The Causal Relationship between PCSK9 Inhibitors and Malignant Tumors: A Mendelian Randomization Study Based on Drug Targeting
Abstract
:1. Introduction
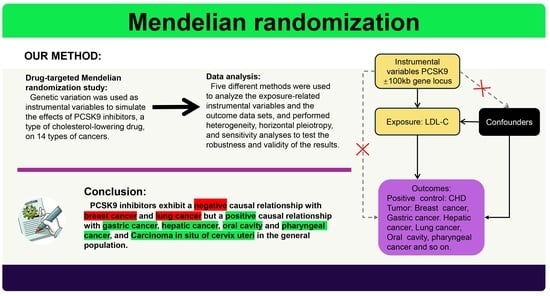
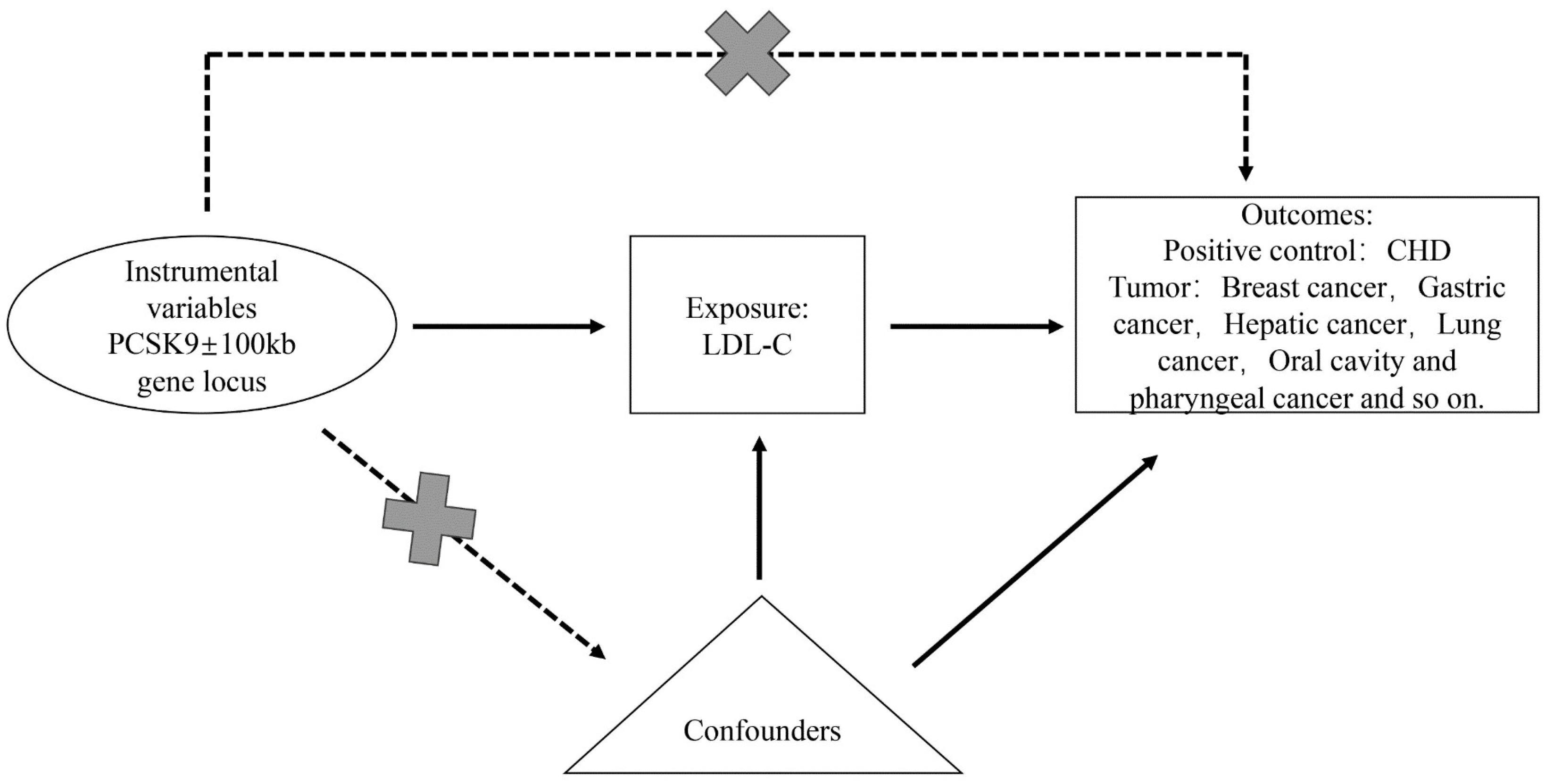
2. Materials and Methods
2.1. Study Design
2.2. Selection of Instrumental Variables
2.3. Sources of Outcome Data
2.4. Reverse Mendelian Randomization Validation
2.5. Data Analysis
3. Result
3.1. Causal Relationship between PCSK9 Inhibitors and CHD
3.2. Causal Relationship between PCSK9 Inhibitors and Tumors
3.3. Sensitivity, Heterogeneity, and Horizontal Pleiotropy Analysis
3.4. Reverse Mendelian Randomization Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Pandya, P.; Afshar, S. Therapeutic Advances in Oncology. Int. J. Mol. Sci. 2021, 22, 2008. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Yang, E.J.; Shim, J.S. Cholesterol Trafficking: An Emerging Therapeutic Target for Angiogenesis and Cancer. Cells 2019, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Revilla, G.; Cedó, L.; Tondo, M.; Moral, A.; Pérez, J.I.; Corcoy, R.; Lerma, E.; Fuste, V.; Reddy, S.T.; Blanco-Vaca, F.; et al. LDL, HDL and endocrine-related cancer: From pathogenic mechanisms to therapies. Semin. Cancer Biol. 2021, 73, 134–157. [Google Scholar] [CrossRef] [PubMed]
- Murtola, T.J.; Syvälä, H.; Pennanen, P.; Bläuer, M.; Solakivi, T.; Ylikomi, T.; Tammela, T.L.J. The Importance of LDL and Cholesterol Metabolism for Prostate Epithelial Cell Growth. PLoS ONE 2012, 7, e39445. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.Y.; Ko, J.; Um, J.; Chinnathambi, A.; Alharbi, S.A.; Sethi, G.; Ahn, K.S. LDL cholesterol promotes the proliferation of prostate and pancreatic cancer cells by activating the STAT3 pathway. J. Cell. Physiol. 2021, 236, 5253–5264. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, A.; Watts, E.L.; Perez-Cornago, A.; Platz, E.A.; Mills, I.G.; Key, T.J.; Travis, R.C.; Tsilidis, K.K.; Zuber, V. The relationship between lipoprotein A and other lipids with prostate cancer risk: A multivariable Mendelian randomisation study. PLoS Med. 2022, 19, e1003859. [Google Scholar] [CrossRef]
- Bull, C.J.; Bonilla, C.; Holly, J.M.P.; Perks, C.M.; Davies, N.; Haycock, P.; Yu, O.H.Y.; Richards, J.B.; Eeles, R.; Easton, D.; et al. Blood lipids and prostate cancer: A Mendelian randomization analysis. Cancer Med. 2016, 5, 1125–1136. [Google Scholar] [CrossRef]
