosis, Incidence, and Newborn Screening in Japan. Int. J. Neonatal Screen. 2021, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Deng, S.; Chiriboga, C.A.; Kay, D.M.; Irumudomon, O.; Laureta, E.; Delfiner, L.; Treidler, S.O.; Anziska, Y.; Sakonju, A.; et al. Newborn Screening for Spinal Muscular Atrophy in New York State: Clinical Outcomes from the First 3 Years. Neurology 2022, 99, e1527–e1537. [Google Scholar] [CrossRef] [PubMed]
- Wirth, B. An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum. Mutat. 2000, 15, 228–237. [Google Scholar] [CrossRef]
- Fallini, C.; Donlin-Asp, P.G.; Rouanet, J.P.; Bassell, G.J.; Rossoll, W. Deficiency of the Survival of Motor Neuron Protein Impairs mRNA Localization and Local Translation in the Growth Cone of Motor Neurons. J. Neurosci. 2016, 36, 3811–3820. [Google Scholar] [CrossRef] [Green Version]
- Wirth, B. Spinal Muscular Atrophy: In the Challenge Lies a Solution. Trends Neurosci. 2021, 44, 306–322. [Google Scholar] [CrossRef]
- Monani, U.R.; Lorson, C.L.; Parsons, D.W.; Prior, T.W.; Androphy, E.J.; Burghes, A.H.; McPherson, J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999, 8, 1177–1183. [Google Scholar] [CrossRef]
- Wirth, B.; Brichta, L.; Schrank, B.; Lochmüller, H.; Blick, S.; Baasner, A.; Heller, R. Mildly affected patients with spinal muscular atrophy are partially protected by an increased SMN2 copy number. Hum. Genet. 2006, 119, 422–428. [Google Scholar] [CrossRef]
- Zerres, K.; Rudnik-Schöneborn, S. Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch. Neurol. 1995, 52, 518–523. [Google Scholar] [CrossRef]
- Verhaart, I.E.C.; Robertson, A.; Wilson, I.J.; Aartsma-Rus, A.; Cameron, S.; Jones, C.C.; Cook, S.F.; Lochmüller, H. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy—A literature review. Orphanet J. Rare Dis. 2017, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Lusakowska, A.; Jedrzejowska, M.; Kaminska, A.; Janiszewska, K.; Grochowski, P.; Zimowski, J.; Sierdzinski, J.; Kostera-Pruszczyk, A. Observation of the natural course of type 3 spinal muscular atrophy: Data from the polish registry of spinal muscular atrophy. Orphanet J. Rare Dis. 2021, 16, 150. [Google Scholar] [CrossRef]
- Calucho, M.; Bernal, S.; Alías, L.; March, F.; Venceslá, A.; Rodríguez-Álvarez, F.J.; Aller, E.; Fernández, R.M.; Borrego, S.; Millán, J.M. Correlation between SMA type and SMN2 copy number revisited: An analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscul. Disord. 2018, 28, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Milligan, J.N.; Blasco-Pérez, L.; Costa-Roger, M.; Codina-Solà, M.; Tizzano, E.F. Recommendations for Interpreting and Reporting Silent Carrier and Disease-Modifying Variants in SMA Testing Workflows. Genes 2022, 13, 1657. [Google Scholar] [CrossRef]
- Cuscó, I.; Bernal, S.; Blasco-Pérez, L.; Calucho, M.; Alias, L.; Fuentes-Prior, P.; Tizzano, E.F. Practical guidelines to manage discordant situations of SMN2 copy number in patients with spinal muscular atrophy. Neurol. Genet. 2020, 6, e530. [Google Scholar] [CrossRef] [PubMed]
