Investigating the Migratory Behavior of Soybean Looper, a Major Pest of Soybean, through Comparisons with the Corn Pest Fall Armyworm Using Mitochondrial Haplotypes and a Sex-Linked Marker
Abstract
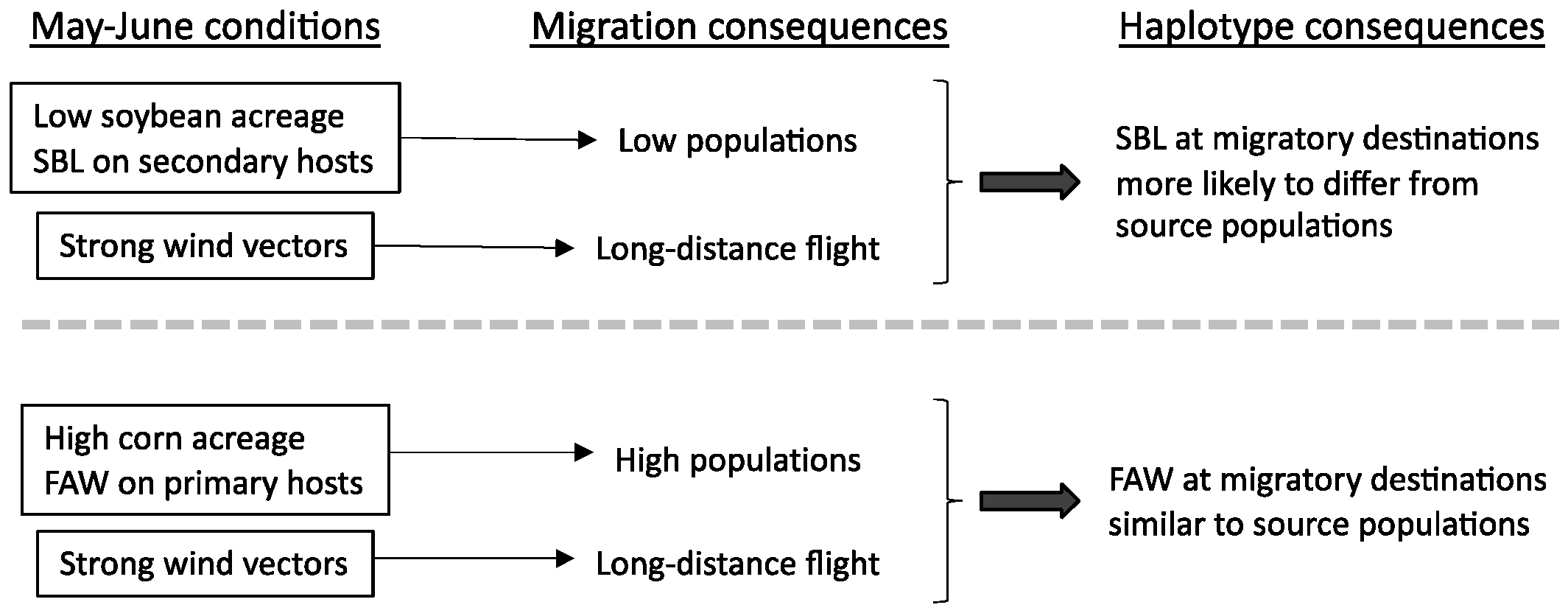
:1. Introduction
