Genome-Wide Identification of Trehalose-6-phosphate Synthase (TPS) Gene Family Reveals the Potential Role in Carbohydrate Metabolism in Peach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification of PpTPS Gene Family in Peach
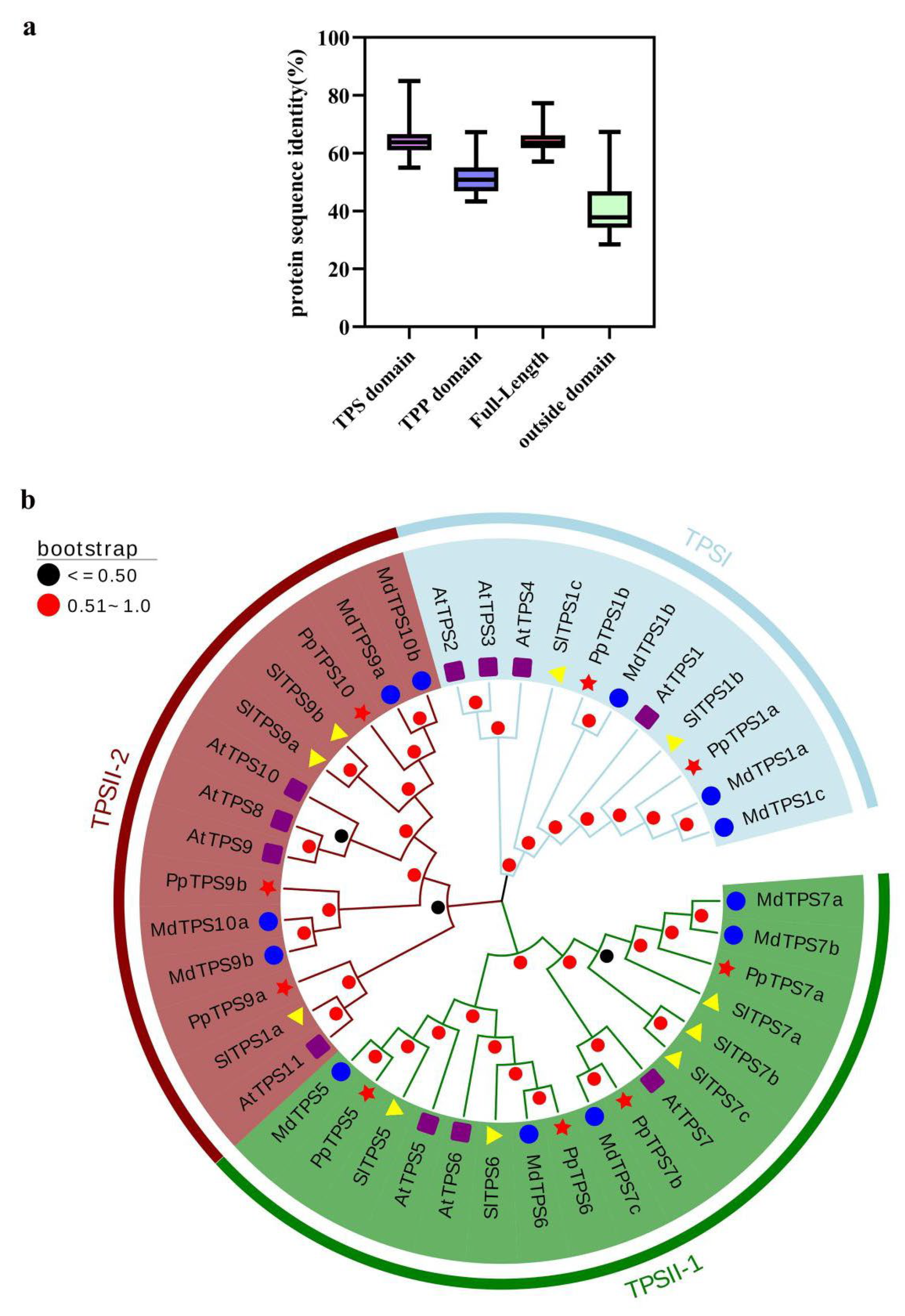
2.2. Collinearity Analysis and System Evolution Analysis
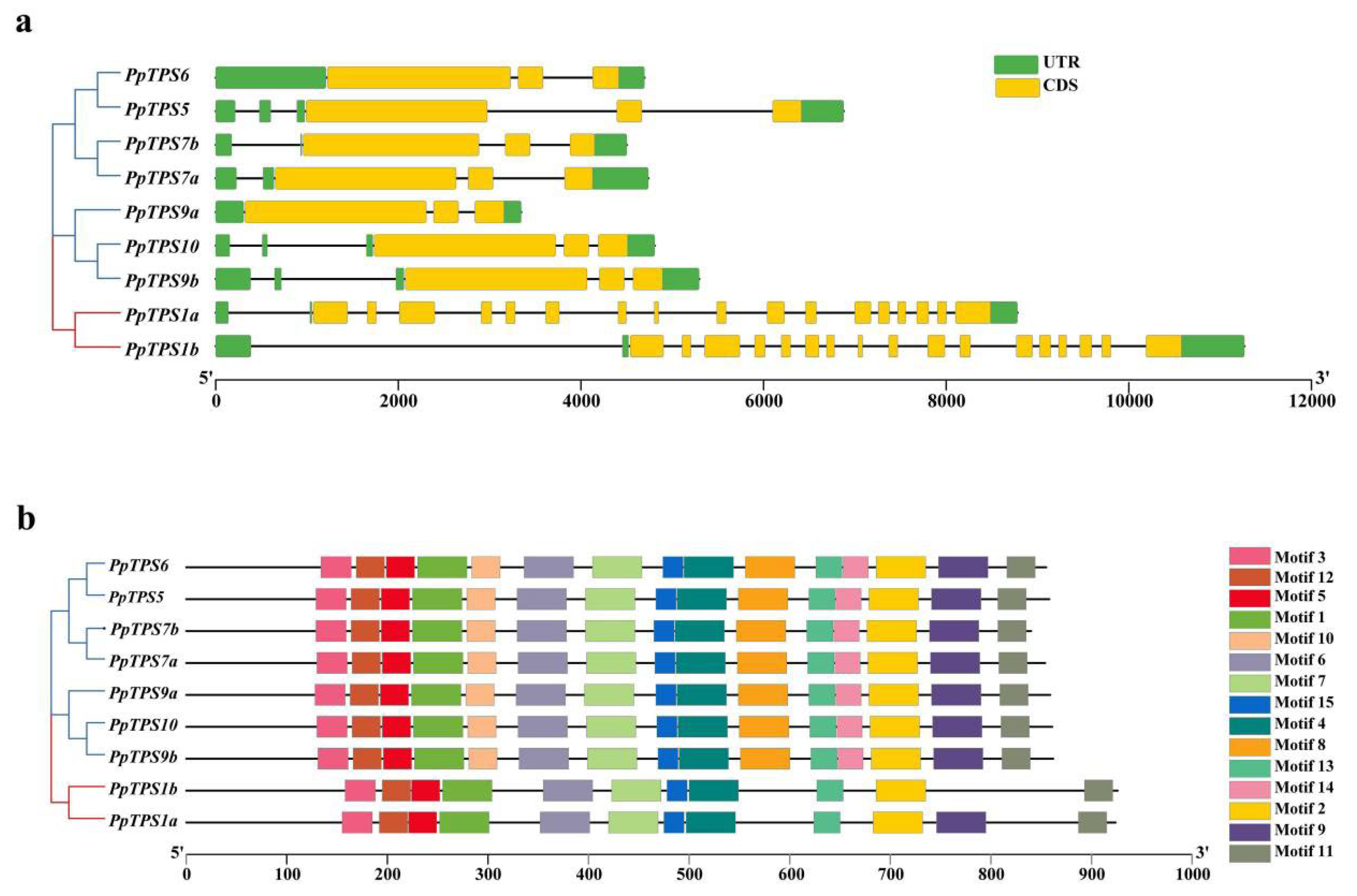
2.3. Analysis of Gene Structure and Conserved Domain
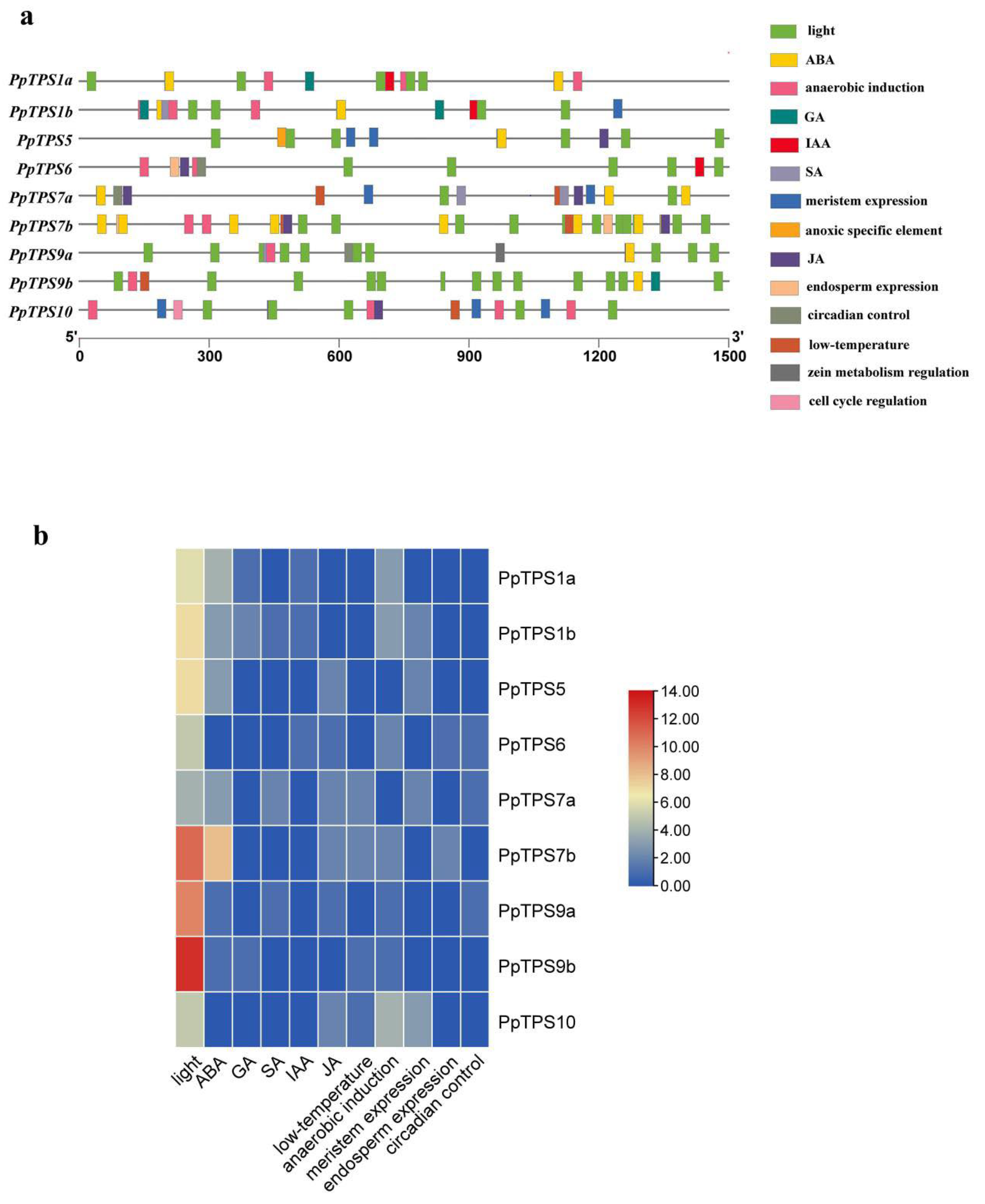
2.4. Promoter Element Analysis
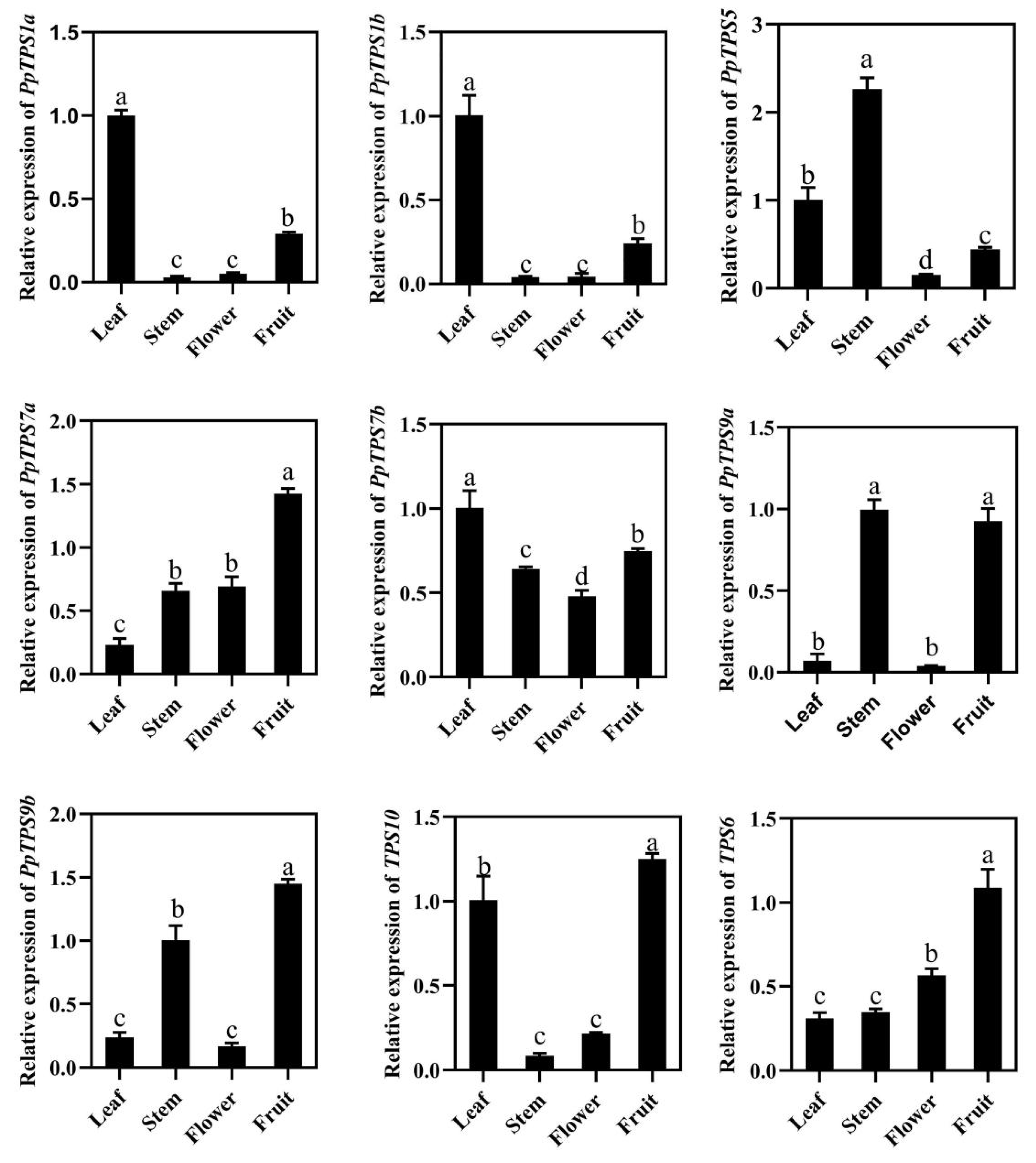
2.5. Plant Material Acquisition, RNA Extraction and qRT-PCR
2.6. Determination of T6P Content and SnRK1 Activity
2.7. Transient Overexpression and Silencing in Peach Fruit
3. Results
3.1. Identification of PpTPS Gene Family in Peach
3.2. Gene Collinearity Analysis of PpTPS Genes
3.3. Multiple Sequence Alignment and Phylogenetic Analysis
3.4. Gene Structure and Protein Domain of PpTPS
3.5. Promoter Element Analysis
3.6. Tissue Specific Expression Pattern of PpTPS Genes in Peach
3.7. Expression Profile of PpTPS during Peach Fruit Development
3.8. Functional Validation of PpTPS7a in Peach Fruit
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiemken, A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Leeuwenhoek 1990, 58, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Mitchell, W.J.; Rafii, F. Effect of Trehalose and Trehalose Transport on the Tolerance of Clostridium perfringens to Environmental Stress in a Wild Type Strain and Its Fluoroquinolone-Resistant Mutant. Int. J. Microbiol. 2016, 2016, 4829716. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A. The function of trehalose biosynthesis in plants. Phytochemistry 2002, 60, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Boller, T.; Wiemken, A. Trehalose and trehalase in plants: Recent developments. Plant Sci. 1995, 112, 1–9. [Google Scholar] [CrossRef]