- Oskoui, M.; Levy, G.; Garland, C.J.; Gray, J.M.; O’Hagen, J.; De Vivo, D.C.; Kaufmann, P. The changing natural history of spinal muscular atrophy type 1. Neurology 2007, 69, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Wijngaarde, C.A.; Veldhoen, E.S.; van Eijk, R.P.A.; Stam, M.; Otto, L.A.M.; Asselman, F.-L.; Wösten-van Asperen, R.M.; Hulzebos, E.H.J.; Verweij-van den Oudenrijn, L.P.; Bartels, B.; et al. Natural history of lung function in spinal muscular atrophy. Orphanet J. Rare Dis. 2020, 15, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vivo, D.C.; Topaloglu, H.; Swoboda, K.J.; Bertini, E.; Hwu, W.-L.; Crawford, T.O.; Foster, R.; Bhan, I.; Fradette, S.; Farwell, W.; et al. Nusinersen in Infants Who Initiate Treatment in a Presymptomatic Stage of Spinal Muscular Atrophy (SMA): Interim Efficacy and Safety Results from the Phase 2 NURTURE Study (S25.001). Neurology 2019, 92 (Suppl. 15), S25.001. [Google Scholar]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene abeparvovec for presymptomatic infants with three copies of SMN2 at risk for spinal muscular atrophy: The Phase III SPR1NT trial. Nat. Med. 2022, 28, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: The Phase III SPR1NT trial. Nat. Med. 2022, 28, 1381–1389. [Google Scholar] [CrossRef]
- Boemer, F.; Caberg, J.-H.; Dideberg, V.; Dardenne, D.; Bours, V.; Hiligsmann, M.; Dangouloff, T.; Servais, L. Newborn screening for SMA in Southern Belgium. Neuromuscul. Disord. 2019, 29, 343–349. [Google Scholar] [CrossRef]
- Vill, K.; Kölbel, H.; Schwartz, O.; Blaschek, A.; Olgemöller, B.; Harms, E.; Burggraf, S.; Röschinger, W.; Durner, J.; Gläser, D.; et al. One year of newborn screening for SMA–Results of a German pilot project. J. Neuromuscul. Dis. 2019, 6, 503–515. [Google Scholar] [CrossRef] [Green Version]
- Gailite, L.; Sterna, O.; Konika, M.; Isakovs, A.; Isakova, J.; Micule, I.; Setlere, S.; Diriks, M.; Auzenbaha, M. New-Born Screening for Spinal Muscular Atrophy: Results of a Latvian Pilot Study. Int. J. Neonatal Screen. 2022, 8, 15. [Google Scholar] [CrossRef]
- Hale, K.; Ojodu, J.; Singh, S. Landscape of Spinal Muscular Atrophy Newborn Screening in the United States: 2018–2021. Int. J. Neonatal Screen. 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Dangouloff, T.; Vrščaj, E.; Servais, L.; Osredkar, D. SMA NBS World Study Group. Newborn screening programs for spinal muscular atrophy worldwide: Where we stand and where to go. Neuromuscul. Disord. 2021, 31, 574–582. [Google Scholar] [CrossRef]
- Dangouloff, T.; Burghes, A.; Tizzano, E.F.; Servais, L.; NBS SMA Study Group. 244th ENMC international workshop: Newborn screening in spinal muscular atrophy May 10–12, 2019, Hoofdorp, The Netherlands. Neuromuscul. Disord. 2020, 30, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Boemer, F.; Caberg, J.-H.; Beckers, P.; Dideberg, V.; di Fiore, S.; Bours, V.; Marie, S.; Dewulf, J.; Marcelis, L.; Deconinck, N. Three years pilot of spinal muscular atrophy newborn screening turned into official program in Southern Belgium. Sci. Rep. 2021, 11, 19922. [Google Scholar] [CrossRef] [PubMed]