2. Materials and Methods
2.1. Specimen Collections
2.2. DNA Preparation
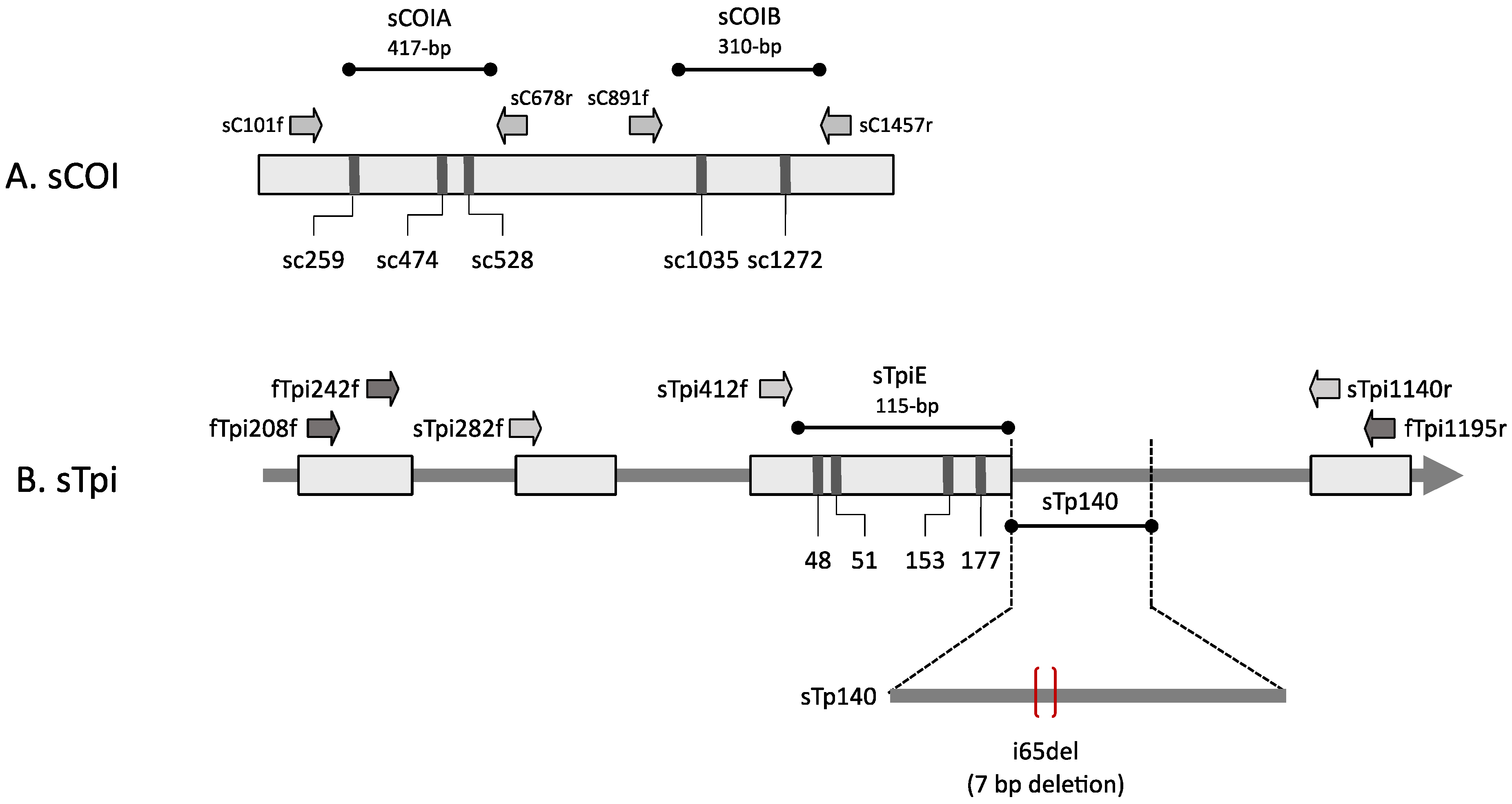
2.3. Isolation of the DNA Segments by Polymerase Chain Reaction (PCR)
2.4. Characterization of Haplotypes