- Olsson, C.; Jansson, H.; Swenson, J. The Role of Trehalose for the Stabilization of Proteins. J. Phys. Chem. B 2016, 120, 4723–4731. [Google Scholar] [CrossRef] [PubMed]
- Cabib, E.; Leloir, L.F. The biosynthesis of trehalose phosphate. J. Biol. Chem. 1958, 231, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose metabolism in plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef]
- Ramon, M.; Rolland, F. Plant development: Introducing trehalose metabolism. Trends Plant Sci. 2007, 12, 185–188. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, X.; Yang, X.; Zhao, T.; An, X.; Chen, Z. Characterization and expression pattern of the trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase gene families in Populus. Int. J. Biol. Macromol. 2021, 187, 9–23. [Google Scholar] [CrossRef]
- Vandesteene, L.; Ramon, M.; Le Roy, K.; Van Dijck, P.; Rolland, F. A Single Active Trehalose-6-P Synthase (TPS) and a Family of Putative Regulatory TPS-Like Proteins in Arabidopsis. Mol. Plant 2010, 3, 406–419. [Google Scholar] [CrossRef]
- Zang, B.; Li, H.; Li, W.; Deng, X.W.; Wang, X. Analysis of trehalose-6-phosphate synthase (TPS) gene family suggests the formation of TPS complexes in rice. Plant Mol. Biol. 2011, 76, 507–522. [Google Scholar] [CrossRef]
- Eastmond, P.J.; van Dijken, A.J.; Spielman, M.; Kerr, A.; Tissier, A.F.; Dickinson, H.G.; Jones, J.D.; Smeekens, S.C.; Graham, I.A. Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. Plant J. 2002, 29, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Ramon, M.; De Smet, I.; Vandesteene, L.; Naudts, M.; Leyman, B.; Van Dijck, P.; Rolland, F.; Beeckman, T.; Thevelein, J.M. Extensive expression regulation and lack of heterologous enzymatic activity of the Class II trehalose metabolism proteins from Arabidopsis thaliana. Plant Cell Environ. 2009, 32, 1015–1032. [Google Scholar] [CrossRef] [PubMed]
