Multiplex Real-Time PCR-Based Newborn Screening for Severe Primary Immunodeficiency and Spinal Muscular Atrophy in Osaka, Japan: Our Results after 3 Years
Abstract
:1. Introduction
2. Materials and Methods
2.1. Optional Screening for PID and SMA in Osaka
2.2. Consent for the Optional NBS Program of OWCH
2.3. Workflow for the Optional NBS Program
2.3.1. From Collecting DBS Samples to Performing the Screening Tests
2.3.2. Workflow for the PID-NBS Program
2.3.3. Workflow for the SMA-NBS Program
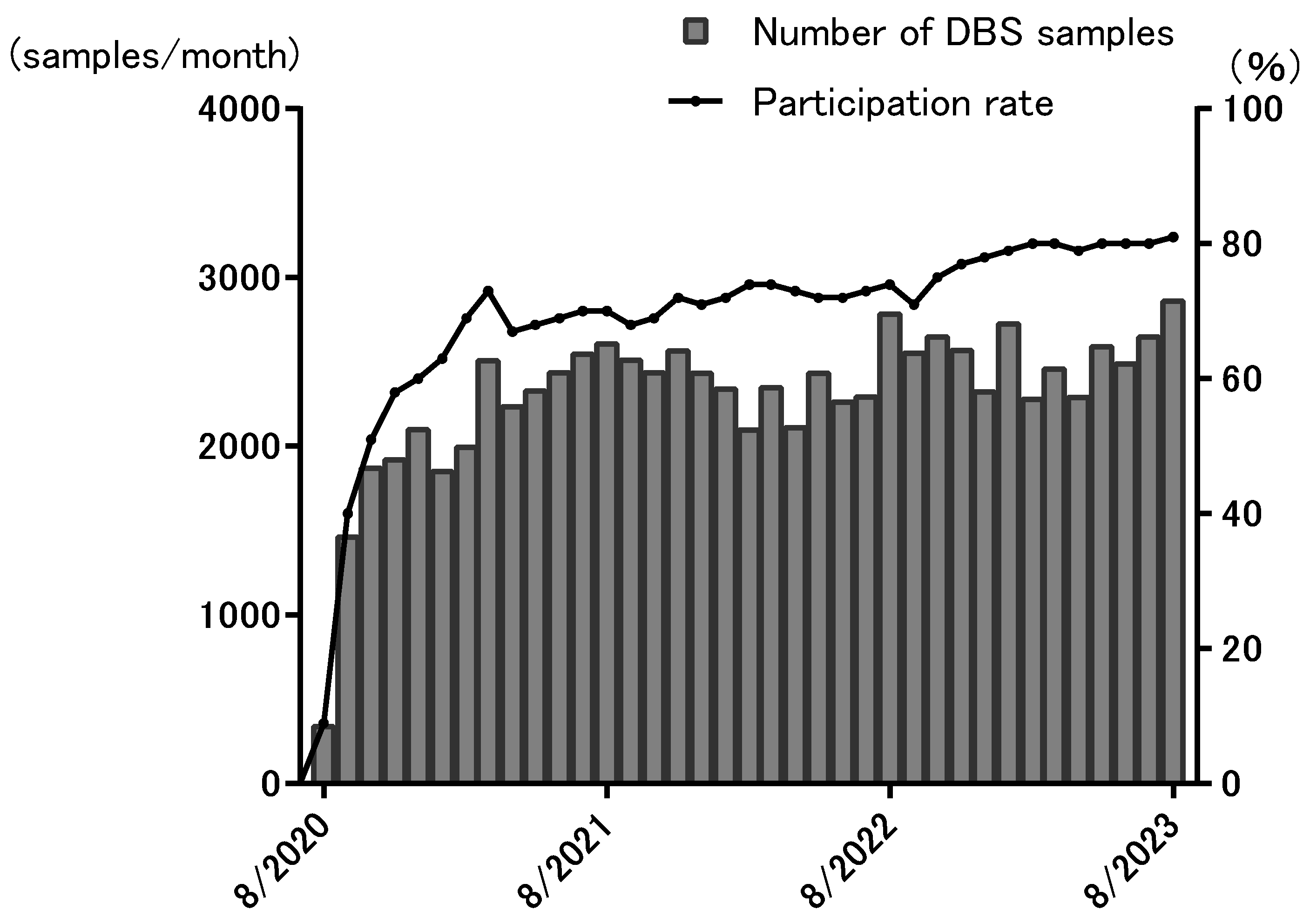
3. Results
4. Discussion
4.1. PID Screening in Osaka and the Merits of the Simultaneous Assessment of TREC and KREC Levels
4.2. SMA-NBS in Osaka and the Merits of Screening for PID and SMA Simultaneously
4.3. Issues with PID-NBS and SMA-NBS
4.3.1. PID-NBS
4.3.2. SMA-NBS
4.4. Limitations of Our Project and Optional NBS Programs in Japan
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, J.M.G.; Jungner, G.; World Health Organization. Principles and Practice of Screening for Disease; World Health Organization: Geneva, Switzerland, 1968.