2.5. DNA Sequence Analysis, Statistics, and Data Availability
3. Results
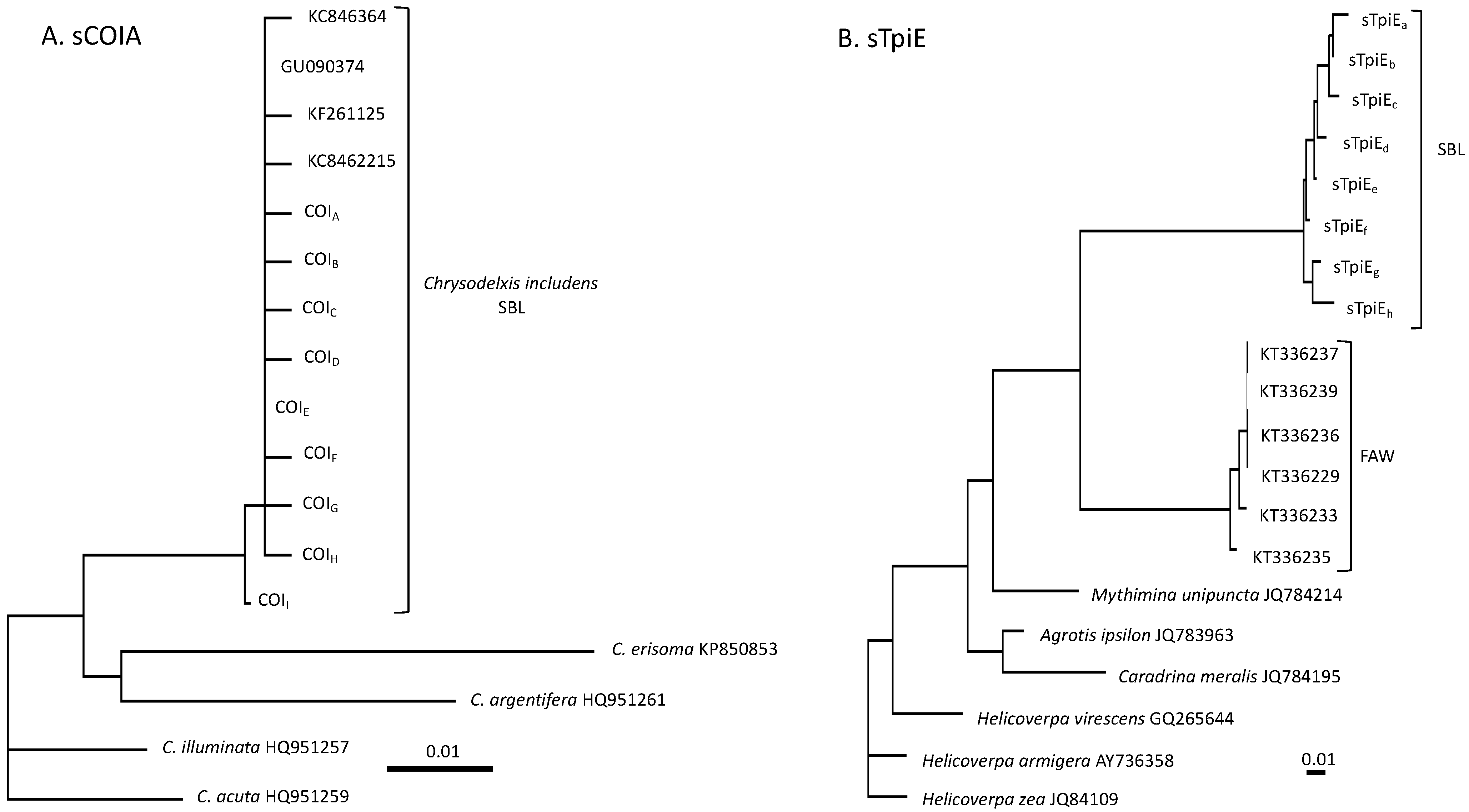
3.1. Characterization of SBL Genetic Markers
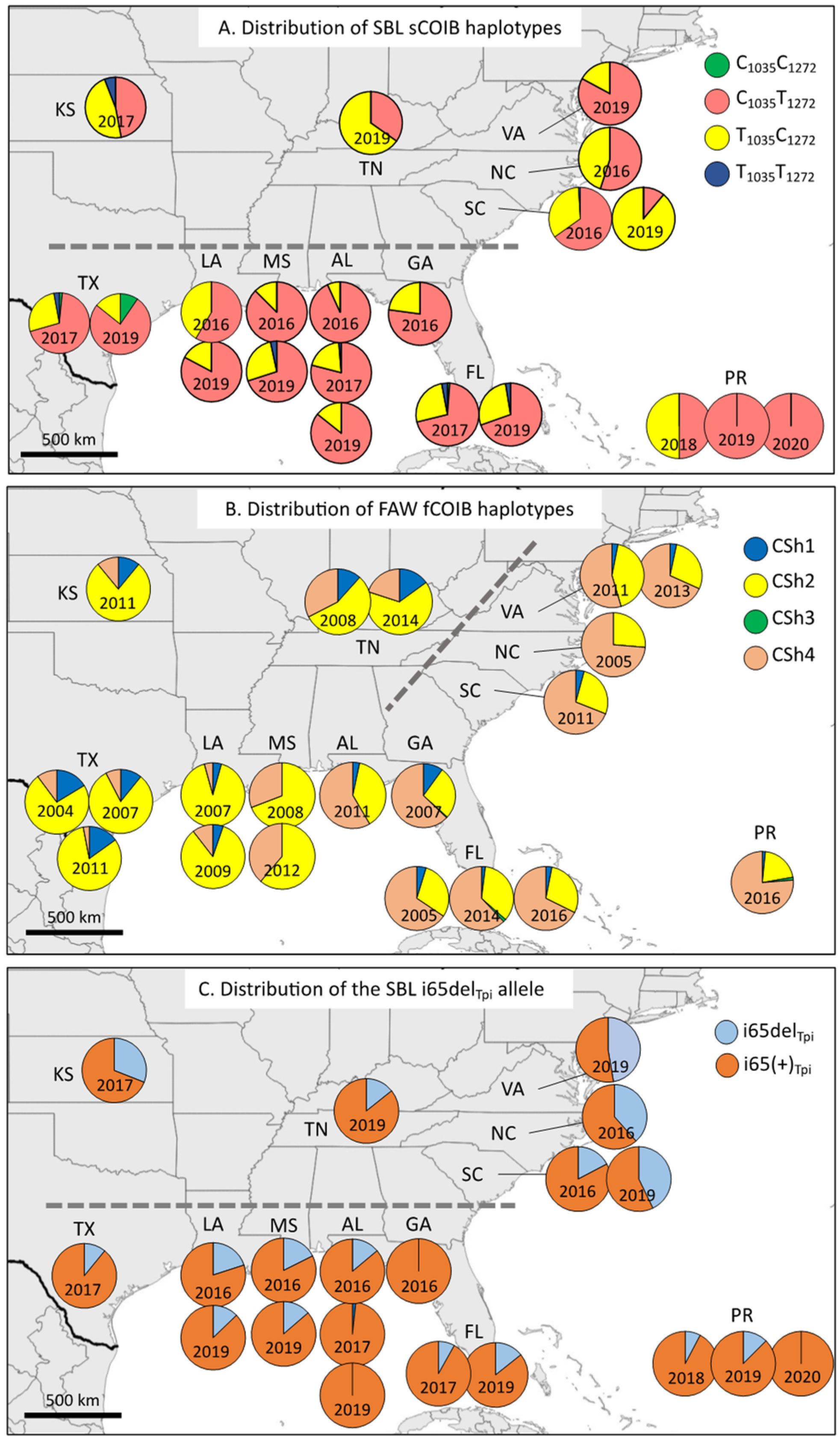
3.2. Comparisons between the SBL and FAW COIB Haplotype Profiles
