Two New Mitogenomes of Bibionidae and Their Comparison within the Infraorder Bibionomorpha (Diptera)
Abstract
:1. Introduction
2. Material and Methods
2.1. Sample Collecting and Mitogenome Sequencing
2.2. Mitogenome Assembly and Annotation
2.3. Mitogenome Characteristics Analysis
2.4. Phylogenetic Analysis
2.5. Divergence Time Estimation
3. Results
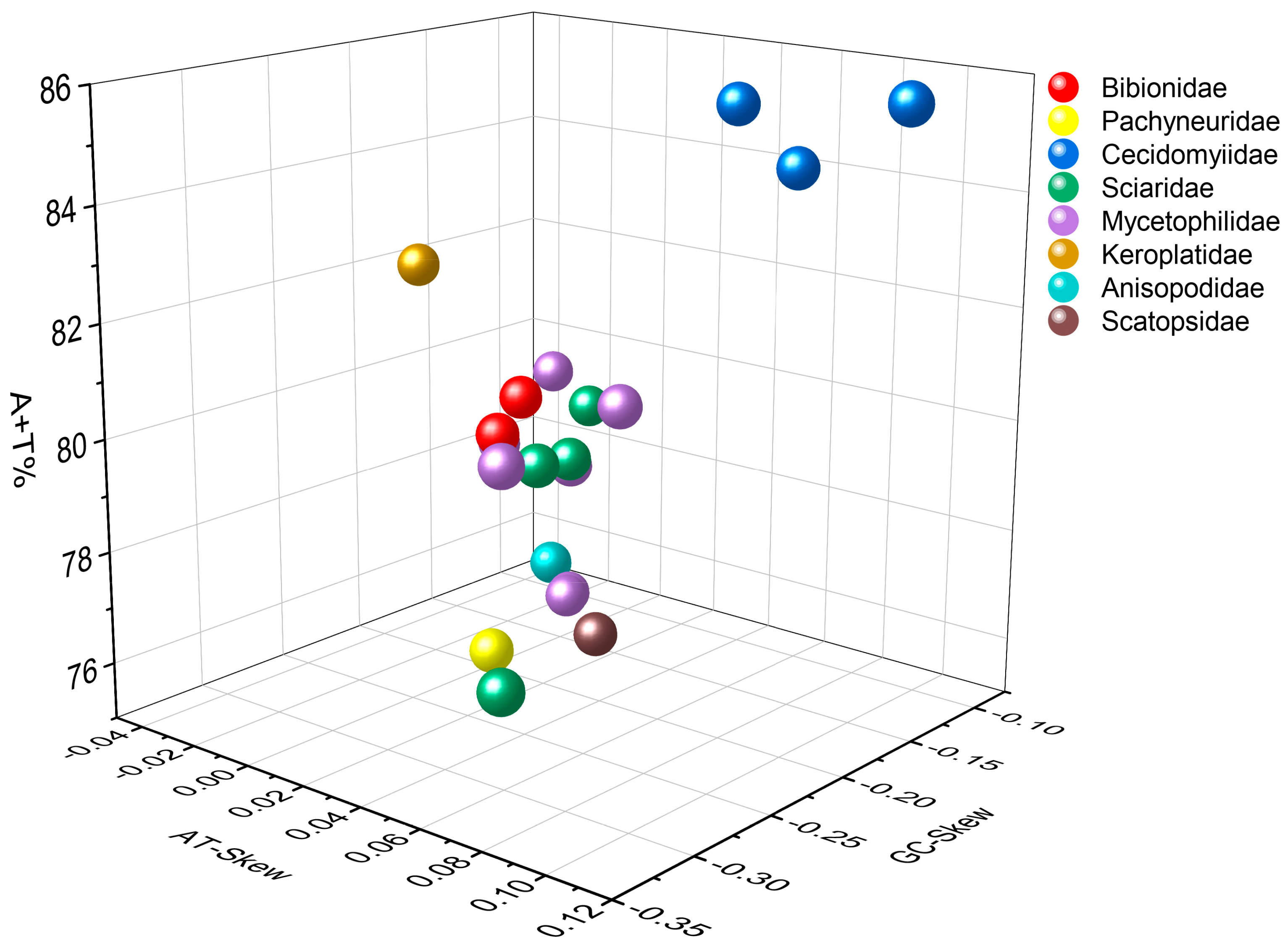
3.1. Mitogenome Characteristics of Bibionomorpha
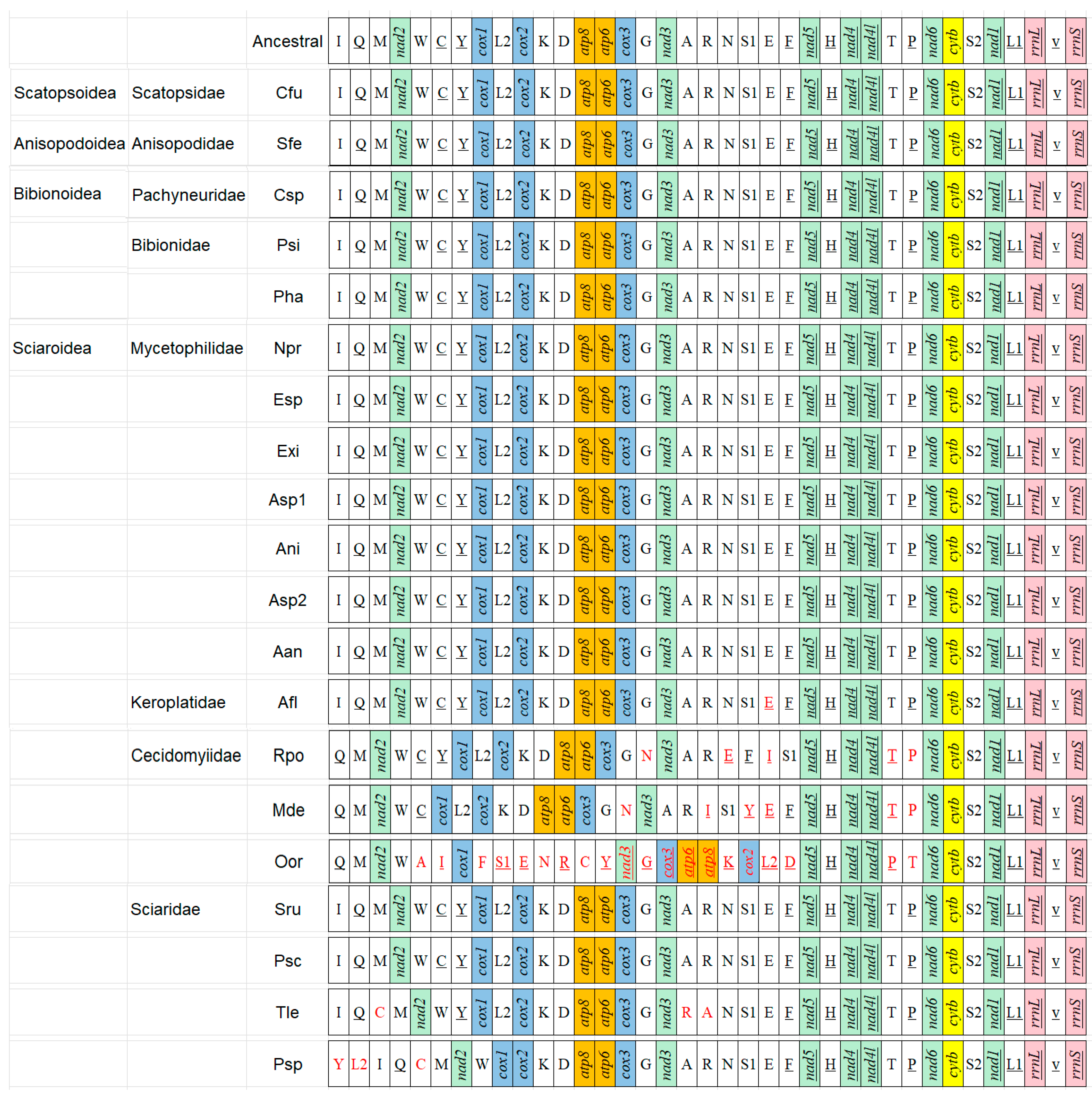
3.2. Gene Rearrangement Events of Bibionomorpha
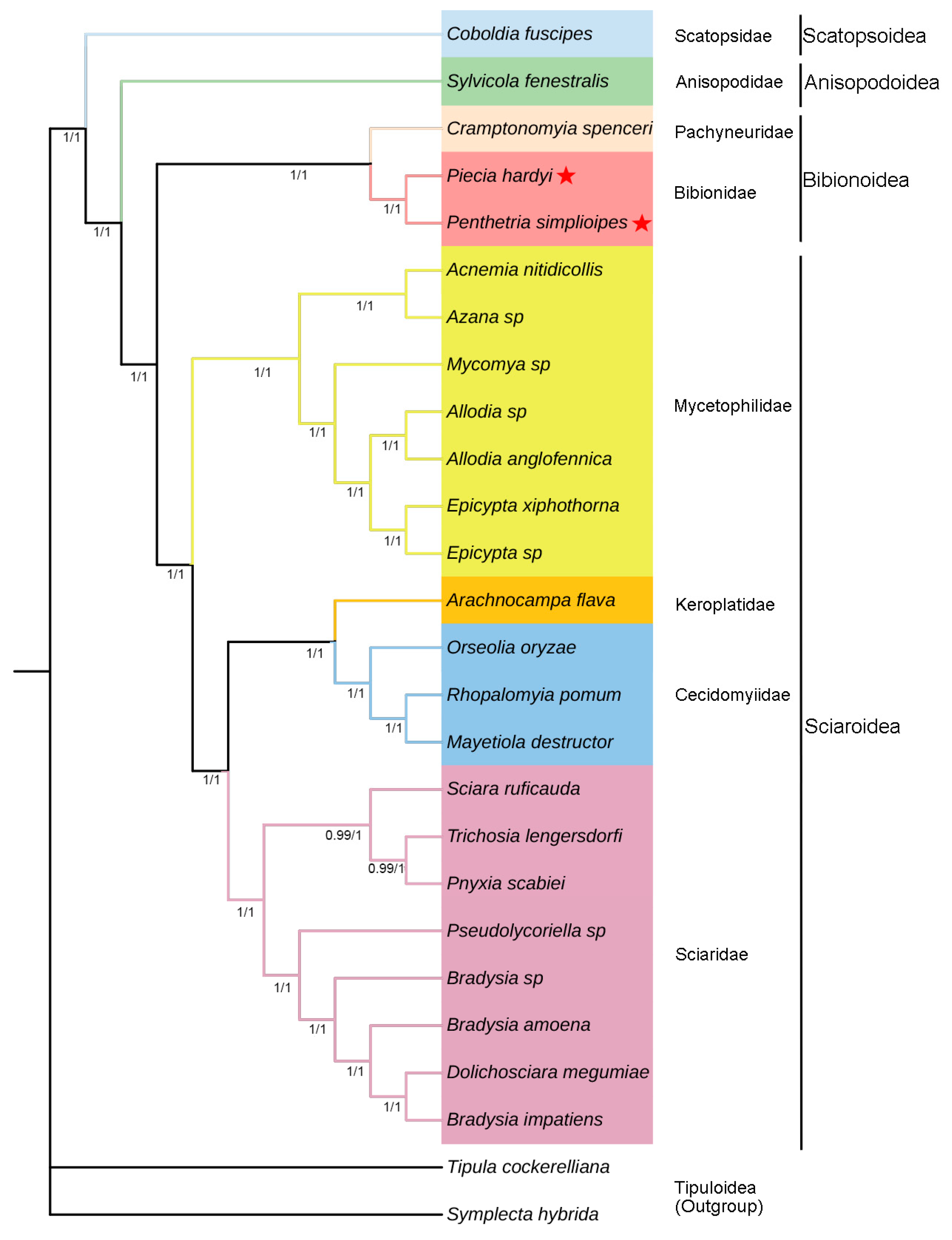
3.3. Phylogenetic Relationships and Divergence Time
4. Discussion
4.1. Mitogenome Organization and Characteristics
4.2. Gene Rearrangement of Mitogenomes
4.3. Phylogenetics and Evolution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van Nieukerken, E.J.; Kaila, L.; Kitching, I.J.; Kristensen, N.P.; Lees, D.C.; Minet, J.; Mitter, C.; Mutanen, M.; Regier, J.C.; Simonsen, T.J.; et al. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa 2011, 3148, 222–229. [Google Scholar]
- Matile, L. Phylogeny and evolution of the larval diet in the Sciaroidea (Diptera, Bibionomorpha) since the Mesozoic, The origin of biodiversity in insects: Phylogenetic tests of evolutionary scenarios. Mém. Mus. Natl. Hist. Nat. Paris Sér. A 1997, 173, 273–303. [Google Scholar]
- Arimoto, M.; Uesugi, R.; Hinomoto, N.; Sueyoshi, M.; Yoshimatsu, S.-I. Molecular marker to identify the fungus gnat, Bradysia sp. (Diptera: Sciaridae), a new pest of Welsh onion and carrot in Japan. Appl. Entomol. Zool. 2018, 53, 419–424. [Google Scholar] [CrossRef]
- Scarlett, K.; Tesoriero, L.; Daniel, R.; Guest, D. Sciarid and shore flies as aerial vectors of Fusarium oxysporumf. sp. cucumerinum in greenhouse cucumbers. Appl. Entomol. Zool. 2014, 138, 368–377. [Google Scholar] [CrossRef]
- Elmer, W.H. Preventing spread of Fusarium wilt of Hiemalis begonias in the greenhouse. Crop. Prot. 2008, 27, 1078–1083. [Google Scholar] [CrossRef]
- Hennig, W. Flügelgeäder und System der Dipteren unter Berücksichtigung der ausdem Mesozoikum beschriebenen Fossilien. Beitr. Zur Entomol. 1954, 4, 245–388. [Google Scholar]
