Dysregulation of the Skin–Liver Axis in Prurigo Nodularis: An Integrated Genomic, Transcriptomic, and Population-Based Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population-Level Analysis
2.2. Skin RNA Sequencing
2.3. Statistical Analysis
2.4. Disease Associations in FinnGen
3. Results
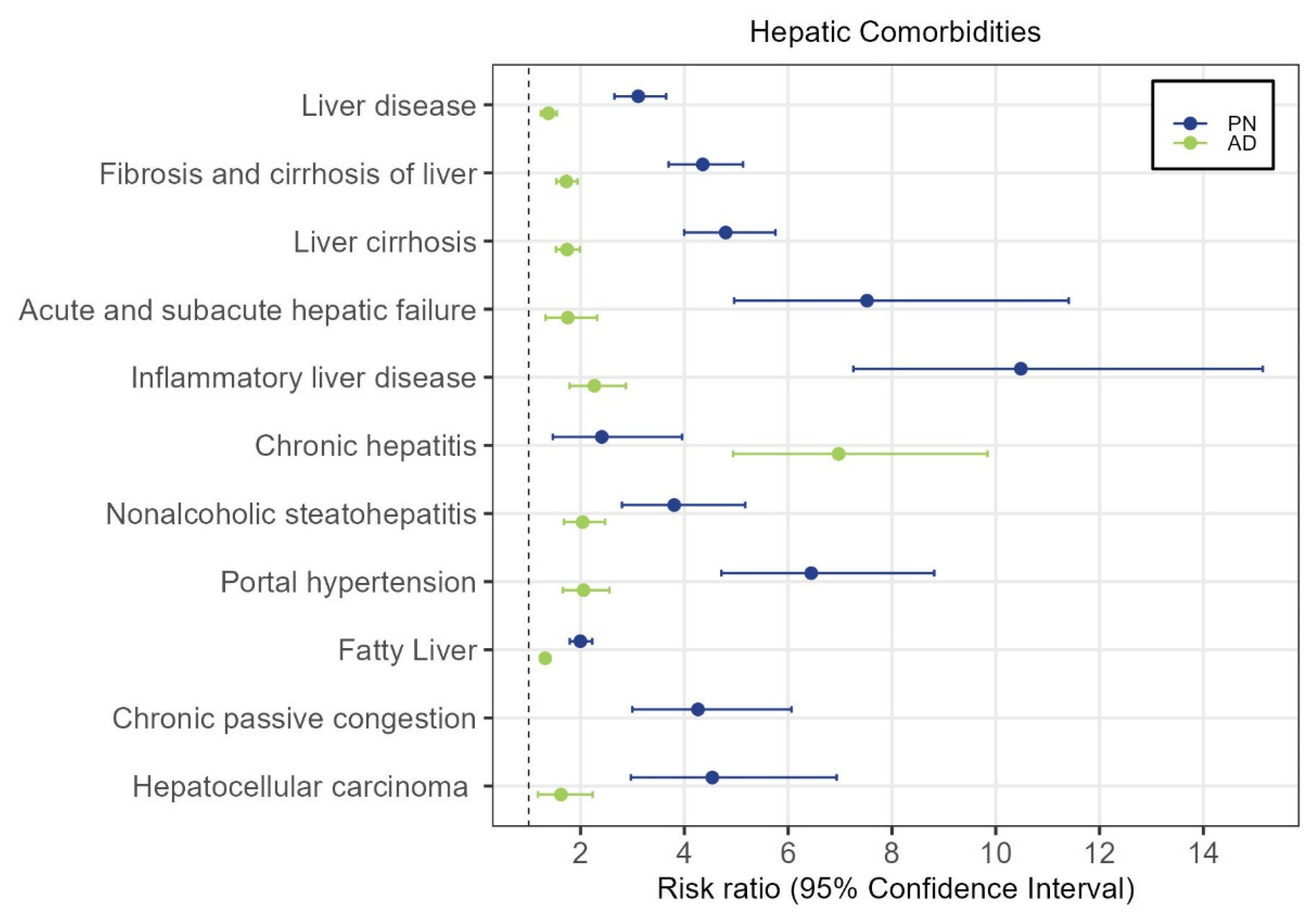
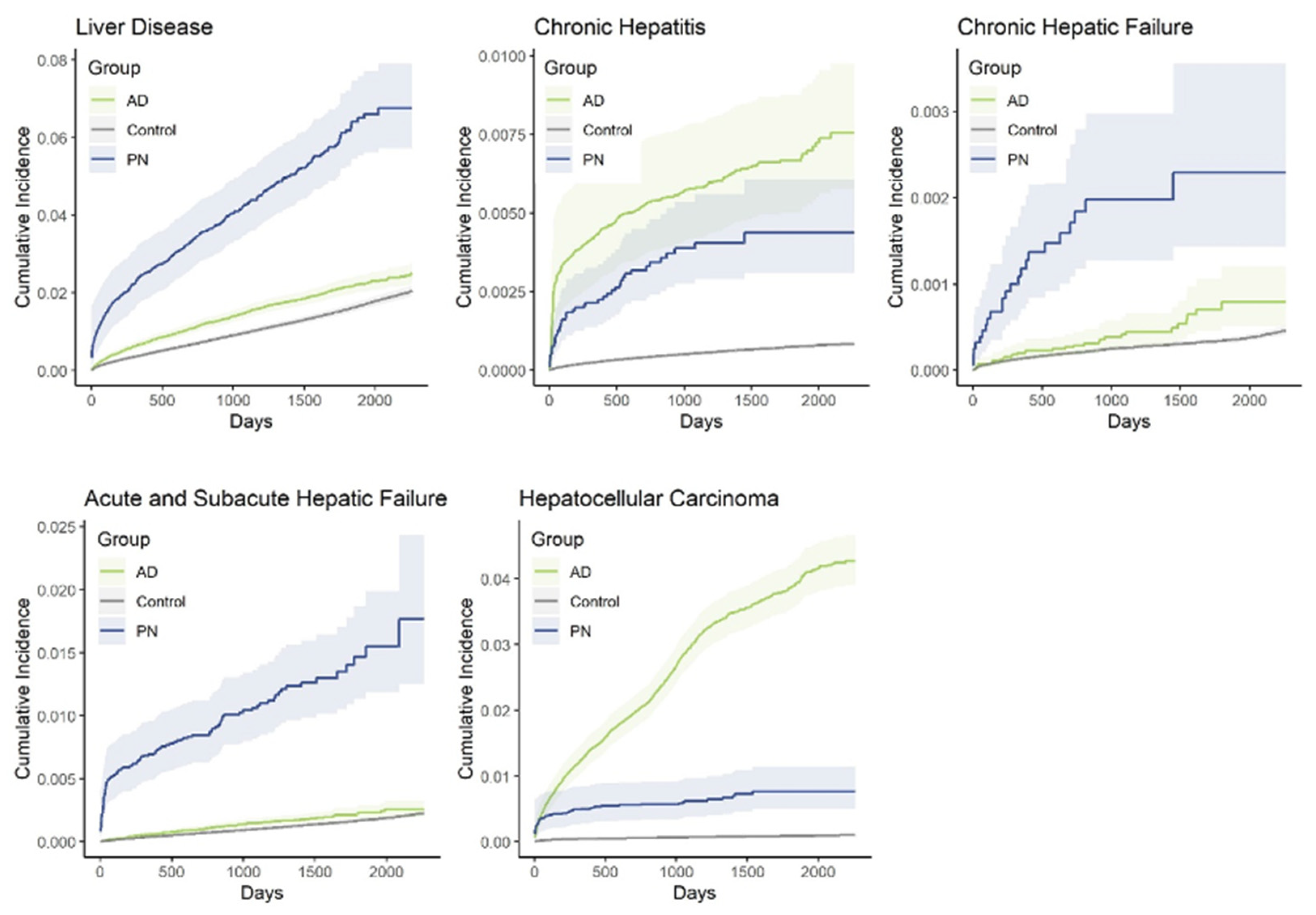
3.1. Population-Level Analysis of PN
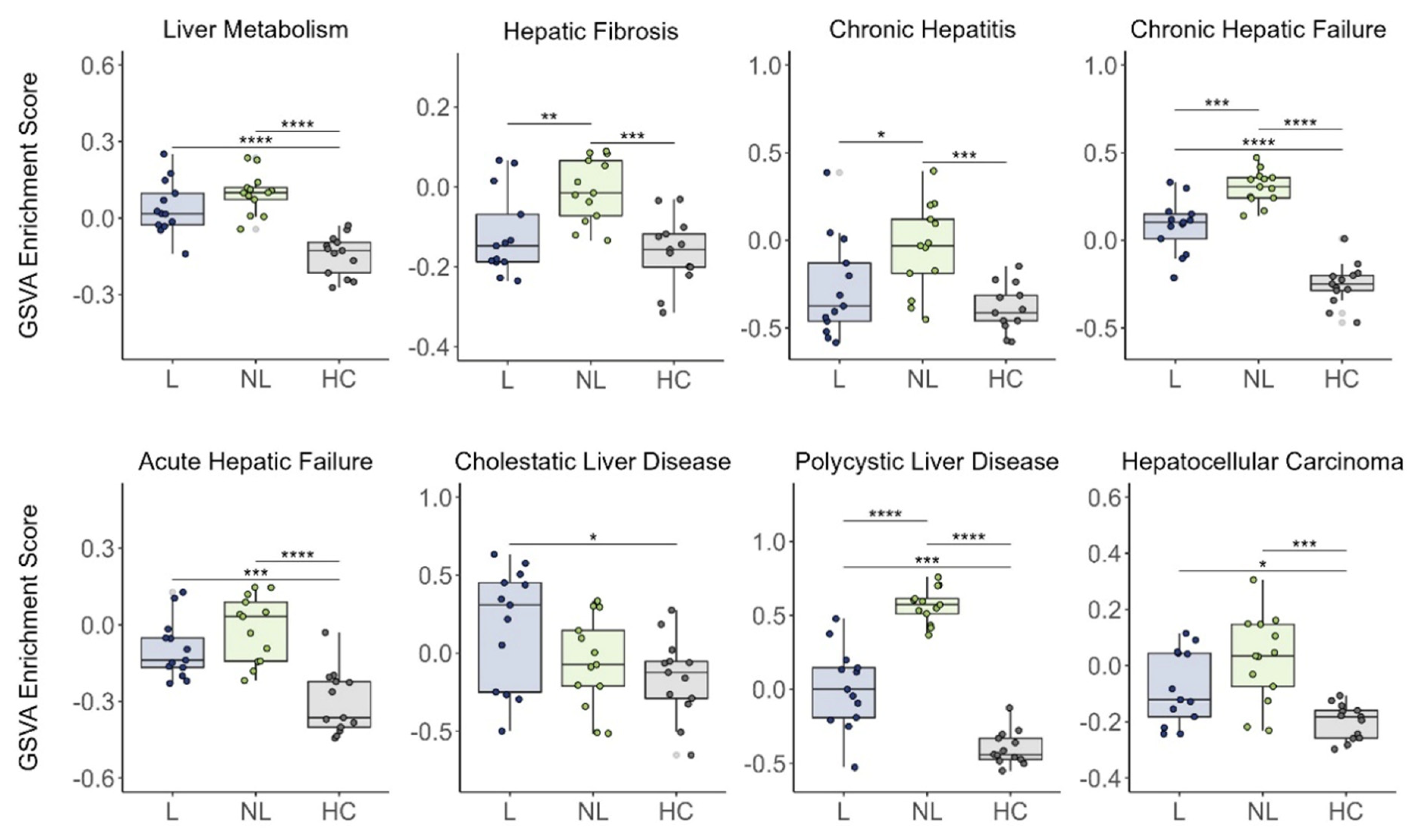
3.2. Cutaneous Expression of Hepatic Failure-Related Genes in PN
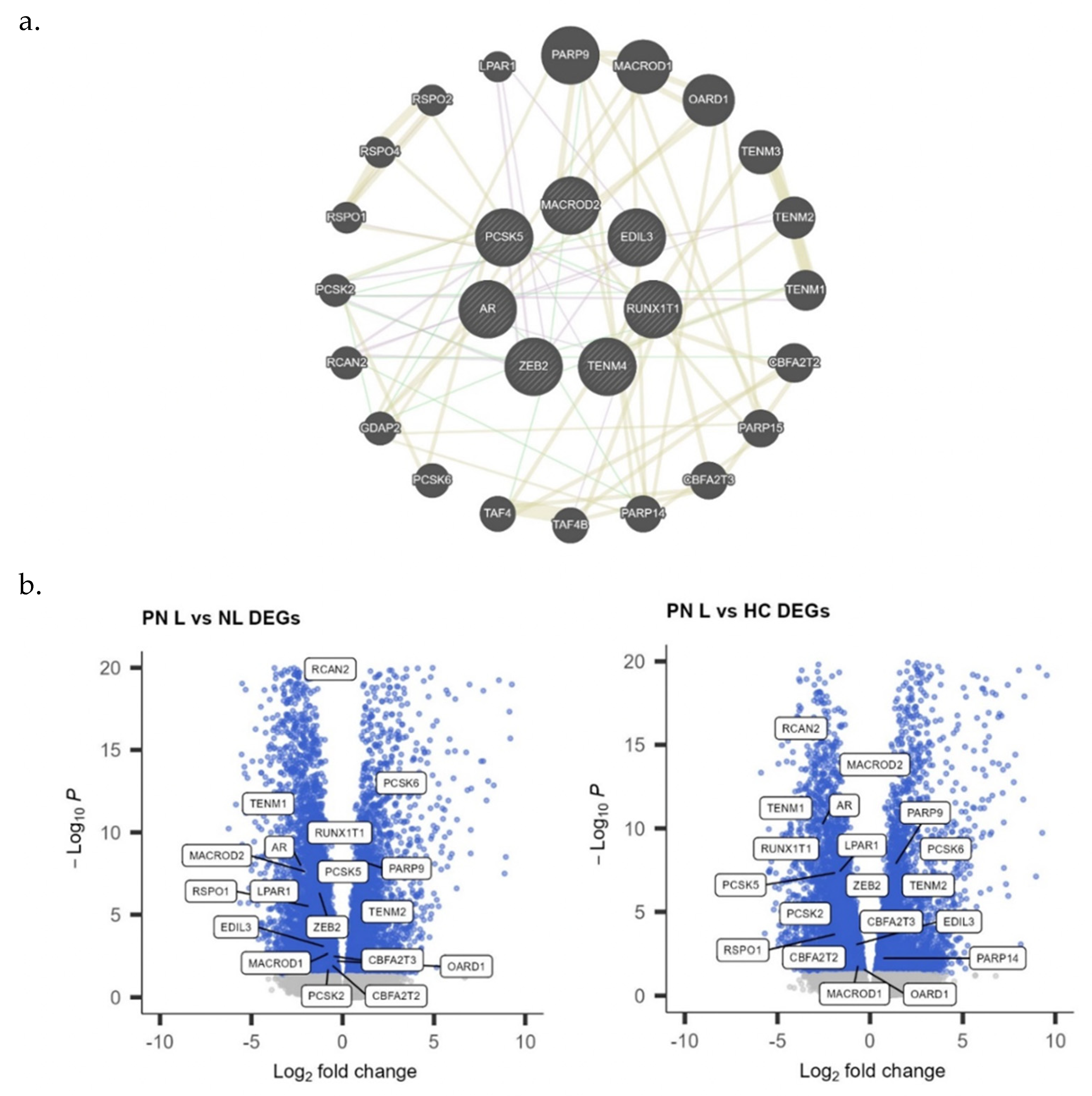
3.3. Genome-Wide Association Analysis for PN and Liver Disease
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwatra, S.G. Breaking the Itch–Scratch Cycle in Prurigo Nodularis. N. Engl. J. Med. 2020, 382, 757–758. [Google Scholar] [CrossRef] [PubMed]
- Whang, K.A.; Le, T.K.; Khanna, R.; Williams, K.A.; Roh, Y.S.; Sutaria, N.; Choi, J.; Gabriel, S.; Chavda, R.; Semenov, Y.; et al. Health-related quality of life and economic burden of prurigo nodularis. J. Am. Acad. Dermatol. 2022, 86, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Steinke, S.; Zeidler, C.; Riepe, C.; Bruland, P.; Soto-Rey, I.; Storck, M.; Augustin, M.; Bobko, S.; Garcovich, S.; Legat, F.J.; et al. Humanistic burden of chronic pruritus in patients with inflammatory dermatoses: Results of the European Academy of Dermatology and Venereology Network on Assessment of Severity and Burden of Pruritus (PruNet) cross-sectional trial. J. Am. Acad. Dermatol. 2018, 79, 457–463.e5. [Google Scholar] [CrossRef] [PubMed]
- Fostini, A.C.; Girolomoni, G.; Tessari, G. Prurigo nodularis: An update on etiopathogenesis and therapy. J. Dermatolog. Treat. 2013, 24, 458–462. [Google Scholar] [CrossRef]
- Whang, K.A.; Khanna, R.; Thomas, J.; Aguh, C.; Kwatra, S.G. Racial and Gender Differences in the Presentation of Pruritus. Medicines 2019, 6, 98. [Google Scholar] [CrossRef]
- Joel, M.Z.; Hydol-Smith, J.; Kambala, A.; Cornman, H.L.; Kwatra, S.G. Prevalence and comorbidity burden of prurigo nodularis in United States adults enrolled in the All of Us research program. J. Am. Acad. Dermatol. 2023, 89, 1056–1058. [Google Scholar] [CrossRef]