3.3. Using an Intron Segment from Tpi as a Genetic Marker
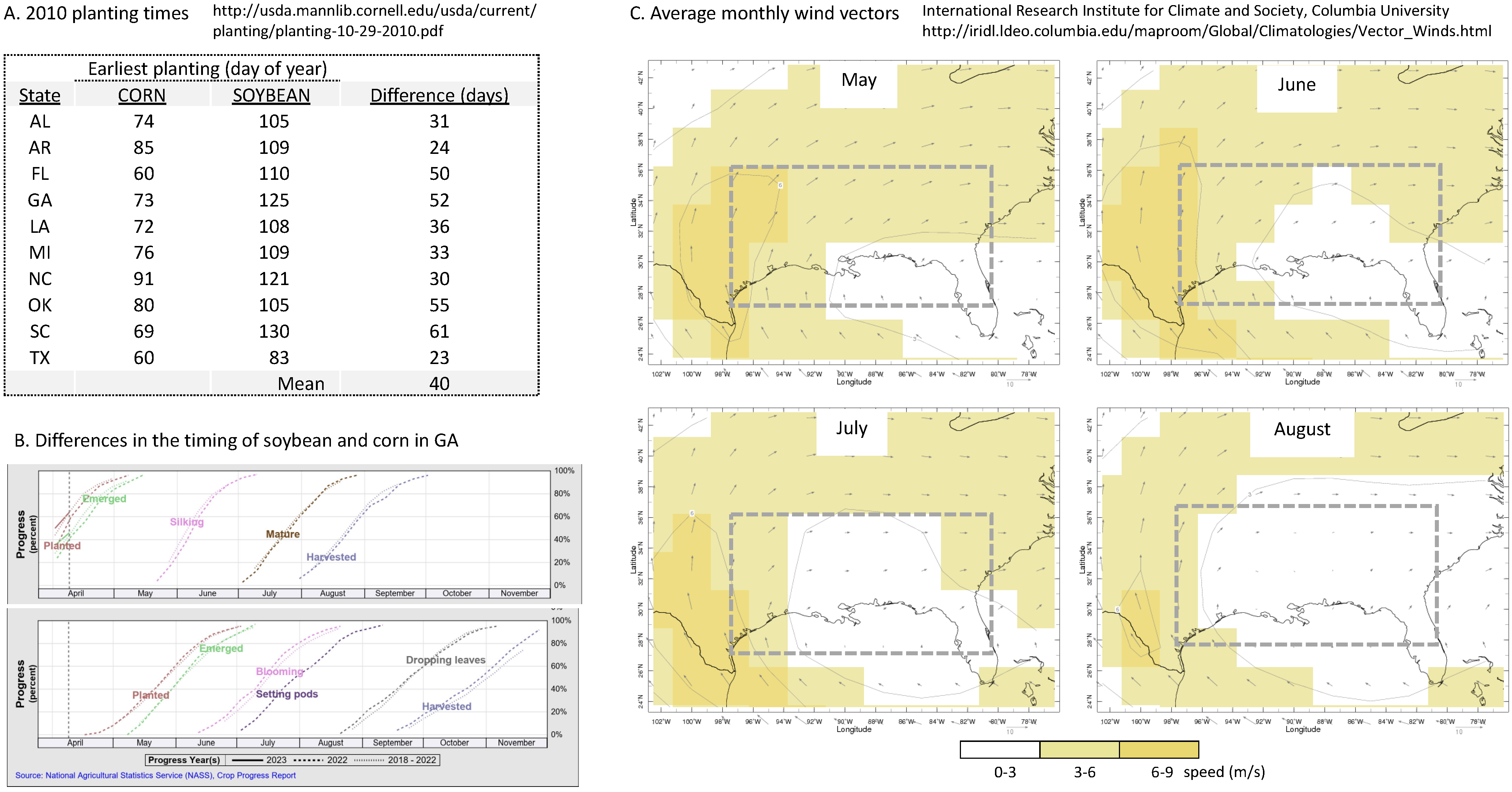
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NASS. Southern Region News Release. Available online: https://www.nass.usda.gov/Statistics_by_State/Regional_Office/Southern/includes/Publications/Crop_Releases/Acreage/ACREAGE2022.pdf#:~:text=The%20U.S.%20Department%20of%20Agriculture%27s%20National%20Agricultural%20Statistics,million%20acres%2C%20up%201%20percent%20from%20last%20year. (accessed on 30 June 2022).