- Oosterbroek, P.; Courtney, G. Phylogeny of the nematocerous families of Diptera (Insecta). Zool. J. Linn. Soc. 1995, 115, 267–311. [Google Scholar] [CrossRef]
- Bertone, M.A.; Courtney, G.W.; Wiegmann, B.M. Phylogenetics and temporal diversification of the earliest true flies (Insecta: Diptera) based on multiple nuclear genes. Syst. Èntomol. 2008, 33, 668–687. [Google Scholar] [CrossRef]
- Hennig, W. Ordnung Diptera (Zweiflügler). Handb. Zool. 1973, 4, 1–227. [Google Scholar]
- Ševčík, J.; Kaspřák, D.; Mantič, M.; Fitzgerald, S.; Ševčíková, T.; Tóthová, A.; Jaschhof, M. Molecular phylogeny of the megadiverse insect infraorder Bibionomorpha sensu lato (Diptera). PeerJ 2016, 4, e2563. [Google Scholar] [CrossRef] [Green Version]
- Beckenbach, A.T. Mitochondrial Genome Sequences of Nematocera (Lower Diptera): Evidence of Rearrangement following a Complete Genome Duplication in a Winter Crane Fly. Genome Biol. Evol. 2011, 4, 89–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzgerald, S.J. Evolution and classification of Bibionidae (Diptera: Bibionomorpha). Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2004. [Google Scholar]
- Lambkin, C.L.; Sinclair, B.J.; Pape, T.; Courtney, G.W.; Skevington, J.H.; Meier, R.; Yeates, D.K.; Blagoderov, V.; Wiegmann, B.M. The phylogenetic relationships among infraorders and superfamilies of Diptera based on morphological evidence. Syst. Èntomol. 2012, 38, 164–179. [Google Scholar] [CrossRef]
- Mantič, M.; Sikora, T.; Burdíková, N.; Blagoderov, V.; Kjærandsen, J.; Kurina, O.; Ševčík, J. Hidden in Plain Sight: Comprehensive Molecular Phylogeny of Keroplatidae and Lygistorrhinidae (Diptera) Reveals Parallel Evolution and Leads to a Revised Family Classification. Insects 2020, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.M.; Borkent, A. Phylogeny and classifification of the Nematocera. Man. Nearctic Diptera 1989, 117, 1333–1370. [Google Scholar]
- Sikora, T.; Jaschhof, M.; Mantič, M.; Kaspřák, D.; Ševčík, J. Considerable congruence, enlightening conflict: Molecular analysis largely supports morphology-based hypotheses on Cecidomyiidae (Diptera) phylogeny. Zool. J. Linn. Soc. 2018, 185, 98–110. [Google Scholar] [CrossRef]