- Kuntz, N.L. Nusinersen Effect in Infants in the Presymptomatic Stage of SMA: 4.9-Year Interim of the NURTURE Study. In Proceedings of the Annual Cure SMA Research and Clinical Care Meeting, Anaheim, CA, USA, 15–17 June 2022. [Google Scholar]
- Butterfield, R.J. Spinal Muscular Atrophy Treatments, Newborn Screening, and the Creation of a Neurogenetics Urgency. Semin. Pediatr. Neurol. 2021, 38, 100899. [Google Scholar] [CrossRef] [PubMed]
- Kucera, K.S.; Taylor, J.L.; Robles, V.R.; Clinard, K.; Migliore, B.; Boyea, B.L.; Okoniewski, K.C.; Duparc, M.; Rehder, C.W.; Shone, S.M.; et al. A Voluntary Statewide Newborn Screening Pilot for Spinal Muscular Atrophy: Results from Early Check. Int. J. Neonatal Screen. 2021, 7, 20. [Google Scholar] [CrossRef]
- Hale, J.E.; Darras, B.T.; Swoboda, K.J.; Estrella, E.; Chen, J.Y.H.; Abbott, M.-A.; Hay, B.N.; Kumar, B.; Counihan, A.M.; Gerstel-Thompson, J.; et al. Massachusetts’ Findings from Statewide Newborn Screening for Spinal Muscular Atrophy. Int. J. Neonatal Screen. 2021, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Finkel, R.; Farrar, M.; Vlodavets, D.; Zanoteli, E.; Al-Muhaizea, M.; Nelson, L.; Prufer, A.; Servais, L.; Wang, Y.; Fisher, C.; et al. RAINBOWFISH: Preliminary efficacy and safety data in risdiplam-treated infants with presymptomatic SMA. Neurology 2022, 98, 1636. [Google Scholar]
- Elkins, K.; Wittenauer, A.; Hagar, A.F.; Logan, R.; Sekul, E.; Xiang, Y.; Verma, S.; Wilcox, W.R. Georgia state spinal muscular atrophy newborn screening experience: Screening assay performance and early clinical outcomes. Am. J. Med. Genet. Part C Semin. Med. Genet. 2022, 190, 187–196. [Google Scholar] [CrossRef]
- Noguchi, Y.; Bo, R.; Nishio, H.; Matsumoto, H.; Matsui, K.; Yano, Y.; Sugawara, M.; Ueda, G.; Wijaya, Y.O.S.; Niba, E.T.E.; et al. PCR-Based Screening of Spinal Muscular Atrophy for Newborn Infants in Hyogo Prefecture, Japan. Genes 2022, 13, 2110. [Google Scholar] [CrossRef]
- Matteson, J.; Wu, C.H.; Mathur, D.; Tang, H.; Sciortino, S.; Feuchtbaum, L.; Bishop, T.; Sharma, S.C.; Neogi, P.; Fitzgibbon, I.; et al. California’s experience with SMA newborn screening: A successful path to early intervention. J. Neuromuscul. Dis. 2022, 9, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Blaschek, A.; Kölbel, H.; Schwartz, O.; Köhler, C.; Gläser, D.; Eggermann, K.; Hannibal, I.; Schara-Schmidt, U.; Müller-Felber, W.; Vill, K. Newborn Screening for SMA-Can a Wait-and-See Strategy be Responsibly Justified in Patients With Four SMN2 Copies? J. Neuromuscul. Dis. 2022, 9, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, O.; Kölbel, H.; Blaschek, A.; Gläser, D.; Burggraf, S.; Röschinger, W.; Schara, U.; Müller-Felber, W.; Vill, K. Spinal Muscular Atrophy-Is Newborn Screening Too Late for Children with Two SMN2 Copies? J. Neuromuscul. Dis. 2022, 9, 389–396. [Google Scholar] [CrossRef]