- Meese, B.G.; Carter, P.R.; Oplinger, E.S.; Pendleton, J.W. Corn soybean rotation effect as influenced by tillage, nitrogen, and hybrid cultivar. J. Prod. Agric. 1991, 4, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Wallner, W.E. Factors affecting insect population-dynamics—Differences between outbreak and non-outbreak species. Annu. Rev. Entomol. 1987, 32, 317–340. [Google Scholar] [CrossRef]
- Soares, J.R.S.; da Silva, R.S.; Ramos, R.S.; Picanco, M.C. Distribution and invasion risk assessments of Chrysodeixis includens (Walker, [1858]) (Lepidoptera: Noctuidae) using CLIMEX. Int. J. Biometeorol. 2021, 65, 1137–1149. [Google Scholar] [CrossRef]
- Timilsena, B.P.; Niassy, S.; Kimathi, E.; Abdel-Rahman, E.M.; Seidl-Adams, I.; Wamalwa, M.; Tonnang, H.E.Z.; Ekesi, S.; Hughes, D.P.; Rajotte, E.G.; et al. Potential distribution of fall armyworm in Africa and beyond, considering climate change and irrigation patterns. Sci. Rep. 2022, 12, 539. [Google Scholar] [CrossRef]
- Sparks, A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 82–86. [Google Scholar] [CrossRef]
- Barros, E.M.; Torres, J.B.; Ruberson, J.R.; Oliveira, M.D. Development of Spodoptera frugiperda on different hosts and damage to reproductive structures in cotton. Entomol. Exp. Appl. 2010, 137, 237–245. [Google Scholar] [CrossRef]
- Westbrook, J.; Fleischer, S.; Jairam, S.; Meagher, R.; Nagoshi, R. Multigenerational migration of fall armyworm, a pest insect. Ecosphere 2019, 10, e02919. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.F.; McCracken, G.F. Dietary variation of Brazilian free-tailed bats links to migratory populations of pest insects. J. Mammal. 2005, 86, 67–76. [Google Scholar] [CrossRef]
- Westbrook, J.K.; Raulston, J.R.; Wolf, W.W.; Pair, S.D.; Eyster, R.S.; Lingren, P.D. Field observations and simulations of atmospheric transport of Noctuids from northeastern Mexico and the south-central US. Southwest Entomol. Suppl. 1995, 18, 25–44. [Google Scholar]
- Westbrook, J.K. Noctuid migration in Texas within the nocturnal aeroecological boundary layer. Integr. Comp. Biol. 2008, 48, 99–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, W.W.; Westbrook, J.K.; Raulston, J.R.; Pair, S.D.; Lingren, P.D. Radar observations of orientation of Noctuids migrating from corn fields in the lower Rio-Grande valley. Southwest Entomol. 1995, 18, 45–61. [Google Scholar]
- Nagoshi, R.N.; Fleischer, S.J.; Meagher, R.L. Texas is the overwintering source of fall armyworm in central Pennsylvania: Implications for migration into the northeastern United States. Environ. Entomol. 2009, 38, 1546–1554. [Google Scholar] [CrossRef] [Green Version]
- Nagoshi, R.N.; Meagher, R.L.; Hay-Roe, M. Inferring the annual migration patterns of fall armyworm (Lepidoptera: Noctuidae) in the United States from mitochondrial haplotypes. Ecol. Evol. 2012, 2, 1458–1467. [Google Scholar] [CrossRef]
- Tessnow, A.; Nagoshi, R.N.; Meagher, R.; Westbrook, J.K.; Fleischer, S.J. Revisiting Fall Armyworm Population Movement in the US. Front. Insect Sci. 2023; in press. [Google Scholar] [CrossRef]
- Mitchell, E.R.; Chalfant, R.B.; Green, G.L.; Creighton, C.S. Soybean looper Lepidoptera-Noctuidae—Populations in Florida, Georgia, and South Carolina, as determined with pheromone-baited Bl Traps. J. Econ. Entomol. 1975, 68, 747–750. [Google Scholar] [CrossRef]
- Alford, A.R.; Hammond, A.M. Plusiinae (Lepidoptera, Noctuidae) Populations in Louisiana Soybean Ecosystems as Determined with Looplure-Baited Traps. J. Econ. Entomol. 1982, 75, 647–650. [Google Scholar] [CrossRef]