- Jamnagerwalla, J.; Howard, L.E.; Allott, E.H.; Vidal, A.C.; Moreira, D.M.; Castro-Santamaria, R.; Andriole, G.L.; Freeman, M.R.; Freedland, S.J. Serum cholesterol and risk of high-grade prostate cancer: Results from the REDUCE study. Prostate Cancer Prostatic Dis. 2018, 21, 252–259. [Google Scholar] [CrossRef]
- YuPeng, L.; YuXue, Z.; PengFei, L.; Cheng, C.; YaShuang, Z.; DaPeng, L.; Chen, D. Cholesterol Levels in Blood and the Risk of Prostate Cancer: A Meta-analysis of 14 Prospective Studies. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1086–1093. [Google Scholar] [CrossRef]
- Martin, L.J.; Melnichouk, O.; Huszti, E.; Connelly, P.W.; Greenberg, C.V.; Minkin, S.; Boyd, N.F. Serum Lipids, Lipoproteins, and Risk of Breast Cancer: A Nested Case-Control Study Using Multiple Time Points. JNCI J. Natl. Cancer Inst. 2015, 107, djv032. [Google Scholar] [CrossRef] [PubMed]
- Lashgari, N.-A.; Roudsari, N.M.; Zadeh, S.S.T.; Momtaz, S.; Abbasifard, M.; Reiner, Ž.; Abdolghaffari, A.H.; Sahebkar, A. Statins block mammalian target of rapamycin pathway: A possible novel therapeutic strategy for inflammatory, malignant and neurodegenerative diseases. Inflammopharmacology 2023, 31, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Fathi, F.; Reiner, Ž.; Banach, M.; Sahebkar, A. The role of statins in lung cancer. Arch. Med. Sci. 2022, 18, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Rosoff, D.B.; Bell, A.S.; Jung, J.; Wagner, J.; Mavromatis, L.A.; Lohoff, F.W. Mendelian Randomization Study of PCSK9 and HMG-CoA Reductase Inhibition and Cognitive Function. J. Am. Coll. Cardiol. 2022, 80, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Wu, J.-L.; Ji, F.; Kang, W.; Bian, X.; Chen, H.; Chan, L.-S.; Luk, S.T.Y.; Tong, S.; Xu, J.; et al. The cholesterol uptake regulator PCSK9 promotes and is a therapeutic target in APC/KRAS-mutant colorectal cancer. Nat. Commun. 2022, 13, 3971. [Google Scholar] [CrossRef]
- Pitteri, S.J.; Kelly-Spratt, K.S.; Gurley, K.E.; Kennedy, J.; Buson, T.B.; Chin, A.; Wang, H.; Zhang, Q.; Wong, C.-H.; Chodosh, L.A.; et al. Tumor Microenvironment–Derived Proteins Dominate the Plasma Proteome Response during Breast Cancer Induction and Progression. Cancer Res. 2011, 71, 5090–5100. [Google Scholar] [CrossRef]
- Zia, S.; Batool, S.; Shahid, R. Could PCSK9 be a new therapeutic target of Eugenol? In vitro and in silico evaluation of hypothesis. Med. Hypotheses 2020, 136, 109513. [Google Scholar] [CrossRef]