- Joel, M.Z.; Taylor, M.T.; Cornman, H.L.; Kambala, A.; Reddy, S.V.; Gabriel, S.; Kwatra, S.G. Risk of incident sleep disorders in patients with prurigo nodularis: A population-level analysis using The Health Improvement Network. JAAD Int. 2023, 13, 39–45. [Google Scholar] [CrossRef]
- Sutaria, N.; Marani, M.; Choi, J.; Roh, Y.S.; Parthasarathy, V.; Deng, J.; Bordeaux, Z.A.; Taylor, M.T.; Lee, K.K.; Pritchard, T.; et al. Racial differences in dysregulation of the renin-angiotensin-aldosterone system in patients with prurigo nodularis. J. Dermatol. Sci. 2022, 105, 130–136. [Google Scholar] [CrossRef]
- Boozalis, E.; Tang, O.; Patel, S.; Semenov, Y.R.; Pereira, M.P.; Stander, S.; Kang, S.; Kwatra, S.G. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J. Am. Acad. Dermatol. 2018, 79, 714–719.e3. [Google Scholar] [CrossRef]
- Sutaria, N.; Parthasarathy, V.; Roh, Y.S.; Choi, J.; Bordeaux, Z.A.; Trinh, P.; Le, T.K.; Semenov, Y.R.; Kwatra, S.G. Itch in skin of colour: A multicentre cross-sectional study. Br. J. Dermatol. 2021, 185, 652–654. [Google Scholar] [CrossRef]
- Mettang, T.; Vonend, A.; Raap, U. Prurigo nodularis: Its association with dermatoses and systemic disorders. Hautarzt 2014, 65, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Shimizu, Y. Recent advances in the management of pruritus in chronic liver diseases. World J. Gastroenterol. 2017, 23, 3418–3426. [Google Scholar] [CrossRef] [PubMed]
- Bergasa, N.V.; Mehlman, J.K.; Jones, E.A. Pruritus and fatigue in primary biliary cirrhosis. Baillieres Best Pract. Res. Clin. Gastroenterol. 2000, 14, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Kiwi, M.L.; Boparai, N.; Price, L.L.; Guyatt, G. Cholestatic liver diseases and health-related quality of life. Am. J. Gastroenterol. 2000, 95, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Koulentaki, M.; Ioannidou, D.; Stefanidou, M.; Maraki, S.; Drigiannakis, I.; Dimoulios, P.; Melono, J.M.E.; Tosca, A.; Kouroumalis, E.A. Dermatological manifestations in primary biliary cirrhosis patients: A case control study. Am. J. Gastroenterol. 2006, 101, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Asano, T.; Morino, M.; Matsumoto, K.; Kashima, H.; Koito, Y.; Miura, T.; Takahashi, Y.; Tsuboi, R.; Ishii, T.; et al. Pruritus is common in patients with chronic liver disease and is improved by nalfurafine hydrochloride. Sci. Rep. 2021, 11, 3015. [Google Scholar] [CrossRef] [PubMed]