- Li, H.W.; Zang, B.S.; Deng, X.W.; Wang, X.P. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice. Planta 2011, 234, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Zacharaki, V.; Ponnu, J.; Crepin, N.; Langenecker, T.; Hagmann, J.; Skorzinski, N.; Musialak-Lange, M.; Wahl, V.; Rolland, F.; Schmid, M. Impaired KIN10 function restores developmental defects in the Arabidopsis trehalose 6-phosphate synthase1 (tps1) mutant. New Phytol. 2022, 235, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Xie, Z.; Lu, C.; Hao, X.; Wu, S.; Huang, Y.; Li, D.; Chen, L. The trehalose-6-phosphate synthase TPS5 negatively regulates ABA signaling in Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Chary, S.N.; Hicks, G.R.; Choi, Y.G.; Carter, D.; Raikhel, N.V. Trehalose-6-phosphate synthase/phosphatase regulates cell shape and plant architecture in Arabidopsis. Plant Physiol. 2008, 146, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Vishal, B.; Krishnamurthy, P.; Ramamoorthy, R.; Kumar, P.P. OsTPS8 controls yield-related traits and confers salt stress tolerance in rice by enhancing suberin deposition. New Phytol. 2019, 221, 1369–1386. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fu, L.; Qin, P.; Sun, Y.; Liu, J.; Wang, X. Overexpression of the wheat trehalose 6-phosphate synthase 11 gene enhances cold tolerance in Arabidopsis thaliana. Gene 2019, 710, 210–217. [Google Scholar] [CrossRef]
- Yadav, U.P.; Ivakov, A.; Feil, R.; Duan, G.Y.; Walther, D.; Giavalisco, P.; Piques, M.; Carillo, P.; Hubberten, H.M.; Stitt, M.; et al. The sucrose-trehalose 6-phosphate (Tre6P) nexus: Specificity and mechanisms of sucrose signalling by Tre6P. J. Exp. Bot. 2014, 65, 1051–1068. [Google Scholar] [CrossRef]