- Nagashima, S.; Morishima, K.; Okamoto, H.; Ishibashi, S. Possible involvement of PCSK9 overproduction in hyperlipoproteinemia associated with hepatocellular carcinoma: A case report. J. Clin. Lipidol. 2016, 10, 1045–1049. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Fang, Y.; Zou, Y.; Song, D.; Zhang, S.; Cai, Y. Proprotein Convertase Subtilisin/Kexin Type 9 Promotes Gastric Cancer Metastasis and Suppresses Apoptosis by Facilitating MAPK Signaling Pathway Through HSP70 Up-Regulation. Front. Oncol. 2020, 10, 609663. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Chowdhury, A.; Chaudhury, K.; Shukla, P.C. Proprotein convertase subtilisin/kexin type 9 (PCSK9): A potential multifaceted player in cancer. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2021, 1876, 188581. [Google Scholar] [CrossRef]
- Chong, E.W.; Joncas, F.-H.; Seidah, N.G.; Calon, F.; Diorio, C.; Gangloff, A. Circulating levels of PCSK9, ANGPTL3 and Lp(a) in stage III breast cancers. BMC Cancer 2022, 22, 1049. [Google Scholar] [CrossRef]
- Zhou, B.; Zhou, Z.; Chen, Y.; Deng, H.; Cai, Y.; Rao, X.; Yin, Y.; Rong, L. Plasma proteomics-based identification of novel biomarkers in early gastric cancer. Clin. Biochem. 2020, 76, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Momtazi-Borojeni, A.A.; Nik, M.E.; Jaafari, M.R.; Banach, M.; Sahebkar, A. Effects of immunisation against PCSK9 in mice bearing melanoma. Arch. Med. Sci. 2020, 16, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Oza, P.P.; Kashfi, K. The evolving landscape of PCSK9 inhibition in cancer. Eur. J. Pharmacol. 2023, 949, 175721. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Lee, S.P.; Linsley, P.S.; Gersuk, V.; Yeh, M.M.; Chen, Y.; Peng, Y.; Dutta, M.; Mascarinas, G.; Molla, B.; et al. Pcsk9 Deletion Promotes Murine Nonalcoholic Steatohepatitis and Hepatic Carcinogenesis: Role of Cholesterol. Hepatol. Commun. 2022, 6, 780–794. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.G.; Sanderson, E.; Palmer, T.M.; Ala-Korpela, M.; Ference, B.A.; Smith, G.D.; Holmes, M.V. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020, 17, e1003062. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, W.; Wang, F.; Yang, X.; Ling, H.; Fan, Y.; Tao, J.; Shuai, Z.; Zhang, L.; Ye, D.; et al. Telomere Length and Development of Systemic Lupus Erythematosus: A Mendelian Randomization Study. Arthritis Rheumatol. 2022, 74, 1984–1990. [Google Scholar] [CrossRef]