- Hippa, H.; Vilkamaa, P. Phylogeny of the Sciaroidea (Diptera): The implication of additional taxa and character data. Zootaxa 2006, 1132, 63–68. [Google Scholar] [CrossRef]
- Amorim, D.D.S.; Rindal, E. Phylogeny of the Mycetophiliformia, with proposal of the subfamilies Heterotrichinae, Ohakuneinae, and Chiletrichinae for the Rangomaramidae (Diptera, Bibionomorpha). Zootaxa 2007, 1535, 1–92. [Google Scholar] [CrossRef]
- Hippa, H.; Vilkamaa, P. The genus Sciarotricha gen. n. (Sciaridae) and the phylogeny of recent and Sciarotricha gen. n. (Sciaridae) and the phylogeny of recent and Sciarotricha fossil Sciaroidea (Diptera). Insect Syst. Evol. 2005, 36, 121–144. [Google Scholar]
- Cameron, S.L. Insect Mitochondrial Genomics: Implications for Evolution and Phylogeny. Annu. Rev. Èntomol. 2014, 59, 95–117. [Google Scholar] [CrossRef] [Green Version]
- Beckenbach, A.T.; Joy, J.B. Evolution of the Mitochondrial Genomes of Gall Midges (Diptera: Cecidomyiidae): Rearrangement and Severe Truncation of tRNA Genes. Genome Biol. Evol. 2009, 1, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brehm, A.; Harris, D.J.; Hernández, M.; Cabrera, V.M.; Larruga, J.M.; Pinto, F.M.; González, A.M. Structure and evolution of the mitochondrial DNA complete control region in the Drosophila subobscura subgroup. Insect Mol. Biol. 2001, 10, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, A.C.M.; Lessinger, A.C.; Torres, T.T.; da Silva, F.R.; Vettore, A.L.; Arruda, P.; Espin, A.M.L.A. The mitochondrial genome of the blowfly Chrysomya chloropyga (Diptera: Calliphoridae). Gene 2004, 339, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Huang, J.; Menzel, F.; Wang, Q.; Wei, Q.; Lin, X.-L.; Wu, H. Five mitochondrial genomes of black fungus gnats (Sciaridae) and their phylogenetic implications. Int. J. Biol. Macromol. 2020, 150, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yan, Z.; Si, F.; Li, X.; Mao, Q.; Asghar, S.; Chen, B. Mitochondrial genes associated with pyrethroid resistance revealed by mitochondrial genome and transcriptome analyses in the malaria vector Anopheles sinensis (Diptera: Culicidae). Pest Manag. Sci. 2020, 76, 769–778. [Google Scholar] [CrossRef]