- Mela, M.; Mancuso, A.; Burroughs, A.K. Review article: Pruritus in cholestatic and other liver diseases. Aliment. Pharmacol. Ther. 2003, 17, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Nakajima, K.; Takaishi, M.; Ohko, K.; Serada, S.; Fujimoto, M.; Naka, T.; Sano, S. The Skin-Liver Axis Modulates the Psoriasiform Phenotype and Involves Leucine-Rich α-2 Glycoprotein. J. Immunol. 2021, 206, 1469–1477. [Google Scholar] [CrossRef]
- Vasavda, C.; Wan, G.; Szeto, M.D.; Marani, M.; Sutaria, N.; Rajeh, A.; Lu, C.; Lee, K.K.; Nguyen, N.T.T.; Adawi, W.; et al. A Polygenic Risk Score for Predicting Racial and Genetic Susceptibility to Prurigo Nodularis. J. Investig. Dermatol. 2023, 143, 2416. [Google Scholar] [CrossRef]
- Belzberg, M.; Alphonse, M.P.; Brown, I.; Williams, K.A.; Khanna, R.; Ho, B.; Wongvibulsin, S.; Pritchard, T.; Roh, Y.S.; Sutaria, N.; et al. Prurigo Nodularis Is Characterized by Systemic and Cutaneous T Helper 22 Immune Polarization. J. Investig. Dermatol. 2021, 141, 2208–2218.e14. [Google Scholar] [CrossRef]
- Ständer, S.; Yosipovitch, G.; Legat, F.J.; Lacour, J.; Paul, C.; Narbutt, J.; Bieber, T.; Misery, L.; Wollenberg, A.; Reich, A.; et al. Trial of Nemolizumab in Moderate-to-Severe Prurigo Nodularis. N. Engl. J. Med. 2020, 382, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Hänzelmann, S.; Castelo, R.; Guinney, J. GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013, 14, 7. [Google Scholar] [CrossRef]
- Watanabe, K.; Taskesen, E.; van Bochoven, A.; Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 2017, 8, 1826. [Google Scholar] [CrossRef] [PubMed]
- GTEx Consortium. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef] [PubMed]
- Bonacini, M. Pruritus in patients with chronic human immunodeficiency virus, hepatitis B and C virus infections. Dig. Liver Dis. 2000, 32, 621–625. [Google Scholar] [CrossRef]