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 42, 1473–1507. [Google Scholar] [CrossRef]
- Pai, S.Y.; Logan, B.R.; Griffith, L.M.; Buckley, R.H.; Parrott, R.E.; Dvorak, C.C.; Kapoor, N.; Hanson, I.C.; Filipovich, A.H.; Jyonouchi, S.; et al. Transplantation Outcomes for Severe Combined Immunodeficiency, 2000–2009. N. Engl. J. Med. 2014, 371, 434–446. [Google Scholar] [CrossRef]
- Plebani, A.; Soresina, A.; Rondelli, R.; Amato, G.M.; Azzari, C.; Cardinale, F.; Cazzola, G.; Consolini, R.; De Mattia, D.; Dell’Erba, G.; et al. Clinical, Immunological, and Molecular Analysis in a Large Cohort of Patients with X-Linked Agammaglobulinemia: An Italian Multicenter Study. Clin. Immunol. 2002, 104, 221–230. [Google Scholar] [CrossRef]
- Hazenberg, M.D.; Verschuren, M.C.; Hamann, D.; Miedema, F.; van Dongen, J.J. T Cell Receptor Excision Circles as Markers for Recent Thymic Emigrants: Basic Aspects, Technical Approach, and Guidelines for Interpretation. J. Mol. Med. 2001, 79, 631–640. [Google Scholar] [CrossRef]
- Morinishi, Y.; Imai, K.; Nakagawa, N.; Sato, H.; Horiuchi, K.; Ohtsuka, Y.; Kaneda, Y.; Taga, T.; Hisakawa, H.; Miyaji, R.; et al. Identification of Severe Combined Immunodeficiency by T-cell Receptor Excision Circles Quantification Using Neonatal Guthrie Cards. J. Pediatr. 2009, 155, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Siminovitch, K.A.; Bakhshi, A.; Goldman, P.; Korsmeyer, S.J. A Uniform Deleting Element Mediates the Loss of kappa Genes in Human B Cells. Nature 1985, 316, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, N.; Imai, K.; Kanegane, H.; Sato, H.; Yamada, M.; Kondoh, K.; Okada, S.; Kobayashi, M.; Agematsu, K.; Takada, H.; et al. Quantification of kappa-Deleting Recombination Excision Circles in Guthrie Cards for the Identification of Early B-cell Maturation Defects. J. Allergy Clin. Immunol. 2011, 128, 223–225.e222. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.; Puck, J.M. Development of Population-Based Newborn Screening for Severe Combined Immunodeficiency. J. Allergy Clin. Immunol. 2005, 115, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, M.; Puck, J. Newborn Screening for Severe Combined Immunodeficiency in the US: Current Status and Approach to Management. Int. J. Neonatal Screen. 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Kutlug, S.; Karadag Alpaslan, M.; Hancioglu, G.; Elif Ozyazici Ozkan, S.; Cemile Yesilirmak, D.; Bulut, H.; Aygun, C.; Ogur, G.; Yildiran, A. Multiplex PCR-Based Newborn Screening for Severe T and B-Cell Lymphopenia: The first Pilot Study in Turkey. Sisli Etfal Hast. Tip. Bul. 2021, 55, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Fisfalen, M.E.; DeGroot, L.J.; Quintans, J.; Franklin, W.A.; Soltani, K. Microsomal Antigen-Reactive Lymphocyte Lines and Clones Derived from Thyroid Tissue of Patients with Graves’ Disease. J. Clin. Endocrinol. Metab. 1988, 66, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Gizewska, M.; Durda, K.; Winter, T.; Ostrowska, I.; Oltarzewski, M.; Klein, J.; Blankenstein, O.; Romanowska, H.; Krzywinska-Zdeb, E.; Patalan, M.F.; et al. Newborn Screening for SCID and Other Severe Primary Immunodeficiency in the Polish-German Transborder Area: Experience From the First 14 Months of Collaboration. Front. Immunol. 2020, 11, 1948. [Google Scholar] [CrossRef] [PubMed]