- Zhang, Y.; Primavesi, L.F.; Jhurreea, D.; Andralojc, P.J.; Mitchell, R.A.; Powers, S.J.; Schluepmann, H.; Delatte, T.; Wingler, A.; Paul, M.J. Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol. 2009, 149, 1860–1871. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, B.; Moraes, T.A.; Mengin, V.; Margalha, L.; Vicente, R.; Feil, R.; Höhne, M.; Sousa, A.G.G.; Lilue, J.; Stitt, M.; et al. Impact of the SnRK1 protein kinase on sucrose homeostasis and the transcriptome during the diel cycle. Plant Physiol. 2021, 187, 1357–1373. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Peng, F.; Wang, W.; Liang, J.; Xiao, Y.; Yuan, X. SnRK1 phosphorylation of SDH positively regulates sorbitol metabolism and promotes sugar accumulation in peach fruit. Tree Physiol. 2021, 41, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Shulaev, V.; Korban, S.S.; Sosinski, B.; Abbott, A.G.; Aldwinckle, H.S.; Folta, K.M.; Iezzoni, A.; Main, D.; Arús, P.; Dandekar, A.M.; et al. Multiple models for Rosaceae genomics. Plant Physiol. 2008, 147, 985–1003. [Google Scholar] [CrossRef] [PubMed]
- Verde, I.; Abbott, A.G.; Scalabrin, S.; Jung, S.; Shu, S.; Marroni, F.; Zhebentyayeva, T.; Dettori, M.T.; Grimwood, J.; Cattonaro, F.; et al. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat. Genet. 2013, 45, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Arús, P.; Verde, I.; Sosinski, B.; Zhebentyayeva, T.; Abbott, A.G. The peach genome. Tree Genet. Genomes 2012, 8, 531–547. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Finn, R.D.; Tate, J.; Mistry, J.; Coggill, P.C.; Sammut, S.J.; Hotz, H.R.; Ceric, G.; Forslund, K.; Eddy, S.R.; Sonnhammer, E.L.; et al. The Pfam protein families database. Nucleic Acids Res. 2008, 36, D281–D288. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2002, 2, 2–3. [Google Scholar] [CrossRef]
- Yockteng, R.; Almeida, A.M.; Morioka, K.; Alvarez-Buylla, E.R.; Specht, C.D. Molecular evolution and patterns of duplication in the SEP/AGL6-like lineage of the Zingiberales: A proposed mechanism for floral diversification. Mol. Biol. Evol. 2013, 30, 2401–2422. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Rombauts, S.; Déhais, P.; Van Montagu, M.; Rouzé, P. PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res. 1999, 27, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Huan, C.; Jiang, L.; An, X.; Yu, M.; Xu, Y.; Ma, R.; Yu, Z. Potential role of reactive oxygen species and antioxidant genes in the regulation of peach fruit development and ripening. Plant Physiol. Biochem. 2016, 104, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Derveaux, S.; Vandesompele, J.; Hellemans, J. How to do successful gene expression analysis using real-time PCR. Methods 2010, 50, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Senthil-Kumar, M.; Dai, X.; Ramu, V.S.; Lee, S.; Mysore, K.S.; Zhao, P.X. pssRNAit: A Web Server for Designing Effective and Specific Plant siRNAs with Genome-Wide Off-Target Assessment. Plant Physiol. 2020, 184, 65–81. [Google Scholar] [CrossRef]
- Glinski, M.; Weckwerth, W. Differential multisite phosphorylation of the trehalose-6-phosphate synthase gene family in Arabidopsis thaliana: A mass spectrometry-based process for multiparallel peptide library phosphorylation analysis. Mol. Cell. Proteom. 2005, 4, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, M.A.; Santos, E.; Flores, C.L.; Martínez-Zapater, J.M.; Salinas, J.; Gancedo, C. Isolation and molecular characterization of the Arabidopsis TPS1 gene, encoding trehalose-6-phosphate synthase. Plant J. 1998, 13, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Fu, F.-L.; Zhang, S.-Z.; Wu, L.; Li, W.-C. Cloning and Characterization of Functional Trehalose-6-Phosphate Synthase Gene in Maize. J. Plant Biol. 2010, 53, 134–141. [Google Scholar] [CrossRef]
- Jin, X.F.; Xiong, A.S.; Peng, R.H.; Liu, J.G.; Gao, F.; Chen, J.M.; Yao, Q.H. OsAREB1, an ABRE-binding protein responding to ABA and glucose, has multiple functions in Arabidopsis. BMB Rep. 2010, 43, 34–39. [Google Scholar] [CrossRef]