- Boernel, D.J.; Mink, J.S.; Wier, A.T.; Thomas, J.D.; Leonard, B.R.; Gallardo, F. Management of insecticide resistant soybean loopers (Pseudoplusia includens) in the southern United States. In Pest Management in Soybean; Springer: Dordrecht, The Netherlands, 1992; pp. 66–87. [Google Scholar]
- Mascarenhas, R.N.; Boethel, D.J. Responses of Field-Collected Strains of Soybean Looper (Lepidoptera: Noctuidae) to Selected Insecticides Using an Artificial Diet Overlay Bioassay. J. Econ. Entomol. 1997, 90, 1117–1124. [Google Scholar] [CrossRef]
- Portillo, H.; Felland, C.; Pitre, H.; Porter, R. Pyrethroid resistance levels in soybean looper (Lepidoptera: Noctuidae) in Mississippi. Fla. Entomol. 1993, 76, 577–584. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L.; Hay-Roe, M. Assessing the resolution of haplotype distributions to delineate fall armyworm (Lepidoptera: Noctuidae) migratory behaviors. J. Econ. Entomol. 2014, 107, 1462–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagoshi, R.N.; Silvie, P.; Meagher, R.L. Comparison of haplotype frequencies differentiate fall armyworm (Lepidoptera: Noctuidae) corn-strain populations from Florida and Brazil. J. Econ. Entomol. 2007, 100, 954–961. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; DeWaard, J.R. Biological identifications through DNA barcodes. P. Roy Soc. B-Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L.; Flanders, K.; Gore, J.; Jackson, R.; Lopez, J.; Armstrong, J.S.; Buntin, G.D.; Sansone, C.; Leonard, B.R. Using haplotypes to monitor the migration of fall armyworm (Lepidoptera:Noctuidae) corn-strain populations from Texas and Florida. J. Econ. Entomol. 2008, 101, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Nagoshi, R.N. The fall armyworm Triosephosphate isomerase (Tpi) gene as a marker of strain identity and interstrain mating. Ann. Entomol. Soc. Am. 2010, 103, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Nagoshi, R.N.; Vizuete, J.L.A.; Murua, M.G.; Garces-Carrera, S. Comparisons of fall armyworm haplotypes between the Galapagos Islands and mainland Ecuador indicate limited migration to and between islands. Sci. Rep. 2021, 11, 3457. [Google Scholar] [CrossRef]
- Drummond, A.J.; Ashton, B.; Buxton, S.; Cheung, M.; Cooper, A.; Duran, C.; Field, M.; Heled, J.; Kearse, M.; Markowitz, S.; et al. Geneious, v5.5; Geneious: Auckland, New Zealand, 2010. [Google Scholar]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef]
- Palma, J.; Maebe, K.; Guedes, J.V.C.; Smagghe, G. Molecular variability and genetic structure of Chrysodeixis includens (Lepidoptera: Noctuidae), an important soybean defoliator in Brazil. PLoS ONE 2015, 10, e0121260. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.S.; Cordeiro, E.M.G.; de Paiva, J.B.; Dourado, P.M.; Carvalho, R.A.; Head, G.; Martinelli, S.; Correa, A.S. Population expansion and genomic adaptation to agricultural environments of the soybean looper, Chrysodeixis includens. Evol. Appl. 2020, 13, 2071–2085. [Google Scholar] [CrossRef] [Green Version]
- Nagoshi, R.N. Observations of genetic differentiation between the fall armyworm host strains. PLoS ONE 2022, 1717, e0277510. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Meagher, R.L. Using intron sequence comparisons in the Triose-phosphate isomerase gene to study the divergence of the fall armyworm host strains. Insect Mol. Biol. 2016, 25, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Catchot, A.; Gore, J.; Cook, D. Diamide Insecticides and Soybean Looper. Available online: https://www.mississippi-crops.com/2016/08/12/diamide-insecticides-and-soybean-loopermanagement/ (accessed on 24 April 2023).