- Xie, W.; Li, J.; Du, H.; Xia, J. Causal relationship between PCSK9 inhibitor and autoimmune diseases: A drug target Mendelian randomization study. Arthritis Res. Ther. 2023, 25, 148. [Google Scholar] [CrossRef]
- Park, S.W.; Moon, Y.-A.; Horton, J.D. Post-transcriptional Regulation of Low Density Lipoprotein Receptor Protein by Proprotein Convertase Subtilisin/Kexin Type 9a in Mouse Liver. J. Biol. Chem. 2004, 279, 50630–50638. [Google Scholar] [CrossRef]
- Hackam, D.G.; Hegele, R.A. Lipid-Modifying Therapies and Stroke Prevention. Curr. Neurol. Neurosci. Rep. 2022, 22, 375–382. [Google Scholar] [CrossRef]
- Gallego-Colon, E.; Daum, A.; Yosefy, C. Statins and PCSK9 inhibitors: A new lipid-lowering therapy. Eur. J. Pharmacol. 2020, 878, 173114. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, L.; Lian, J.; Schauer, S.; Vesely, P.W.; Kratky, D.; Hoefler, G.; Lehner, R. Tumor-Induced Hyperlipidemia Contributes to Tumor Growth. Cell Rep. 2016, 15, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, K.S.; Siddique, A.B.; Mohyeldin, M.M.; Qusa, M.H.; Goda, A.A.; Singh, S.S.; Ayoub, N.M.; King, J.A.; Jois, S.D.; El Sayed, K.A. Pseurotin A as a novel suppressor of hormone dependent breast cancer progression and recurrence by inhibiting PCSK9 secretion and interaction with LDL receptor. Pharmacol. Res. 2020, 158, 104847. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xu, J.G.; Wang, Z.; Wang, X.; Zhu, Q.; Zhao, J.; Bian, L. Bioinformatics Identification of Key Genes for the Development and Prognosis of Lung Adenocarcinoma. Inq. J. Health Care Organ. Provis. Financ. 2022, 59, 00469580221096259. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, A.; Subbannayya, Y.; Sahasrabuddhe, N.A.; Balakrishnan, L.; Syed, N.; Sekhar, N.R.; Katte, T.V.; Pinto, S.M.; Srikanth, S.M.; Kumar, P.; et al. SILAC-based quantitative proteomic analysis of gastric cancer secretome. Proteom. Clin. Appl. 2012, 7, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Alannan, M.; Fatrouni, H.; Trézéguet, V.; Dittrich-Domergue, F.; Moreau, P.; Siegfried, G.; Liet, B.; Khatib, A.-M.; Grosset, C.F.; Badran, B.; et al. Targeting PCSK9 in Liver Cancer Cells Triggers Metabolic Exhaustion and Cell Death by Ferroptosis. Cells 2022, 12, 62. [Google Scholar] [CrossRef]