- Cameron, S.L.; Lambkin, C.L.; Barker, S.C.; Whiting, M.F. A mitochondrial genome phylogeny of Diptera: Whole genome sequence data accurately resolve relationships over broad timescales with high precision. Syst. Èntomol. 2007, 32, 40–59. [Google Scholar] [CrossRef]
- Chang, H.; Qiu, Z.; Yuan, H.; Wang, X.; Li, X.; Sun, H.; Guo, X.; Lu, Y.; Feng, X.; Majid, M.; et al. Evolutionary rates of and selective constraints on the mitochondrial genomes of Orthoptera insects with different wing types. Mol. Phylogenet. Evol. 2020, 145, 106734. [Google Scholar] [CrossRef]
- Yuan, M.-L.; Zhang, Q.-L.; Zhang, L.; Guo, Z.-L.; Liu, Y.-J.; Shen, Y.-Y.; Shao, R. High-level phylogeny of the Coleoptera inferred with mitochondrial genome sequences. Mol. Phylogenet. Evol. 2016, 104, 99–111. [Google Scholar] [CrossRef]
- Tang, P.; Zhu, J.-C.; Zheng, B.-Y.; Wei, S.-J.; Sharkey, M.; Chen, X.-X.; Vogler, A.P. Mitochondrial phylogenomics of the Hymenoptera. Mol. Phylogenet. Evol. 2018, 131, 8–18. [Google Scholar] [CrossRef]
- Anderson, N.; Jaron, K.S.; Hodson, C.N.; Couger, M.B.; Ševčík, J.; Weinstein, B.; Pirro, S.; Ross, L.; Roy, S.W. Gene-rich X chromosomes implicate intragenomic conflict in the evolution of bizarre genetic systems. Proc. Natl. Acad. Sci. USA 2022, 119, e2122580119. [Google Scholar] [CrossRef]
- Lu, B.L.; Dong, X.S. Fauna Sinica, Insecta, Diptera: Culicidae 1.8; Science Press: Beijing, China, 1997. [Google Scholar]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Dat. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 6 October 2011).
- Ankevich, B.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2016, 45, e18. [Google Scholar]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Eddy, S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1996, 25, 955–964. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [Green Version]
- Grant, J.R.; Arantes, A.S.; Stothard, P. Comparing thousands of circular genomes using the CGView Comparison Tool. BMC Genom. 2012, 13, 202. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2759. [Google Scholar] [CrossRef] [Green Version]
- Mikrajuddin, A.K. A simple method for determining surface porosity based on SEM images using Origin Pro software. Indones. J. Phys. 2009, 20, 37–41. [Google Scholar]