- Dega, H.; Francès, C.; Dupin, N.; Lebre, C.; Simantov, A.; Callot, C.; Laporte, J.L.; Blot, C.; Opolon, P.; Poynard, T.; et al. Pruritus and the hepatitis C virus. The MULTIVIRC Unit. Ann. Dermatol. Venereol. 1998, 125, 9–12. [Google Scholar]
- Cacoub, P.; Poynard, T.; Ghillani, P.; Charlotte, F.; Olivi, M.; Piette, J.C.; Opolon, P. Extrahepatic manifestations of chronic hepatitis C. MULTIVIRC Group. Multidepartment Virus C. Arthritis Rheumatol. 1999, 42, 2204–2212. [Google Scholar] [CrossRef]
- Jones, E.A.; Bergasa, N.V. Evolving concepts of the pathogenesis and treatment of the pruritus of cholestasis. Can. J. Gastroenterol. 2000, 14, 33–40. [Google Scholar] [CrossRef]
- Thijs, J.; Krastev, T.; Weidinger, S.; Buckens, C.F.; de Bruin-Weller, M.; Bruijnzeel-Koomen, C.; Flohr, C.; Hijnen, D. Biomarkers for atopic dermatitis: A systematic review and meta-analysis. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 453–460. [Google Scholar] [CrossRef]
- Mukai, H.; Noguchi, T.; Kamimura, K.; Nishioka, K.; Nishiyama, S. Significance of elevated serum LDH (lactate dehydrogenase) activity in atopic dermatitis. J. Dermatol. 1990, 17, 477–481. [Google Scholar] [CrossRef]
- Nagasue, N.; Ito, A.; Yukaya, H.; Ogawa, Y. Androgen receptors in hepatocellular carcinoma and surrounding parenchyma. Gastroenterology 1985, 89, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.E.; Xie, X.U.; Lin, Y.; Tan, G.; Zhong, W.U. Androgen receptor in hepatocarcinogenesis: Recent developments and perspectives. Oncol. Lett. 2015, 9, 1983–1988. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Lu, J.; Huo, X.; Gong, Z. Liver-specific androgen receptor knockout attenuates early liver tumor development in zebrafish. Sci. Rep. 2019, 9, 10645. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Yang, X.; Glick, A.B.; Weinstein, M.; Letterio, J.L.; Mizel, D.E.; Anzano, M.; Greenwell-Wild, T.; Wahl, S.M.; Deng, C.; et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1999, 1, 260–266. [Google Scholar] [CrossRef]