- He, M.; Hu, J.; Fang, T.; Tang, W.; Lv, B.; Yang, B.; Xia, J. Protein convertase subtilisin/Kexin type 9 inhibits hepatocellular carcinoma growth by interacting with GSTP1 and suppressing the JNK signaling pathway. Cancer Biol. Med. 2021, 18, 90–103. [Google Scholar] [CrossRef]
- Chen, H.; Tang, X.; Su, W.; Li, S.; Yang, R.; Cheng, H.; Zhang, G.; Zhou, X. Causal effects of lipid-lowering therapies on aging-related outcomes and risk of cancers: A drug-target Mendelian randomization study. Aging 2023, 15, 15228–15242. [Google Scholar] [CrossRef]
- EBioMedicine. PCSK9 Inhibitors: What Lies Beyond Monoclonal Antibodies? eBioMedicine 2015, 2, 1835. [Google Scholar] [CrossRef]
- Olsson, A.G.; Angelin, B.; Assmann, G.; Binder, C.J.; Björkhem, I.; Cedazo-Minguez, A.; Cohen, J.; von Eckardstein, A.; Farinaro, E.; Müller-Wieland, D.; et al. Can LDL cholesterol be too low? Possible risks of extremely low levels. J. Intern. Med. 2017, 281, 534–553. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, W.; Tang, J. Editorial: Functional screening for cancer drug discovery: From experimental approaches to data integration. Front. Genet. 2023, 14, 1201454. [Google Scholar] [CrossRef] [PubMed]
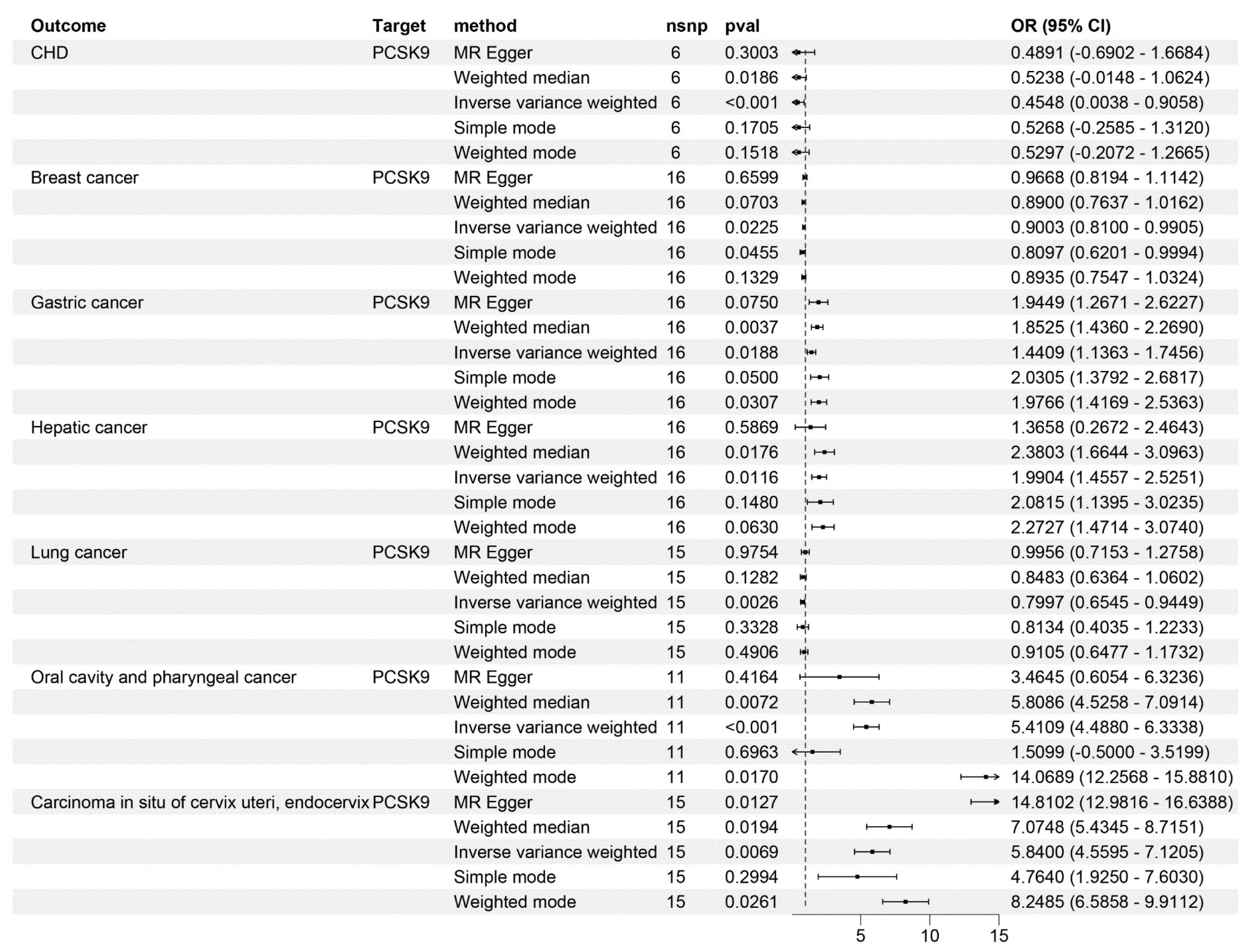



| Outcome | Method | nsnp | pval | or | or_lci95 | or_uci95 |
|---|---|---|---|---|---|---|
| CHD | IVW | 9 | 0.000105 | 0.412278 | 0.03543 | 0.859984 |
| Breast cancer | IVW | 33 | 0.046465 | 0.915973 | 0.829577 | 1.002369 |
| Gastric cancer | IVW | 33 | 0.009436 | 1.466226 | 1.177273 | 1.755179 |
| Hepatic cancer | IVW | 33 | 0.031913 | 1.748559 | 1.238079 | 2.259039 |
| Lung cancer | IVW | 29 | 0.001287 | 0.770876 | 0.612424 | 0.929328 |
| Oral cavity and pharyngeal cancer | IVW | 26 | 4.74 × 10−5 | 12.95632 | 11.7222 | 14.19044 |
| Carcinoma in situ of cervix uteri, endocervix | IVW | 29 | 0.006413 | 6.024705 | 4.733405 | 7.316005 |
| Malignant neoplasm of brain | IVW | 29 | 0.13796 | 0.584619 | 0.12463 | 1.293866 |
| Bladder cancer | IVW | 33 | 0.817092 | 1.000201 | 0.9985 | 1.001901 |
| Thyroid cancer | IVW | 33 | 0.640864 | 1.11934 | 0.645657 | 1.593023 |
| Malignant neoplasm of kidney, except renal pelvis | IVW | 29 | 0.806225 | 0.941211 | 0.457094 | 1.425327 |
| Malignant neoplasm of esophagus | IVW | 29 | 0.613284 | 1.284654 | 0.31321 | 2.256097 |
| Pancreatic cancer | IVW | 4 | 0.246173 | 0.343108 | −1.46481 | 2.151024 |