- Truck, J.; Prader, S.; Natalucci, G.; Hagmann, C.; Brotschi, B.; Kelly, J.; Bassler, D.; Steindl, K.; Rauch, A.; Baumgartner, M.; et al. Swiss Newborn Screening for Severe T and B cell Deficiency with a Combined TREC/KREC Assay—Management Recommendations. Swiss Med. Wkly. 2020, 150, w20254. [Google Scholar] [CrossRef]
- Lefebvre, S.; Burglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and Characterization of a Spinal Muscular Atrophy-Determining Gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Calucho, M.; Bernal, S.; Alias, L.; March, F.; Vencesla, A.; Rodriguez-Alvarez, F.J.; Aller, E.; Fernandez, R.M.; Borrego, S.; Millan, J.M.; et al. Correlation between SMA Type and SMN2 Copy Number Revisited: An Analysis of 625 Unrelated Spanish Patients and a Compilation of 2834 Reported Cases. Neuromuscul. Disord. NMD 2018, 28, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Verhaart, I.E.C.; Robertson, A.; Wilson, I.J.; Aartsma-Rus, A.; Cameron, S.; Jones, C.C.; Cook, S.F.; Lochmuller, H. Prevalence, Incidence and Carrier Frequency of 5q-Linked Spinal Muscular Atrophy—A Literature Review. Orphanet J. Rare Dis. 2017, 12, 124. [Google Scholar] [CrossRef]
- Arnold, W.D.; Kassar, D.; Kissel, J.T. Spinal Muscular Atrophy: Diagnosis and Management in a New Therapeutic Era. Muscle Nerve 2015, 51, 157–167. [Google Scholar] [CrossRef]
- Keinath, M.C.; Prior, D.E.; Prior, T.W. Spinal Muscular Atrophy: Mutations, Testing, and Clinical Relevance. Appl. Clin. Genet. 2021, 14, 11–25. [Google Scholar] [CrossRef]
- Shorrock, H.K.; Gillingwater, T.H.; Groen, E.J.N. Overview of Current Drugs and Molecules in Development for Spinal Muscular Atrophy Therapy. Drugs 2018, 78, 293–305. [Google Scholar] [CrossRef]
- De Vivo, D.C.; Bertini, E.; Swoboda, K.J.; Hwu, W.L.; Crawford, T.O.; Finkel, R.S.; Kirschner, J.; Kuntz, N.L.; Parsons, J.A.; Ryan, M.M.; et al. Nusinersen Initiated in Infants during the Presymptomatic Stage of Spinal Muscular Atrophy: Interim Efficacy and Safety Results from the Phase 2 NURTURE Study. Neuromuscul. Disord. NMD 2019, 29, 842–856. [Google Scholar] [CrossRef]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene Abeparvovec for Presymptomatic Infants with Two Copies of SMN2 at Risk for Spinal Muscular Atrophy Type 1: The Phase III SPR1NT Trial. Nat. Med. 2022, 28, 1381–1389. [Google Scholar] [CrossRef]
- Strauss, K.A.; Farrar, M.A.; Muntoni, F.; Saito, K.; Mendell, J.R.; Servais, L.; McMillan, H.J.; Finkel, R.S.; Swoboda, K.J.; Kwon, J.M.; et al. Onasemnogene Abeparvovec for Presymptomatic Infants with Three Copies of SMN2 at Risk for Spinal Muscular Atrophy: The Phase III SPR1NT Trial. Nat. Med. 2022, 28, 1390–1397. [Google Scholar] [CrossRef]