- Mast, B.A.; Schultz, G.S. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. 1996, 4, 411–420. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Xu, J.; Zanvit, P.; Hu, L.; Tseng, P.; Liu, N.; Wang, F.; Liu, O.; Zhang, D.; Jin, W.; Guo, N.; et al. The Cytokine TGF-β Induces Interleukin-31 Expression from Dermal Dendritic Cells to Activate Sensory Neurons and Stimulate Wound Itching. Immunity 2020, 53, 371–383.e5. [Google Scholar] [CrossRef]
- Yeh, K.; Chen, T.; Yang, H.; Chou, J.; Chen, L.; Yeh, C.; Chen, Y.; Lin, R.; Su, H.; Chen, G.C.; et al. Aberrant TGFβ/SMAD4 signaling contributes to epigenetic silencing of a putative tumor suppressor, RunX1T1, in ovarian cancer. Epigenetics 2011, 6, 727–739. [Google Scholar] [CrossRef]
- Helms, C.; Cao, L.; Krueger, J.G.; Wijsman, E.M.; Chamian, F.; Gordon, D.; Heffernan, M.; Daw, J.A.W.; Robarge, J.; Ott, J.; et al. A putative RUNX1 binding site variant between SLC9A3R1 and NAT9 is associated with susceptibility to psoriasis. Nat. Genet. 2003, 35, 349–356. [Google Scholar] [CrossRef]
- Nasir, A.; Helm, J.; Turner, L.; Chen, D.; Strosberg, J.; Hafez, N.; Henderson-Jackson, E.B.; Hodul, P.; Bui, M.M.; Nasir, N.A.; et al. RUNX1T1: A Novel Predictor of Liver Metastasis in Primary Pancreatic Endocrine Neoplasms. Pancreas 2011, 40, 627–633. [Google Scholar] [CrossRef]
- Lotem, J.; Levanon, D.; Negreanu, V.; Bauer, O.; Hantisteanu, S.; Dicken, J.; Groner, Y. Runx3 at the interface of immunity, inflammation and cancer. Biochim. Biophys. Acta 2015, 1855, 131–143. [Google Scholar] [CrossRef]
- Rada, M.; Kapelanski-Lamoureux, A.; Petrillo, S.; Tabariès, S.; Siegel, P.; Reynolds, A.R.; Lazaris, A.; Metrakos, P. Runt related transcription factor-1 plays a central role in vessel co-option of colorectal cancer liver metastases. Commun. Biol. 2021, 4, 950. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Muller, A.; Lauerma, A.I.; Pivarcsi, A.; Soto, H.; Kemeny, L.; Alenius, H.; Dieu-Nosjean, M.; Meller, S.; Rieker, J.; et al. IL-31: A new link between T cells and pruritus in atopic skin inflammation. J. Allergy Clin. Immunol. 2006, 117, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Furue, M.; Ulzii, D.; Vu, Y.; Tsuji, G.; Kido-Nakahara, M.; Nakahara, T. Pathogenesis of Atopic Dermatitis: Current Paradigm. Iran. J. Immunol. 2019, 16, 97–107. [Google Scholar]