| Colorectal cancer | IVW | 33 | 0.703725 | 1.039738 | 0.838898 | 1.240578 |
| Outcome | Heterogeneity Test (Q-Value) | Pleiotropy Test (p-Value) | Outlier Test (p-Value) | |
|---|---|---|---|---|
| MR-Egger | IVW | |||
| CHD | 0.89 | 0.81 | 0.25 | NA |
| Breast cancer | 0.49 | 0.46 | 0.25 | NA |
| Gastric cancer | 0.74 | 0.74 | 0.35 | NA |
| Hepatic cancer | 0.73 | 0.75 | 0.45 | NA |
| Lung cancer | 0.72 | 0.60 | 0.08 | NA |
| Oral cavity and pharyngeal cancer | 0.75 | 0.61 | 0.70 | NA |
| Carcinoma in situ of cervix uteri, endocervix | 0.87 | 0.79 | 0.18 | NA |
| Malignant neoplasm of brain | 0.63 | 0.41 | 0.073 | NA |
| Bladder cancer | 0.97 | 0.98 | 0.55 | NA |
| Thyroid cancer | 0.97 | 0.98 | 0.82 | NA |
| Malignant neoplasm of kidney, except renal pelvis | 0.85 | 0.87 | 0.56 | NA |
| Malignant neoplasm of esophagus | 0.82 | 0.84 | 0.48 | NA |
| Pancreatic cancer | 0.97 | 0.97 | 0.72 | NA |
| Colorectal cancer | 0.25 | 0.17 | 0.13 | NA |
| Outcome | Heterogeneity Test (Q-Value) | Pleiotropy Test (p-Value) | Outlier Test (p-Value) | |
|---|---|---|---|---|
| MR-Egger | IVW | |||
| CHD | 0.76 | 0.87 | 0.90 | NA |
| Breast cancer | 0.71 | 0.75 | 0.85 | NA |
| Gastric cancer | 0.67 | 0.70 | 0.61 | NA |
| Hepatic cancer | 0.60 | 0.65 | 0.92 | NA |
| Lung cancer | 0.12 | 0.13 | 0.44 | NA |
| Oral cavity and pharyngeal cancer | 0.60 | 0.63 | 0.48 | NA |
| Carcinoma in situ of cervix uteri, endocervix | 0.90 | 0.86 | 0.14 | NA |
| Malignant neoplasm of brain | 0.49 | 0.36 | 0.075 | NA |
| Bladder cancer | 0.99 | 0.99 | 0.62 | NA |
| Thyroid cancer | 0.98 | 0.99 | 0.79 | NA |
| Malignant neoplasm of kidney, except renal pelvis | 0.42 | 0.45 | 0.52 | NA |
| Malignant neoplasm of esophagus | 0.69 | 0.60 | 0.11 | NA |
| Pancreatic cancer | 0.96 | 0.95 | 0.99 | NA |
| Colorectal cancer | 0.19 | 0.21 | 0.51 | NA |
| Exposure | Method | nsnp | pval | or | or_lci95 | or_uci95 |
|---|---|---|---|---|---|---|
| CHD | IVW | 41 | 0.373 | 1.029 | 0.966 | 1.095 |
| Breast cancer | IVW | 240 | 0.978 | 0.998 | 0.987 | 1.013 |
| Gastric cancer | IVW | 24 | 0.453 | 0.996 | 0.985 | 1.006 |
| Hepatic cancer | IVW | 23 | 0.149 | 0.989 | 0.974 | 1.003 |
| Lung cancer | IVW | 53 | 0.554 | 0.994 | 0.976 | 1.012 |
| Oral cavity and pharyngeal cancer | IVW | 16 | 0.229 | 1.005 | 0.996 | 1.014 |
| Carcinoma in situ of cervix uteri, endocervix | IVW | 6 | 0.773 | 1.000 | 0.996 | 1.004 |