- Jang, I.C.; Oh, S.J.; Seo, J.S.; Choi, W.B.; Song, S.I.; Kim, C.H.; Kim, Y.S.; Seo, H.S.; Choi, Y.D.; Nahm, B.H.; et al. Expression of a bifunctional fusion of the Escherichia coli genes for trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase in transgenic rice plants increases trehalose accumulation and abiotic stress tolerance without stunting growth. Plant Physiol. 2003, 131, 516–524. [Google Scholar] [CrossRef]
- Yang, H.L.; Liu, Y.J.; Wang, C.L.; Zeng, Q.Y. Molecular evolution of trehalose-6-phosphate synthase (TPS) gene family in Populus, Arabidopsis and rice. PLoS ONE 2012, 7, e42438. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, Y.; Mattson, N.; Yang, L.; Jin, Q. Genome-wide analysis of the Solanum tuberosum (potato) trehalose-6-phosphate synthase (TPS) gene family: Evolution and differential expression during development and stress. BMC Genom. 2017, 18, 926. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.; Marquez, Y.; Kalyna, M.; Barta, A. Complexity of the alternative splicing landscape in plants. Plant Cell 2013, 25, 3657–3683. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Lu, X.K.; Wang, J.J.; Wang, D.L.; Yin, Z.J.; Wang, S.; Fan, W.L.; Ye, W.W. Genome-wide Identification and analysis of the stress-resistance function of the TPS (Trehalose-6-Phosphate Synthase) gene family in cotton. BMC Genet. 2016, 17, 54. [Google Scholar] [CrossRef]
- Miranda, J.A.; Avonce, N.; Suárez, R.; Thevelein, J.M.; Van Dijck, P.; Iturriaga, G. A bifunctional TPS-TPP enzyme from yeast confers tolerance to multiple and extreme abiotic-stress conditions in transgenic Arabidopsis. Planta 2007, 226, 1411–1421. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Li, M.; Sun, M.; Jiang, X.; Qiao, F. Plant hormone signals regulate trehalose accumulation against osmotic stress in watermelon cells. Protoplasma 2022, 259, 1351–1369. [Google Scholar] [CrossRef] [PubMed]