- Sawada, T.; Kido, J.; Sugawara, K.; Yoshida, S.; Ozasa, S.; Nomura, K.; Okada, K.; Fujiyama, N.; Nakamura, K. Newborn screening for spinal muscular atrophy in Japan: One year of experience. Mol. Genet. Metab. Rep. 2022, 32, 100908. [Google Scholar] [CrossRef]
- Kariyawasam, D.S.; D’Silva, A.M.; Sampaio, H.; Briggs, N.; Herbert, K.; Wiley, V.; Farrar, M.A. Newborn screening for spinal muscular atrophy in Australia: A non-randomised cohort study. Lancet Child Adolesc. Health 2023, 7, 159–170. [Google Scholar] [CrossRef]
- WHO Multicentre Growth Reference Study Group; Onis, M. WHO Motor Development Study: Windows of achievement for six gross motor development milestones: Windows of achievement for motor milestones. Acta Paediatr. 2007, 95, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Glascock, J.; Sampson, J.; Haidet-Phillips, A.; Connolly, A.; Darras, B.; Day, J.; Finkel, R.; Howell, R.R.; Klinger, K.; Kuntz, N.; et al. Treatment Algorithm for Infants Diagnosed with Spinal Muscular Atrophy through Newborn Screening. J. Neuromuscul. Dis. 2018, 5, 145–158. [Google Scholar] [CrossRef] [Green Version]
- Kaufmann, P.; McDermott, M.P.; Darras, B.T.; Finkel, R.S.; Sproule, D.M.; Kang, P.B.; Oskoui, M.; Constantinescu, A.; Gooch, C.L.; Foley, A.R.; et al. Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurology 2012, 79, 1889–1897. [Google Scholar] [CrossRef]
- Pane, M.; Donati, M.A.; Cutrona, C.; De Sanctis, R.; Pirinu, M.; Coratti, G.; Ricci, M.; Palermo, C.; Berti, B.; Leone, D.; et al. Neurological assessment of newborns with spinal muscular atrophy identified through neonatal screening. Eur. J. Pediatr. 2022, 181, 2821–2829. [Google Scholar] [CrossRef]
- Lee, B.H.; Waldrop, M.A.; Connolly, A.M.; Ciafaloni, E. Time is muscle: A recommendation for early treatment for preterm infants with spinal muscular atrophy. Muscle Nerve 2021, 64, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Glascock, J.; Sampson, J.; Connolly, A.M.; Darras, B.T.; Day, J.W.; Finkel, R.; Howell, R.R.; Klinger, K.W.; Kuntz, N.; Prior, T.; et al. Revised Recommendations for the Treatment of Infants Diagnosed with Spinal Muscular Atrophy Via Newborn Screening Who Have 4 Copies of SMN2. J. Neuromuscul. Dis. 2020, 7, 97–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller-Felber, W.; Vill, K.; Schwartz, O.; Gläser, D.; Nennstiel, U.; Wirth, B.; Burggraf, S.; Röschinger, W.; Becker, M.; Durner, J.; et al. Infants Diagnosed with Spinal Muscular Atrophy and 4 SMN2 Copies through Newborn Screening-Opportunity or Burden? J. Neuromuscul. Dis. 2020, 7, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dangouloff, T.; Botty, C.; Beaudart, C.; Servais, L.; Hiligsmann, M. Systematic literature review of the economic burden of spinal muscular atrophy and economic evaluations of treatments. Orphanet J. Rare Dis. 2021, 16, 47. [Google Scholar] [CrossRef]