- Julio, R.; Albert, F.M.; Carlos, J.S.D.; Sara, G.R.; Pablo, L.; Sebastián, E.R.O.; Alejandro, S.G. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minh, B.Q.; Nguyen, M.A.T.; Von Haeseler, A. Ultrafast Approximation for Phylogenetic Bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, FigTree (Version 1.4.3). Available online: https://tree.bio.ed.ac.uk/software/figtree/.2009 (accessed on 10 September 2020).
- Letunic, P.B. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, 242–245. [Google Scholar] [CrossRef]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- Guo, Z.; Yan, W.; Fu, H.; Yuan, X.L.; Chen, B. Complete mitogenomes of Anopheles peditaeniatus and Anopheles nitidus and phylogenetic relationships within the genus Anopheles inferred from mitogenomes. Parasite. Vector. 2021, 14, 452. [Google Scholar] [CrossRef]
- Hao, Y.-J.; Zou, Y.-L.; Ding, Y.-R.; Xu, W.-Y.; Yan, Z.-T.; Li, X.-D.; Fu, W.-B.; Li, T.-J.; Chen, B. Complete mitochondrial genomes of Anopheles stephensi and An. dirus and comparative evolutionary mitochondriomics of 50 mosquitoes. Sci. Rep. 2017, 7, 7666. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, J.; Wu, H. Mitogenomes provide insights into the phylogeny of Mycetophilidae (Diptera: Sciaroidea). Gene 2021, 783, 145564. [Google Scholar] [CrossRef] [PubMed]
- Ramakodi, M.P.; Singh, B.; Wells, J.D.; Guerrero, F.; Ray, D.A. A 454 sequencing approach to dipteran mitochondrial genome research. Genomics 2015, 105, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Beard, C.B.; Hamm, D.M.; Collins, F.H. The mitochondrial genome of the mosquito Anopheles gambiae: DNA sequence, genome organization, and comparisons with mitochondrial sequences of other insects. Insect Mol. Biol. 1993, 2, 103–124. [Google Scholar] [CrossRef]
- Wiegmann, B.M.; Trautwein, M.D.; Winkler, I.S.; Barr, N.B.; Kim, J.-W.; Lambkin, C.; Bertone, M.A.; Cassel, B.K.; Bayless, K.M.; Heimberg, A.M.; et al. Episodic radiations in the fly tree of life. Proc. Natl. Acad. Sci. USA 2011, 108, 5690–5695. [Google Scholar] [CrossRef]