- Avonce, N.; Leyman, B.; Mascorro-Gallardo, J.O.; Van Dijck, P.; Thevelein, J.M.; Iturriaga, G. The Arabidopsis trehalose-6-P synthase AtTPS1 gene is a regulator of glucose, abscisic acid, and stress signaling. Plant Physiol. 2004, 136, 3649–3659. [Google Scholar] [CrossRef]
- Song, J.; Mao, H.; Cheng, J.; Zhou, Y.; Chen, R.; Zeng, L.; Li, H.; Wang, Y. Identification of the trehalose-6-phosphate synthase gene family in Medicago truncatula and expression analysis under abiotic stresses. Gene 2021, 787, 145641. [Google Scholar] [CrossRef]
- Dan, Y.; Niu, Y.; Wang, C.; Yan, M.; Liao, W. Genome-wide identification and expression analysis of the trehalose-6-phosphate synthase (TPS) gene family in cucumber (Cucumis sativus L.). PeerJ 2021, 9, e11398. [Google Scholar] [CrossRef]
- Griffiths, C.A.; Sagar, R.; Geng, Y.; Primavesi, L.F.; Patel, M.K.; Passarelli, M.K.; Gilmore, I.S.; Steven, R.T.; Bunch, J.; Paul, M.J.; et al. Chemical intervention in plant sugar signalling increases yield and resilience. Nature 2016, 540, 574–578. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Paul, M.J. Source/sink interactions underpin crop yield: The case for trehalose 6-phosphate/SnRK1 in improvement of wheat. Front. Plant Sci. 2014, 5, 418. [Google Scholar] [CrossRef]
- O’Hara, L.E.; Paul, M.J.; Wingler, A. How do sugars regulate plant growth and development? New insight into the role of trehalose-6-phosphate. Mol. Plant 2013, 6, 261–274. [Google Scholar] [CrossRef]
- Schluepmann, H.; Berke, L.; Sanchez-Perez, G.F. Metabolism control over growth: A case for trehalose-6-phosphate in plants. J. Exp. Bot. 2011, 63, 3379–3390. [Google Scholar] [CrossRef]
- Van Leene, J.; Eeckhout, D.; Gadeyne, A.; Matthijs, C.; Han, C.; De Winne, N.; Persiau, G.; Van De Slijke, E.; Persyn, F.; Mertens, T.; et al. Mapping of the plant SnRK1 kinase signalling network reveals a key regulatory role for the class II T6P synthase-like proteins. Nat. Plants 2022, 8, 1245–1261. [Google Scholar] [CrossRef]