| Collection | State | Year | Type | Collector |
|---|---|---|---|---|
| AL2016 | Alabama | 2016 | F1 larva | J. Davis |
| AL2017 | Alabama | 2017 | Pheromone trap | R. Meagher |
| AL2019 | Alabama | 2019 | F1–2 larva | F. Musser, B. Catchot |
| FL2017 | Florida | 2017 | Pheromone trap | R. Meagher |
| FL2019 | Florida | 2019 | Pheromone trap | R. Meagher |
| GA2016 | Georgia | 2016 | F1 larva | J. Davis |
| KS2017 | Kansas | 2017 | Pheromone trap | B. McCornack |
| LA2016 | Louisiana | 2016 | F1 larva | J. Davis |
| LA2019 | Louisiana | 2019 | F1–2 larva | F. Musser, B. Catchot |
| MS2016 | Mississippi | 2016 | F1 larva | J. Davis |
| MS2019a | Mississippi | 2019 | Pheromone trap | R. Meagher |
| MS2019b | Mississippi | 2019 | F1–2 larva | F. Musser, B. Catchot |
| NC2016 | N. Carolina | 2016 | F1 larva | J. Davis |
| PR2018 | Puerto Rico | 2018 | larva from field | H. Portillo |
| PR2019 | Puerto Rico | 2019 | larva from field | H. Teran |
| PR2020 | Puerto Rico | 2020 | F1–2 larva | F. Musser, B. Catchot |
| SC2016 | S. Carolina | 2016 | F1 larva | J. Davis |
| SC2019 | S. Carolina | 2019 | F1–2 larva | F. Musser, B. Catchot |
| TN2019 | Tennessee | 2019 | F1–2 larva | F. Musser, B. Catchot |
| TX2017 | Texas | 2017 | Pheromone trap | R. Parker |
| VA2019 | Virginia | 2019 | F1–2 larva | F. Musser, B. Catchot |
| SNP | n | Polymorphism | Frequency |
|---|---|---|---|
| sCOIA474 | 332 | C/T | 99% C |
| sCOIA528 | 332 | C/T | 98% T |
| sCOIB1035 | 316 | C/T | 67% C |
| sCOIB1272 | 316 | C/T | 68% T |
| sTpiE48 | 249 | A/G | 97% A |
| sTpiE51 | 242 | A/G | 90% G |
| sTpiE153 | 259 | C/T | 97% C |
| sTpiE177 | 264 | C/T | 97% C |
| Fall Armyworm (FAW) | Soybean Looper (SBL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Region | East 1 | West 2 | North 3 | South 4 | East 1 | West 2 | North 3 | South 4 |
| Mean M 5 | 0.37 | −0.53 | −0.09 | −0.18 | 0.29 | 0.46 | 0.00 | 0.56 |
| sd | 0.11 | 0.34 | 0.50 | 0.55 | 0.47 | 0.36 | 0.50 | 0.18 |
| East vs. West | North vs. South | East vs. West | North vs. South | |||||
| p | <0.0001 | 0.7306 | 0.3871 | 0.0017 | ||||
| t | 7.180 | 0.3500 | 0.8876 | 3.715 | ||||
| df | 17 | 16 | 17 | 17 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagoshi, R.N.; Davis, J.A.; Meagher, R.L.; Musser, F.R.; Head, G.P.; Portillo, H.; Teran, H. Investigating the Migratory Behavior of Soybean Looper, a Major Pest of Soybean, through Comparisons with the Corn Pest Fall Armyworm Using Mitochondrial Haplotypes and a Sex-Linked Marker. Genes 2023, 14, 1495. https://doi.org/10.3390/genes14071495
Nagoshi RN, Davis JA, Meagher RL, Musser FR, Head GP, Portillo H, Teran H. Investigating the Migratory Behavior of Soybean Looper, a Major Pest of Soybean, through Comparisons with the Corn Pest Fall Armyworm Using Mitochondrial Haplotypes and a Sex-Linked Marker. Genes. 2023; 14(7):1495. https://doi.org/10.3390/genes14071495
Chicago/Turabian StyleNagoshi, Rodney N., Jeffrey A. Davis, Robert L. Meagher, Fred R. Musser, Graham P. Head, Hector Portillo, and Henry Teran. 2023. "Investigating the Migratory Behavior of Soybean Looper, a Major Pest of Soybean, through Comparisons with the Corn Pest Fall Armyworm Using Mitochondrial Haplotypes and a Sex-Linked Marker" Genes 14, no. 7: 1495. https://doi.org/10.3390/genes14071495