- Blagoderov, V.; Grimaldi, D.A.; Fraser, N.C. How Time Flies for Flies: Diverse Diptera from the Triassic of Virginia and Early Radiation of the Order. Am. Mus. Novit. 2007, 3572, 1–39. [Google Scholar] [CrossRef]
- Shcherbakov, D.E.; Lukashevich, E.D.; Blagoderov, V.A. Triassic Diptera and initial radiation of the order. Int. J. Dipt. Res. 1995, 6, 75–115. [Google Scholar]
- Krzeminski, W.; Krzeminska, E. Triassic Diptera: Descriptions, revisions and phylogenetic relations. Acta Zool. Cracov. 2003, 46, 153–184. [Google Scholar]
- Evenhuis, N.L. Catalogue of the Fossil Flies of the World; Backhuys Publishers: Leiden, The Netherlands, 1994; pp. 122–133. [Google Scholar]
- Blagoderov, V.A.; Arillo, A. New Sciaroidea (Insecta: Diptera) in Lower Cretaceous amber from Spain. Stud. Dipterol. 2002, 9, 31–40. [Google Scholar]
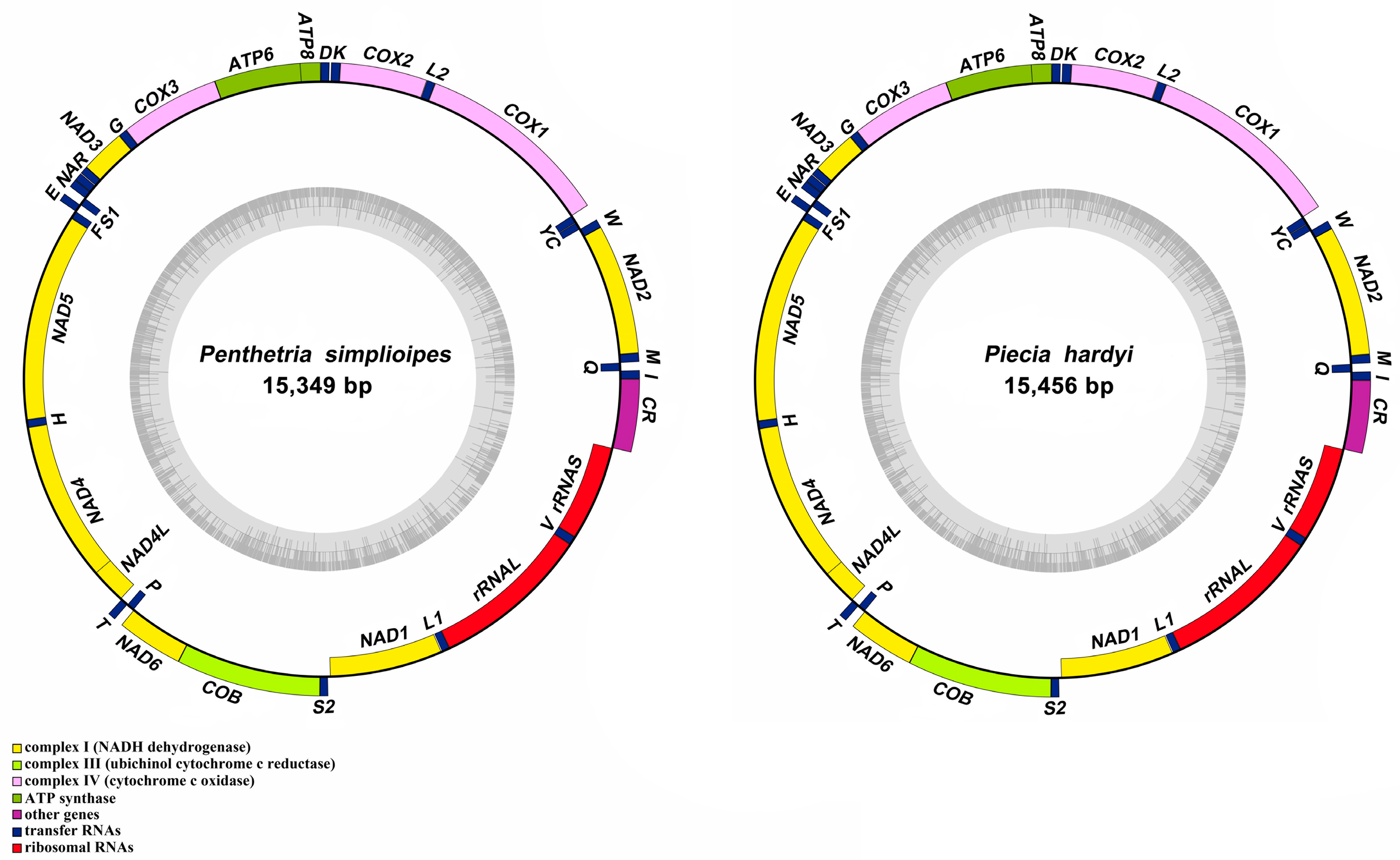




| Gene | Strand | Position (bp) | Length (bp) | Space (+)/Overlap (−) | Start/Stop Codon | ||||
|---|---|---|---|---|---|---|---|---|---|
| PSI | PHA | PSI | PHA | PSI | PHA | PSI | PHA | ||
| trnI | J | 1–67 | 1–71 | 67 | 71 | 0 | 0 | ||
| trnQ | N | 84–152 | 68–138 | 69 | 71 | 16 | −4 | ||
| trnM | J | 211–279 | 136–204 | 69 | 69 | 58 | −3 | ||
| nad2 | J | 279–1325 | 204–1244 | 1047 | 1041 | −1 | −1 | ATA/TAA | ATT/TAA |
| trnW | J | 1326–1395 | 1266–1334 | 70 | 69 | 0 | 21 | ||
| trnC | N | 1394–1459 | 1327–1390 | 66 | 64 | −2 | −8 | ||
| trnY | N | 1465–1533 | 1398–1466 | 69 | 69 | 5 | 7 | ||
| cox1 | J | 1568–3065 | 1501–2998 | 1498 | 1498 | 34 | 34 | ATT/T | ATC/T |
| trnL1 | J | 3065–3132 | 2998–3065 | 68 | 68 | −1 | −1 | ||
| cox2 | J | 3137–3820 | 3069–3761 | 684 | 693 | 4 | 3 | ATT/TAA | ATG/TAA |
| trnK | J | 3824–3896 | 3761–3831 | 73 | 71 | 3 | −1 | ||
| trnD | J | 3903–3974 | 3837–3905 | 72 | 69 | 6 | 5 | ||
| atp8 | J | 3974–4132 | 3906–4067 | 159 | 162 | −1 | 0 | ATT/TAA | ATT/TAA |
| atp6 | J | 4126–4803 | 4061–4735 | 678 | 675 | −7 | −7 | ATG/TAA | ATG/TAA |
| cox3 | J | 4805–5593 | 4739–5527 | 789 | 789 | 1 | 3 | ATG/TAA | ATG/TAA |
| trnG | J | 5594–5660 | 5528–5595 | 67 | 68 | 0 | 0 | ||
| nad3 | J | 5661–6014 | 5596–5947 | 354 | 352 | 0 | 0 | ATT/TAA | ATT/TAA |
| trnR | J | 6020–6085 | 5947–6011 | 66 | 65 | 5 | −1 | ||