- Taylor, J.L.; Lee, F.K.; Yazdanpanah, G.K.; Staropoli, J.F.; Liu, M.; Carulli, J.P.; Sun, C.; Dobrowolski, S.F.; Hannon, W.H.; Vogt, R.F. Newborn Blood Spot Screening Test Using Multiplexed Real-Time PCR to Simultaneously Screen for Spinal Muscular Atrophy and Severe Combined Immunodeficiency. Clin. Chem. 2015, 61, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.H.; Chiang, S.C.; Weng, W.C.; Lee, N.C.; Lin, C.J.; Hsieh, W.S.; Lee, W.T.; Jong, Y.J.; Ko, T.M.; Hwu, W.L. Presymptomatic Diagnosis of Spinal Muscular Atrophy through Newborn Screening. J. Pediatr. 2017, 190, 124–129.e121. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Segawa, O.; Yakabe, M.; Ishida, Y.; Kuroita, T.; Ikeda, K.; Kawakami, B.; Kawamura, Y.; Yohda, M.; Matsunaga, T.; et al. Development of a Novel Method for Operating Magnetic Particles, Magtration Technology, and Its Use for Automating Nucleic Acid Purification. J. Biosci. Bioeng. 2001, 91, 500–503. [Google Scholar] [CrossRef]
- Kimizu, T.; Ida, S.; Oki, K.; Shima, M.; Nishimoto, S.; Nakajima, K.; Ikeda, T.; Mogami, Y.; Yanagihara, K.; Matsuda, K.; et al. Newborn Screening for Spinal Muscular Atrophy in Osaka-Challenges in a Japanese Pilot Study. Brain Dev. 2023, 45, 363–371. [Google Scholar] [CrossRef]
- Patel, N.C.; Hertel, P.M.; Estes, M.K.; de la Morena, M.; Petru, A.M.; Noroski, L.M.; Revell, P.A.; Hanson, I.C.; Paul, M.E.; Rosenblatt, H.M.; et al. Vaccine-Acquired Rotavirus in Infants with Severe Combined Immunodeficiency. N. Engl. J. Med. 2010, 362, 314–319. [Google Scholar] [CrossRef]
- de Felipe, B.; Olbrich, P.; Lucenas, J.M.; Delgado-Pecellin, C.; Pavon-Delgado, A.; Marquez, J.; Salamanca, C.; Soler-Palacin, P.; Gonzalez-Granado, L.I.; Antolin, L.F.; et al. Prospective Neonatal Screening for Severe T- and B-lymphocyte Deficiencies in Seville. Pediatr. Allergy Immunol. 2016, 27, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, M.; Ohlsson, A.; Borte, S.; Jonsson, S.; Zetterstrom, R.H.; King, J.; Winiarski, J.; von Dobeln, U.; Hammarstrom, L. Newborn Screening for Severe Primary Immunodeficiency Diseases in Sweden-a 2-Year Pilot TREC and KREC Screening Study. J. Clin. Immunol. 2017, 37, 51–60. [Google Scholar] [CrossRef]
- Chien, Y.H.; Chiang, S.C.; Chang, K.L.; Yu, H.H.; Lee, W.I.; Tsai, L.P.; Hsu, L.W.; Hu, M.H.; Hwu, W.L. Incidence of Severe Combined Immunodeficiency through Newborn Screening in a Chinese Population. J. Formos. Med. Assoc. 2015, 114, 12–16. [Google Scholar] [CrossRef]
- Amatuni, G.S.; Currier, R.J.; Church, J.A.; Bishop, T.; Grimbacher, E.; Nguyen, A.A.; Agarwal-Hashmi, R.; Aznar, C.P.; Butte, M.J.; Cowan, M.J.; et al. Newborn Screening for Severe Combined Immunodeficiency and T-cell Lymphopenia in California, 2010–2017. Pediatrics 2019, 143, e20182300. [Google Scholar] [CrossRef] [PubMed]
- Nourizadeh, M.; Shakerian, L.; Borte, S.; Fazlollahi, M.; Badalzadeh, M.; Houshmand, M.; Alizadeh, Z.; Dalili, H.; Rashidi-Nezhad, A.; Kazemnejad, A.; et al. Newborn Screening Using TREC/KREC Assay for Severe T and B Cell Lymphopenia in Iran. Scand. J. Immunol. 2018, 88, e12699. [Google Scholar] [CrossRef]