- Dangouloff, T.; Hiligsmann, M.; Deconinck, N.; D’Amico, A.; Seferian, A.M.; Boemer, F.; Servais, L. Financial cost and quality of life of patients with spinal muscular atrophy identified by symptoms or newborn screening. Dev. Med. Child Neurol. 2022, 65, 67–77. [Google Scholar] [CrossRef]
- Velikanova, R.; van der Schans, S.; Bischof, M.; van Olden, R.W.; Postma, M.; Boersma, C. Cost-Effectiveness of Newborn Screening for Spinal Muscular Atrophy in The Netherlands. Value Health 2022, 25, 1696–1704. [Google Scholar] [CrossRef]
- Shih, S.T.F.; Keller, E.; Wiley, V.; Farrar, M.A.; Wong, M.; Chambers, G.M. Modelling the Cost-Effectiveness and Budget Impact of a Newborn Screening Program for Spinal Muscular Atrophy and Severe Combined Immunodeficiency. Int. J. Neonatal Screen. 2022, 8, 45. [Google Scholar] [CrossRef]
- Weidlich, D.; Servais, L.; Kausar, I.; Howells, R.; Bischof, M. Cost Effectiveness of Newborn Screening for Spinal Muscular Atrophy in England. Neurol. Ther. 2023. [Google Scholar] [CrossRef]
- Prior, T.W.; Snyder, P.J.; Rink, B.D.; Pearl, D.K.; Pyatt, R.E.; Mihal, D.C.; Conlan, T.; Schmalz, B.; Montgomery, L.; Ziegler, K.; et al. Newborn and carrier screening for spinal muscular atrophy. Am. J. Med. Genet. A 2010, 152, 1608–1616. [Google Scholar] [CrossRef]
- Jedrzejowska, M.; Borkowska, J.; Zimowski, J.; Kostera-Pruszczyk, A.; Milewski, M.; Jurek, M.; Sielska, D.; Kostyk, E.; Nyka, W.; Zaremba, J.; et al. Unaffected patients with a homozygous absence of the SMN1 gene. Eur. J. Hum. Genet. 2008, 16, 930–934. [Google Scholar] [CrossRef] [Green Version]
- Schorling, D.C.; Becker, J.; Pechmann, A.; Langer, T.; Wirth, B.; Kirschner, J. Discrepancy in redetermination of SMN2 copy numbers in children with SMA. Neurology 2019, 93, 267–269. [Google Scholar] [CrossRef] [PubMed]
| Article Author and Year [Ref.] | Location | Period | Study Description | n a |
|---|---|---|---|---|
| DeVivo 2019, Kuntz 2022 [17,27] | USA, Australia, Canada, Germany, Italy, Qatar, Taiwan, Turkey | 05.2015–02.2021 | Preliminary results from NURTURE clinical trial of nusinersen | 25 b |
| Butterfield 2021 [28] | Utah, USA | 2018 | Two patients with two copies of SMN2 | 2 |
| Kucera 2021 [29] | North Carolina, USA | 10.2018–12.2020 | Results from 2 years of NBS | 1 |
| Hale 2021 [30] | Massachusetts, USA | 01.2018–01.2021 | Results from 3 years of NBS | 9 |
| Boemer 2021 [26] | Southern Belgium | 03.2018–02.2021 | Results from 3 years of NBS | 9 (10) c |
| Vill 2021 [1] | Germany | 01.2018–01.2020 | Results from 2 years of NBS | 42 (43) c |
| Finkel 2022 [31] | USA, Australia, Belgium, Brazil, China, Poland, Russia, Taiwan | 08.2019–07.2021 | Preliminary results from RAINBOWFISH trial of risdiplam | 7 |
| Elkins 2022 [32] | Georgia, USA | 02.2019–02.2021 | Results from 2 years of NBS | 15 (16) c |