| trnA | J | 6083–6149 | 6011–6079 | 67 | 69 | −3 | −1 | ||
| trnN | J | 6149–6218 | 6079–6147 | 70 | 69 | −1 | −1 | ||
| trnS2 | N | 6218–6286 | 6152–6207 | 69 | 56 | −1 | 4 | ||
| trnE | J | 6286–6356 | 6216–6281 | 71 | 66 | −1 | 8 | ||
| trnF | N | 6355–6421 | 6281–6352 | 67 | 72 | −2 | −1 | ||
| nad5 | N | 6421–8152 | 6352–8083 | 1732 | 1732 | −1 | −1 | ATT/T | ATT/T |
| trnH | N | 8153–8219 | 8084–8148 | 67 | 65 | 0 | 0 | ||
| nad4 | N | 8219–9554 | 8149–9489 | 1336 | 1341 | −1 | 0 | ATT/TAA | ATG/TAA |
| nad4L | N | 9548–9844 | 9480–9776 | 297 | 297 | −7 | −10 | ATG/TAA | ATG/TAA |
| trnT | J | 9852–9914 | 9785–9849 | 63 | 65 | 7 | 8 | ||
| trnP | N | 9914–9981 | 9849–9916 | 68 | 68 | −1 | −1 | ||
| nad6 | J | 9983–10,501 | 9918–10,436 | 519 | 519 | 1 | 1 | ATT/TAA | ATA/TAA |
| cytb | J | 10,501–11,637 | 10,445–11,581 | 1137 | 1137 | −1 | 8 | ATG/TAA | ATG/TAA |
| trnS1 | J | 11,641–11,712 | 11,584–11,654 | 72 | 71 | 3 | 2 | ||
| nad1 | N | 11,726–12,658 | 11,672–12,628 | 933 | 957 | 13 | 17 | TTG/TAG | ATT/TAG |
| trnL2 | N | 12,677–12,745 | 12,629–12,700 | 69 | 72 | 18 | 0 | ||
| rrnL | N | 12,746–13,752 | 12,701–14,050 | 1007 | 1350 | 0 | 0 | ||
| trnV | N | 13,753–13,826 | 14,051–14,124 | 74 | 74 | 0 | 0 | ||
| rrnS | N | 13,827–14,611 | 14,125–14,909 | 785 | 785 | 0 | 0 | ||
| CR | 14,612–15,349 | 14,910–15,465 | 738 | 556 | 0 | 0 | |||
| Superfamily | Species | Total | PCGs | tRNA | rRNA | CR | GenBank |
|---|---|---|---|---|---|---|---|
| /Family | Size (bp) | Size (bp) | Size (bp) | Size (bp) | Size (bp) | ID | |
| Bibionoidea | |||||||
| Bibionidae | Penthetria simplioipes | 15,349 | 11,163 | 1513 | 1792 | 738 | MT511121 |
| Piecia hardyi | 15,456 | 11,193 | 1493 | 2084 | 556 | MT511122 | |
| Pachyneuridae | Cramptonomyia spenceri | 16,274 | 11,223 | 1477 | 2138 | 1068 | NC016203 |
| Scatopsoidea | |||||||
| Scatopsidae | Coboldia fuscipes | 15,309 | 11,167 | 1470 | 2111 | 510 | MZ567016 |
| Anisopodoidea | |||||||
| Anisopodidae | Sylvicola fenestralis | 16,234 | 11,237 | 1432 | 2133 | 1232 | NC016176 |
| Sciaroidea | |||||||
| Cecidomyiidae | Mayetiola destructor | 14,759 | 11,209 | 1474 | 2048 | 604 | NC013066 |
| Orseoli aoryzae | 15,286 | 10,794 | 1474 | 1988 | 578 | NC027680 | |