- Cornelissen, C.; Marquardt, Y.; Czaja, K.; Wenzel, J.; Frank, J.; Lüscher-Firzlaff, J.; Lüscher, B.; Baron, J.M. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. J. Allergy Clin. Immunol. 2012, 129, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Feld, M.; Garcia, R.; Buddenkotte, J.; Katayama, S.; Lewis, K.; Muirhead, G.; Hevezi, P.; Plesser, K.; Schrumpf, H.; Krjutskov, K.; et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J. Allergy Clin. Immunol. 2016, 138, 500–508.e24. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Caballero-Díaz, D. Transforming Growth Factor-β-Induced Cell Plasticity in Liver Fibrosis and Hepatocarcinogenesis. Front. Oncol. 2018, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Dooley, S.; ten Dijke, P. TGF-β in progression of liver disease. Cell Tissue Res. 2012, 347, 245–256. [Google Scholar] [CrossRef]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef]
- Friedman, S.L. Hepatic Stellate Cells: Protean, Multifunctional, and Enigmatic Cells of the Liver. Physiol. Rev. 2008, 88, 125–172. [Google Scholar] [CrossRef]
- Kawada, N. Human hepatic stellate cells are resistant to apoptosis: Implications for human fibrogenic liver disease. Gut 2006, 55, 1073–1074. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Q.; Cao, S.; Xu, T.; Li, X.; Zhou, D.; Pan, L.; Li, C.; Huang, C.; Meng, X.; et al. MicroRNA-145 Increases the Apoptosis of Activated Hepatic Stellate Cells Induced by TRAIL through NF-κB Signaling Pathway. Front. Pharmacol. 2018, 8, 980. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Fan, F.; Liu, P. BTXA regulates the epithelial–mesenchymal transition and autophagy of keloid fibroblasts via modulating miR-1587/miR-2392 targeted ZEB2. Biosci. Rep. 2019, 39, BSR20190679. [Google Scholar] [CrossRef] [PubMed]
- Tatari, M.N.; De Craene, B.; Soen, B.; Taminau, J.; Vermassen, P.; Goossens, S.; Haigh, K.; Cazzola, S.; Lambert, J.; Huylebroeck, D.; et al. ZEB2-transgene expression in the epidermis compromises the integrity of the epidermal barrier through the repression of different tight junction proteins. Cell Mol. Life Sci. 2014, 71, 3599–3609. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, N.; Denecker, G.; Bruneel, K.; Blancke, G.; Akay, Ö.; Taminau, J.; De Coninck, J.; De Smedt, E.; Skrypek, N.; Van Loocke, W.; et al. The EMT Transcription Factor ZEB2 Promotes Proliferation of Primary and Metastatic Melanoma While Suppressing an Invasive, Mesenchymal-Like Phenotype. Cancer Res. 2020, 80, 2983–2995. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef]
- Gasca, J.; Flores, M.L.; Jiménez-Guerrero, R.; Sáez, M.E.; Barragán, I.; Ruíz-Borrego, M.; Tortolero, M.; Romero, F.; Sáez, C.; Japón, M.A. EDIL3 promotes epithelial–mesenchymal transition and paclitaxel resistance through its interaction with integrin αVβ3 in cancer cells. Cell Death Discov. 2020, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Li, X.; Feng, Z.; Han, Q.; Li, J.; Liu, Y.; Zhang, K. EDIL3 and VEGF Synergistically Affect Angiogenesis in Endothelial Cells. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1269–1277. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, Y.; Yang, J.; Li, J.; Feng, M.; Wang, Y.; Yang, X.; He, P.; Tian, G.; Zhang, X.; et al. Overexpressed EDIL3 predicts poor prognosis and promotes anchorage-independent tumor growth in human pancreatic cancer. Oncotarget 2016, 7, 4226–4240. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, K.; Wang, B.; Yang, X.; Zhang, Z. EDIL3 regulates gastric cancer cell migration, invasion and epithelial-mesenchymal transition via TGF-β1/XIST/miR-137 feedback loop. Transl. Cancer Res. 2020, 9, 6313–6330. [Google Scholar] [CrossRef]
- Sun, J.; Liang, X.; Pan, K.; Wang, H.; Zhao, J.; Li, J.; Ma, H.; Chen, Y.; Xia, J. High expression level of EDIL3 in HCC predicts poor prognosis of HCC patients. World J. Gastroenterol. 2010, 16, 4611–4615. [Google Scholar] [CrossRef]
- Zhou, Z.; Luo, C.; Xin, H.; Hu, Z.; Zhu, G.; Li, J.; Zhou, S. MACROD2 deficiency promotes hepatocellular carcinoma growth and metastasis by activating GSK-3β/β-catenin signaling. NPJ Genom. Med. 2020, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Krueger, J.G.; Li, K.; Jabbari, A.; Brodmerkel, C.; Lowes, M.A.; Suárez-Fariñas, M. Meta-Analysis Derived (MAD) Transcriptome of Psoriasis Defines the “Core” Pathogenesis of Disease. PLoS ONE 2012, 7, e44274. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Fariñas, M.; Li, K.; Fuentes-Duculan, J.; Hayden, K.; Brodmerkel, C.; Krueger, J.G. Expanding the psoriasis disease profile: Interrogation of the skin and serum of patients with moderate-to-severe psoriasis. J. Investig. Dermatol. 2012, 132, 2552–2564. [Google Scholar] [CrossRef] [PubMed]
- Peppino, G.; Ruiu, R.; Arigoni, M.; Riccardo, F.; Iacoviello, A.; Barutello, G.; Quaglino, E. Teneurins: Role in Cancer and Potential Role as Diagnostic Biomarkers and Targets for Therapy. Int. J. Mol. Sci. 2021, 22, 2321. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo-Jaramillo, B.; Ziegler, A. Teneurins: An Integrative Molecular, Functional, and Biomedical Overview of Their Role in Cancer. Front. Neurosci. 2018, 12, 937. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, L.; Pang, Y.; Yang, J.; Lv, M.; Liu, F.; Qu, X.; Chen, X.; Gong, H.; Liu, D.; et al. GDF11/BMP11 as a novel tumor marker for liver cancer. Exp. Ther. Med. 2018, 15, 3495–3500. [Google Scholar] [CrossRef]
- Ahmad, W.; Ijaz, B.; Hassan, S. Gene expression profiling of HCV genotype 3a initial liver fibrosis and cirrhosis patients using microarray. J. Transl. Med. 2012, 10, 41. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marani, M.; Madan, V.; Le, T.K.; Deng, J.; Lee, K.K.; Ma, E.Z.; Kwatra, S.G. Dysregulation of the Skin–Liver Axis in Prurigo Nodularis: An Integrated Genomic, Transcriptomic, and Population-Based Analysis. Genes 2024, 15, 146. https://doi.org/10.3390/genes15020146
Marani M, Madan V, Le TK, Deng J, Lee KK, Ma EZ, Kwatra SG. Dysregulation of the Skin–Liver Axis in Prurigo Nodularis: An Integrated Genomic, Transcriptomic, and Population-Based Analysis. Genes. 2024; 15(2):146. https://doi.org/10.3390/genes15020146
Chicago/Turabian StyleMarani, Melika, Vrinda Madan, Thomas K. Le, Junwen Deng, Kevin K. Lee, Emily Z. Ma, and Shawn G. Kwatra. 2024. "Dysregulation of the Skin–Liver Axis in Prurigo Nodularis: An Integrated Genomic, Transcriptomic, and Population-Based Analysis" Genes 15, no. 2: 146. https://doi.org/10.3390/genes15020146