| Strauss 2022 [19] | USA, Australia, Belgium, Canada, Japan, UK | 04.2018–12.2020 | Final results from SPR1NT clinical trial of onasemnogene abeparvovec in patients with two copies of SMN2 | 14 |
| Strauss 2022 [18] | 04.2018–12.2020 | Final results from SPR1NT clinical trial of onasemnogene abeparvovec in patients with three copies of SMN2 | 15 | |
| Lee 2022 [3] | New York, USA | 10.2018–10.2021 | Results from 3 years of NBS | 32 (34) d |
| Noguchi 2022 [33] | Hyogo, Japan | 02.2021–08.2022 | Results from 1 year of NBS | 2 |
| Matteson 2022 [34] | California, USA | 06.2020–12.2021 | Results from 1.5 years of NBS | 16 (34) e |
| Blaschek 2022 [35] | Germany | 01.2018–01.2021 | Outcomes in patients with four copies of SMN2 | 4 (20) d |
| Schwartz 2022 [36] | Germany | 01.2018–01.2021 | Outcomes in patient with two copies of SMN2 | 6 (21) d |
| Sawada 2022 [37] | Kumamoto, Japan | 02.2021–01.2022 | Results from 1 year of NBS | 1 |
| Kariyawasam 2023 [38] | Australia | 08.2018–08.2020 | Results from 2 years of NBS | 14 |
| Total | 214 |
| Study | Population | Number of SMN2 Copies | Total Number of Subjects | Number of Subjects Treated | Number of Subjects Symptomatic at Treatment | Incidence | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ≥4 | ||||||
| [26] | 136,339 | - | 4 | 3 | 2 | 9 | 9 | 4 | 1:13,634 |
| [32] | 301,418 | 2 | 5 | 6 | 2 | 15 | 9 | 1 | 1:18,840 |
| [30] | 179,467 | - | 7 | - | 2 | 9 | 9 | 5 | 1:19,940 |
| [29] | 12,065 | - | 1 | - | - | 1 | 1 | 1 | 1:12,065 |
| [28] | N/A | - | 2 | - | - | 2 | 2 | 1 | N/A |
| [3] | 650,000 | 1 | 17 (18) | 10 (11) | 4 | 32 (34) | 30 | 8 | 1:19,000 |
| [34] | 628,791 | - | 8 | 7 | 1 | 16 | 16 | 3 | 1:18,494 |
| [33] | 8336 | - | 2 | - | - | 2 | 2 | 1 | 1:25,000 |
| [37] | 13,587 | - | - | 1 | - | 1 | 1 | 0 | 1:13,587 |
| [1] | 297,163 | - | 17 | 9 | 16 | 42 | 26 | 6 | 1:6910 |
| [36] | N/A | - | 6 (21) | - | - | 6 (21) | 6 | 2 | N/A |
| [35] | N/A | - | - | - | 4 (20) | 4 (20) | 3 | 0 | N/A |
| [38] | N/A | 8 | 5 | 1 | 14 | 13 | 6 | N/A | |
| Total | 2,227,166 | 3 | 77 | 41 | 32 | 153 | 127 | 38 | 1:14,848 |
| Study | NURTURE | SPR1NT | Rainbowfish |
|---|---|---|---|
| Key inclusion criteria | |||
| Age | <6 weeks | <6 weeks | <6 weeks |
| Gestational age (singleton) | 37–42 weeks | 35–42 weeks | 37–42 weeks |
| Gestational age (twins) | 34–42 weeks | 35–42 weeks | 34–42 weeks |
| SMN2 copies | 2 or 3 | 2 or 3 | >1 |
| CMAP | >1 mV | >2 mV | No limit |
| Weight | not specified | >2 kg and/or 3rd percentile | >3rd percentile |
| Key exclusion criteria | |||
| Any signs or symptoms suggestive of SMA | At screening or immediately prior to the first dosing (Day 1) | At screening or immediately prior to dosing | At screening (or at baseline) |
| Respiratory | Hypoxemia < 96% | Hypoxemia < 96% (or <92% for altitude > 1000 m) | (SaO2 < 95%), requiring invasive ventilation, tracheostomy or awake non-invasive ventilation |