| Rhopalomyia pomum | 14,503 | 10,897 | 1477 | 1995 | 363 | GQ387649 | |
| Sciaridae | Sciara ruficauda | 15,167 | 11,204 | 1476 | 2116 | N/A | MN161586 |
| Bradysia sp. | 15,512 | 11,124 | 1377 | 2130 | N/A | MN161585 | |
| Bradysia amoena | 14,049 | 10,032 | 1484 | 1962 | N/A | NC057971 | |
| Bradysia impatiens | 16,479 | 11,217 | 1450 | 2185 | 370 | MZ202360 | |
| Pnyxia scabiei | 15,437 | 11,215 | 1463 | 2128 | N/A | NC053636 | |
| Trichosia lengersdorfi | 16,171 | 11,222 | 1477 | 2212 | N/A | MN161589 | |
| Pseudolycoriella sp. | 15,981 | 11,224 | 1482 | 2163 | N/A | MN161587 | |
| Dolichosciara megumiae | 15,931 | 11,114 | 1423 | 2132 | N/A | MN161588 | |
| Mycetophilidae | Neoempheria proxima | 15,853 | 11,205 | 1469 | 2112 | 783 | MW116802 |
| Epicypta sp. | 16,279 | 11,199 | 1457 | 2116 | 6145 | MW116800 | |
| Epicypta xiphothorna | 15,902 | 11,196 | 1452 | 2132 | 563 | MW116799 | |
| Azana sp. | 16,654 | 11,226 | 1507 | 2171 | 762 | MW116798 | |
| Acnemia nitidicollis | 16,950 | 11,206 | 1478 | 2132 | N/A | NC050318 | |
| Allodia sp. | 14,899 | 11,199 | 1465 | 2077 | N/A | MN310892 | |
| Allodia anglofennica | 14,897 | 11,196 | 1467 | 2074 | N/A | MN310891 | |
| Keroplatidae | Arachnocampa flava | 16,923 | 11,202 | 1482 | 2103 | 1841 | NC016204 |
| Outgroup (Tipulomorpha) | |||||||
| Tipulidae | Symplecta hybrida | 15,811 | 11,167 | 1456 | 2109 | 897 | NC030519 |
| Tipula cockerelliana | 14,453 | 11,154 | 1378 | 1837 | N/A | NC030520 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, M.-L.; Yuan, H.; Li, T.-J.; Chen, B. Two New Mitogenomes of Bibionidae and Their Comparison within the Infraorder Bibionomorpha (Diptera). Genes 2023, 14, 1485. https://doi.org/10.3390/genes14071485
Xiao M-L, Yuan H, Li T-J, Chen B. Two New Mitogenomes of Bibionidae and Their Comparison within the Infraorder Bibionomorpha (Diptera). Genes. 2023; 14(7):1485. https://doi.org/10.3390/genes14071485
Chicago/Turabian StyleXiao, Mei-Ling, Huan Yuan, Ting-Jing Li, and Bin Chen. 2023. "Two New Mitogenomes of Bibionidae and Their Comparison within the Infraorder Bibionomorpha (Diptera)" Genes 14, no. 7: 1485. https://doi.org/10.3390/genes14071485