| Name | Gene Accession | aa | Chrom | Chr-Start | Chr-End | MW (Da) | pI | GRAVY |
|---|---|---|---|---|---|---|---|---|
| PpTPS1a | Prupe.4G071400 | 926 | Chr.4 | 3497894 | 3506676 | 104,186.14 | 6.88 | −0.285 |
| PpTPS1b | Prupe.4G155900 | 924 | Chr.4 | 8926783 | 8938050 | 104,373.73 | 6.37 | −0.378 |
| PpTPS5 | Prupe.5G031500 | 858 | Chr.5 | 3611189 | 3618103 | 97,218.49 | 5.67 | −0.19 |
| PpTPS6 | Prupe.1G334900 | 855 | Chr.1 | 31647574 | 31652271 | 96,726.25 | 5.9 | −0.19 |
| PpTPS7a | Prupe.3G113100 | 840 | Chr.3 | 9435987 | 9440491 | 95,686.72 | 6.19 | −0.244 |
| PpTPS7b | Prupe.4G067300 | 854 | Chr.4 | 3258754 | 3263493 | 96,553.11 | 5.8 | −0.267 |
| PpTPS9a | Prupe.5G176400 | 862 | Chr.5 | 14959038 | 14964334 | 97,797.31 | 6.39 | −0.185 |
| PpTPS9b | Prupe.1G095500 | 859 | Chr.1 | 7551683 | 7555029 | 97,549.83 | 6.25 | −0.207 |
| PpTPS10 | Prupe.1G256200 | 861 | Chr.1 | 26652471 | 26657279 | 97,224.26 | 5.88 | −0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Wang, Z.; Xiao, Y.; Liang, J.; Zhao, S.; Liu, Y.; Peng, F.; Guo, J. Genome-Wide Identification of Trehalose-6-phosphate Synthase (TPS) Gene Family Reveals the Potential Role in Carbohydrate Metabolism in Peach. Genes 2024, 15, 39. https://doi.org/10.3390/genes15010039
Fan S, Wang Z, Xiao Y, Liang J, Zhao S, Liu Y, Peng F, Guo J. Genome-Wide Identification of Trehalose-6-phosphate Synthase (TPS) Gene Family Reveals the Potential Role in Carbohydrate Metabolism in Peach. Genes. 2024; 15(1):39. https://doi.org/10.3390/genes15010039
Chicago/Turabian StyleFan, Shihao, Zhe Wang, Yuansong Xiao, Jiahui Liang, Shilong Zhao, Yihua Liu, Futian Peng, and Jian Guo. 2024. "Genome-Wide Identification of Trehalose-6-phosphate Synthase (TPS) Gene Family Reveals the Potential Role in Carbohydrate Metabolism in Peach" Genes 15, no. 1: 39. https://doi.org/10.3390/genes15010039