| Prior treatment | Any investigational drug or device, gene or cell therapy, or antisense oligonucleotide | Any investigational drug or device, gene or cell therapy, antisense oligonucleotide, drugs for treatment of myopathy, neuropathy, diabetes mellitus, immunosuppressive or immunomodulators, or plasmapheresis | Any investigational or commercial product, gene therapy, prior antisense oligonucleotide, SMN2-splicing modifier, oral β-2 adrenergic, or drugs with known retinal toxicity during pregnancy |
| Laboratory abnormalities | Clinically significant abnormalities in haematology or clinical chemistry | Clinically significant abnormalities of liver function tests (except for neonatal jaundice), blood count, AAV antibodies | Clinically significant abnormalities in laboratory test results |
| Other | ECG abnormalities | ||
| Study (Drug) | N | Mean Follow-Up (mo) | Follow-Up Range (mo) | Mean Age at Treatment (Days) | Age Range (Days) | Sitter < 9 Months | Sitter < 18 Months | Walker < 18 Months | Walker < 3 Years | |
|---|---|---|---|---|---|---|---|---|---|---|
| Two copies of SMN2 | NURTURE (nusinersen) | 15 | 59 | 47–68 | 19 | 8–41 | 11 (73%) | 15 (100%) | 6 (40%) | 13 (87%) |
| SPR1NT (gene therapy) | 14 | 18 | 18 | 20 | 8–34 | 11 (76%) | 14 (100%) | 5 (36%) | 9 (64%) a | |
| Rainbowfish (risdiplam) | 4 | 12 | 12–15 | 26 | 16–40 | 1 (33%) | 4 (100%) | 1 (33%) | 1 (33%) a | |
| Total | 33 | 36 | 12–68 | 22 | 8–41 | 23 (70%) | 33 (100%) | 12 (36%) | 23 (70%) a | |
| Three copies of SMN2 | NURTURE (nusinersen) | 10 | 59 | 47–68 | 22 | 3–42 | 10 (100%) | 10 (100%) | 10 (100%) | 10 (100%) |
| SPR1NT (gene therapy) | 15 | 24 | 24 | 32 | 9–43 | 11 (78%) | 15 (100%) | 11 (78%) | 14 (93%) a | |
| Rainbowfish (risdiplam) | 3 b | 13 | 12–15 | 26 | 16–40 | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) a | |
| Total | 28 | 35 | 12–68 | 27 | 3–42 | 24 (86%) | 28 (100%) | 24 (86%) | 27 (96%) a |
| Ref | N | Mean Follow-Up (mo.) | Range Follow-Up (mo.) | Total Treated | Mean Age at Treatment (Days) | Age Range at Treatment Initiation (Days) | Symptoms at Treatment | Sitters (Age Range, mo.) | Walkers (Age Range, mo.) | Sitter at 9 Months | Sitter at 18 Months | Walker at 18 Months | Walker at 24 Months | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| two SMN2 copies | [26] a | 4 | 21.5 | 14–32 | 4 (100%) | 38 | 29–54 | 4 (100%) | 4 (6–7) | 0 | 4/4 (100%) | 3/3 (100%) | 0/4 (0%) | 0/4 (0%) |
| [30] | 7 | 14 | 5–42 | 7 (100%) | 21 | 11–38 | 5 (71%) | 5 (7–15) | 1 (12) | 2/5 (40%) | 3/3 (100%) | 1/3 (33%) | 1/1 (100%) | |
| [32] b | 5 | 2.75 | 2.5–3 | 3 (60%) | 45 | 30–60 | 1 (33%) | n/a | n/a | n/a | n/a | n/a | n/a | |
| [28] | 2 | 12 | 12 | 2 (100%) | 22 | 20–24 | 1 (50%) | 1 (9-) | 1 (12) | 1/2 (50%) | n/a | n/a | n/a | |
| [29] | 1 | 3 | n/a | 1 (100%) | 30 | n/a | 1 (100%) | n/a | n/a | n/a | n/a | n/a | n/a | |
| [3] c | 17 | 12 | 1–24 | 17 (100%) | 35 | 12–89 | 8 (47%) | 9 (6-n/a) | 3 (n/a) | 8/11 (72%) | 3/4 (75%) | 2/4 (50%) | 2/2 (100%) | |