- Kamae, C.; Nakagawa, N.; Sato, H.; Honma, K.; Mitsuiki, N.; Ohara, O.; Kanegane, H.; Pasic, S.; Pan-Hammarstrom, Q.; van Zelm, M.C.; et al. Common Variable Immunodeficiency Classification by Quantifying T-cell Receptor and Immunoglobulin kappa-Deleting Recombination Excision Circles. J. Allergy Clin. Immunol. 2013, 131, 1437–1440.e1435. [Google Scholar] [CrossRef] [PubMed]
- Ang, C.; Zetterstrom, R.H.; Ramme, K.; Axelsen, E.; Marits, P.; Sundin, M. Case Report: IKZF1-Related Early-Onset CID is Expected to be Missed in TREC-Based SCID Screening but can Be Identified by Determination of KREC Levels. Front. Immunol. 2023, 14, 1257581. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, K.; Imai, K.; Shigeno, M.; Sato, H.; Tezuka, M.; Okawa, T.; Mitsuiki, N.; Isoda, T.; Tomizawa, D.; Takagi, M.; et al. Cord Blood Transplantation is Associated with Rapid B-cell Neogenesis Compared with BM Transplantation. Bone Marrow Transpl. 2014, 49, 1155–1161. [Google Scholar] [CrossRef]
- Boemer, F.; Caberg, J.H.; Dideberg, V.; Dardenne, D.; Bours, V.; Hiligsmann, M.; Dangouloff, T.; Servais, L. Newborn Screening for SMA in Southern Belgium. Neuromuscul. Disord. NMD 2019, 29, 343–349. [Google Scholar] [CrossRef]
- Tesorero, R.; Janda, J.; Horster, F.; Feyh, P.; Mutze, U.; Hauke, J.; Schwarz, K.; Kunz, J.B.; Hoffmann, G.F.; Okun, J.G. A High-Throughput Newborn Screening Approach for SCID, SMA, and SCD Combining Multiplex qPCR and Tandem Mass Spectrometry. PLoS ONE 2023, 18, e0283024. [Google Scholar] [CrossRef]
- Dangouloff, T.; Vrščaj, E.; Servais, L.; Osredkar, D.; Adoukonou, T.; Aryani, O.; Barisic, N.; Bashiri, F.; Bastaki, L.; Benitto, A.; et al. Newborn Screening Programs for Spinal Muscular Atrophy Worldwide: Where We Stand and where to Go. Neuromuscul. Disord. NMD 2021, 31, 574–582. [Google Scholar] [CrossRef]
- Boemer, F.; Caberg, J.H.; Beckers, P.; Dideberg, V.; di Fiore, S.; Bours, V.; Marie, S.; Dewulf, J.; Marcelis, L.; Deconinck, N.; et al. Three Years Pilot of Spinal Muscular Atrophy Newborn Screening Turned into Official Program in Southern Belgium. Sci. Rep. 2021, 11, 19922. [Google Scholar] [CrossRef]
- Muller-Felber, W.; Blaschek, A.; Schwartz, O.; Glaser, D.; Nennstiel, U.; Brockow, I.; Wirth, B.; Burggraf, S.; Roschinger, W.; Becker, M.; et al. Newbornscreening SMA—From Pilot Project to Nationwide Screening in Germany. J. Neuromuscul. Dis. 2023, 10, 55–65. [Google Scholar] [CrossRef]
- Lee, B.H.; Deng, S.; Chiriboga, C.A.; Kay, D.M.; Irumudomon, O.; Laureta, E.; Delfiner, L.; Treidler, S.O.; Anziska, Y.; Sakonju, A.; et al. Newborn Screening for Spinal Muscular Atrophy in New York State: Clinical Outcomes from the First 3 Years. Neurology 2022, 99, e1527–e1537. [Google Scholar] [CrossRef]
- Kariyawasam, D.S.; D’Silva, A.M.; Sampaio, H.; Briggs, N.; Herbert, K.; Wiley, V.; Farrar, M.A. Newborn Screening for Spinal Muscular Atrophy in Australia: A Non-Randomised Cohort Study. Lancet Child Adolesc. Health 2023, 7, 159–170. [Google Scholar] [CrossRef]