| Malignant neoplasm of brain | IVW | 10 | 0.983 | 0.999 | 0.995 | 1.003 |
| Bladder cancer | IVW | 25 | 0.482 | 2.195 | 0.244 | 19.730 |
| Thyroid cancer | IVW | 13 | 0.683 | 0.998 | 0.989 | 1.007 |
| Malignant neoplasm of kidney, except renal pelvis | IVW | 15 | 0.488 | 1.002 | 0.994 | 1.010 |
| Malignant neoplasm of esophagus | IVW | 8 | 0.221 | 0.997 | 0.993 | 1.001 |
| Pancreatic cancer | IVW | 3 | 0.352 | 1.040 | 0.956 | 1.132 |
| Colorectal cancer | IVW | 69 | 0.364 | 1.009 | 0.989 | 1.028 |
| Exposure | Method | nsnp | pval | or | or_lci95 | or_uci95 |
|---|---|---|---|---|---|---|
| CHD | IVW | 41 | 0.113 | 1.053 | 0.987 | 1.124 |
| Breast cancer | IVW | 240 | 0.728 | 1.002 | 0.990 | 1.013 |
| Gastric cancer | IVW | 24 | 0.944 | 0.999 | 0.990 | 1.008 |
| Hepatic cancer | IVW | 23 | 0.067 | 0.989 | 0.978 | 1.000 |
| Lung cancer | IVW | 53 | 0.949 | 1.000 | 0.984 | 1.016 |
| Oral cavity and pharyngeal cancer | IVW | 16 | 0.133 | 1.006 | 0.997 | 1.015 |
| Carcinoma in situ of cervix uteri, endocervix | IVW | 6 | 0.603 | 1.000 | 0.997 | 1.004 |
| Malignant neoplasm of brain | IVW | 10 | 0.606 | 0.999 | 0.996 | 1.002 |
| Bladder cancer | IVW | 25 | 0.339 | 2.022 | 0.477 | 8.575 |
| Thyroid cancer | IVW | 13 | 0.391 | 0.996 | 0.988 | 1.004 |
| Malignant neoplasm of kidney, except renal pelvis | IVW | 15 | 0.408 | 1.002 | 0.996 | 1.008 |
| Malignant neoplasm of esophagus | IVW | 8 | 0.362 | 0.997 | 0.993 | 1.002 |
| Pancreatic cancer | IVW | 3 | 0.412 | 1.040 | 0.946 | 1.143 |
| Colorectal cancer | IVW | 69 | 0.550 | 1.005 | 0.988 | 1.022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Li, W.; Zhang, D.; Mi, Y.; Zhang, J.; He, G. The Causal Relationship between PCSK9 Inhibitors and Malignant Tumors: A Mendelian Randomization Study Based on Drug Targeting. Genes 2024, 15, 132. https://doi.org/10.3390/genes15010132
Wang W, Li W, Zhang D, Mi Y, Zhang J, He G. The Causal Relationship between PCSK9 Inhibitors and Malignant Tumors: A Mendelian Randomization Study Based on Drug Targeting. Genes. 2024; 15(1):132. https://doi.org/10.3390/genes15010132
Chicago/Turabian StyleWang, Wenxin, Wei Li, Dan Zhang, Yongrun Mi, Jingyu Zhang, and Guoyang He. 2024. "The Causal Relationship between PCSK9 Inhibitors and Malignant Tumors: A Mendelian Randomization Study Based on Drug Targeting" Genes 15, no. 1: 132. https://doi.org/10.3390/genes15010132