| [33] | 2 | 4.5 | 3–6 | 2 (100%) | 23 | 22–25 | 1 (50%) | n/a | n/a | n/a | n/a | n/a | n/a | |
| [36] | 23 | 20 | 5.5–30 | 21 (91%) | 22 | 14–39 | 8 (38%) | 19 (6–17) | 13 (11–24) | 19/21 (90%) | 14/15 (93%) | 10/15 (66%) | 4/8 (50%) | |
| [34] | 8 | 12 | n/a | 8 (100%) | 31 | 17–52 | 3 (38%) | n/a | n/a | n/a | n/a | n/a | n/a | |
| [38] | 8 | 24 | 24 | 8 (100%) | 27 | 19–36 | 5 (63%) | 8 (n/a) | 3 (n/a) | n/a | n/a | n/a | 3/8 (38%) | |
| Total | 77 | 11 | 1–42 | 73 (95%) | 23 | 11–89 | 37 (51%) | 46 (6–17) | 21 (11–24) | 34/43 (79%) | 23/25 (92%) | 13/26 (50%) | 10/23 (43%) | |
| three SMN2 copies | [26] a | 3 | 23 | 12–33 | 3 (100%) | 34 | 30–41 | 0 (0%) | 3 (7-) | 3 (11–15) | 3/3 (100%) | 3/3 (100%) | 3/3 (100%) | 2/2 (100%) |
| [32] b | 6 | 7 | 4–14 | 6 (100%) | 133 | 90–180 | 0 (0%) | 2 (8–n/a) | n/a | n/a | n/a | n/a | n/a | |
| [3] c | 10 | 13 | 1.5–26 | 10 (100%) | 37 | 11–94 | 0 (0%) | 7 (n/a) | 6 (11–n/a) | 6/6 (100%) | 2/2 (100%) | 2/2 (100%) | 2/2 (100%) | |
| [34] | 7 | 12 | n/a | 7 (100%) | 40 | 18–79 | 0 (0%) | n/a | n/a | n/a | n/a | n/a | n/a | |
| [37] | 1 | 11 | n/a | 1 (100%) | 42 | n/a | 0 (0%) | 1 (n/a) | n/a | 1/1 (100%) | n/a | n/a | n/a | |
| [1] d | 9 | 11 | 1.5–23 | 6 (66%) | 24 | 20–29 | 0 (0%) | 4 (7–n/a) | 2 (12–19) | n/a | 2/2 (100%) | 1/2 (50%) | n/a | |
| [38] | 5 | 24 | 24 | 5 (100%) | 104 | 30–400 | 1 (20%) | 5 (n/a) | 5 (n/a) | n/a | 5/5 (100%) | n/a | 5/5 (100%) | |
| Total | 41 | 13 | 1.5–33 | 38 (93%) | 52 | 11–400 | 1 (2%) | 22 (7–n/a) | 16 (11–19) | 10/10 (100%) | 12/12 (100%) | 6/7 (86%) | 9/9 (100%) | |
| four SMN2 copies e | [26] a | 2 | 21 | 20–22 | 2 (100%) | 44 | 39–49 | 0 (0%) | 2 (5–6) | 2 (12-) | 2/2 (100%) | 2/2 (100%) | 2/2 (100%) | 2/2 (100%) |
| [30] | 2 | 16 | 10–22 | 2 (100%) | 90 | 8–171 | 1 (50%) | 2 (7–n/a) | 1 (12-) | 2/2 (100%) | 1/1 (100%) | 1/1 (100%) | n/a | |
| [35] | 18 | 25 | 12–44 | 13 (72%) | 560 | 90–1440 | 5 (27%) | 18 (n/a) | 18 (n/a) | n/a | n/a | n/a | n/a | |
| [3] c | 2 | 8.5 | 7–10 | 1 (50%) | 180 | n/a | 0 (0%) | 2 (n/a) | n/a | 1/1 (100%) | n/a | n/a | n/a | |
| Total | 24 | 23 | 7–44 | 18 (75%) | 219 | 8–1440 | 6 (25%) | 24 (5–n/a) | 21 (12–n/a) | 5/5 (100%) | 3/3 (100%) | 3/3 (100%) | 2/2 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragon-Gawinska, K.; Mouraux, C.; Dangouloff, T.; Servais, L. Spinal Muscular Atrophy Treatment in Patients Identified by Newborn Screening—A Systematic Review. Genes 2023, 14, 1377. https://doi.org/10.3390/genes14071377
Aragon-Gawinska K, Mouraux C, Dangouloff T, Servais L. Spinal Muscular Atrophy Treatment in Patients Identified by Newborn Screening—A Systematic Review. Genes. 2023; 14(7):1377. https://doi.org/10.3390/genes14071377
Chicago/Turabian StyleAragon-Gawinska, Karolina, Charlotte Mouraux, Tamara Dangouloff, and Laurent Servais. 2023. "Spinal Muscular Atrophy Treatment in Patients Identified by Newborn Screening—A Systematic Review" Genes 14, no. 7: 1377. https://doi.org/10.3390/genes14071377