- Dollard, S.C.; Dreon, M.; Hernandez-Alvarado, N.; Amin, M.M.; Wong, P.; Lanzieri, T.M.; Osterholm, E.A.; Sidebottom, A.; Rosendahl, S.; McCann, M.T.; et al. Sensitivity of Dried Blood Spot Testing for Detection of Congenital Cytomegalovirus Infection. JAMA Pediatr. 2021, 175, e205441. [Google Scholar] [CrossRef]
- Groulx-Boivin, E.; Osman, H.; Chakraborty, P.; Lintern, S.; Oskoui, M.; Selby, K.; Van Caeseele, P.; Wyatt, A.; McMillan, H.J. Variability in Newborn Screening Across Canada: Spinal Muscular Atrophy and Beyond. Can. J. Neurol. Sci. 2023, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, Y.O.S.; Purevsuren, J.; Harahap, N.I.F.; Niba, E.T.E.; Bouike, Y.; Nurputra, D.K.; Rochmah, M.A.; Thursina, C.; Hapsara, S.; Yamaguchi, S.; et al. Assessment of Spinal Muscular Atrophy Carrier Status by Determining SMN1 Copy Number Using Dried Blood Spots. Int. J. Neonatal Screen. 2020, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Weidlich, D.; Servais, L.; Kausar, I.; Howells, R.; Bischof, M. Cost-Effectiveness of Newborn Screening for Spinal Muscular Atrophy in England. Neurol. Ther. 2023, 12, 1205–1220. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Scuffham, P.; Byrnes, J.; Downes, M. Cost-Effectiveness Analysis of Gene-Based Therapies for Patients with Spinal Muscular Atrophy Type I in Australia. J. Neurol. 2022, 269, 6544–6554. [Google Scholar] [CrossRef]
- Landfeldt, E. The Cost-Effectiveness of Newborn Screening for Spinal Muscular Atrophy. Dev. Med. Child Neurol. 2023, 65, 8–9. [Google Scholar] [CrossRef]
- Shih, S.T.; Farrar, M.A.; Wiley, V.; Chambers, G. Newborn Screening for Spinal Muscular Atrophy with Disease-Modifying Therapies: A Cost-Effectiveness Analysis. J. Neurol. Neurosurg. Psychiatry 2021, 92, 1296–1304. [Google Scholar] [CrossRef]
- Kwan, A.; Abraham, R.S.; Currier, R.; Brower, A.; Andruszewski, K.; Abbott, J.K.; Baker, M.; Ballow, M.; Bartoshesky, L.E.; Bonilla, F.A.; et al. Newborn Screening for Severe Combined Immunodeficiency in 11 Screening Programs in the United States. JAMA 2014, 312, 729–738. [Google Scholar] [CrossRef]
- Abiusi, E.; Vaisfeld, A.; Fiori, S.; Novelli, A.; Spartano, S.; Faggiano, M.V.; Giovanniello, T.; Angeloni, A.; Vento, G.; Santoloci, R.; et al. Experience of a 2-Year Spinal Muscular Atrophy NBS Pilot Study in Italy: Towards Specific Guidelines and Standard Operating Procedures for the Molecular Diagnosis. J. Med. Genet. 2023, 60, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Yamauchi, A.; Urano, M.; Kato, T.; Matsuo, M.; Nakashima, K.; Saito, K. Epidemiological Investigation of Spinal Muscular Atrophy in Japan. Brain Dev. 2022, 44, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Sonehara, S.; Bo, R.; Nambu, Y.; Iketani, K.; Lee, T.; Shimomura, H.; Ueda, M.; Takeshima, Y.; Iijima, K.; Nozu, K.; et al. Newborn Screening for Spinal Muscular Atrophy: A 2.5-Year Experience in Hyogo Prefecture, Japan. Genes 2023, 14, 2211. [Google Scholar] [CrossRef]
- Sawada, T.; Kido, J.; Sugawara, K.; Yoshida, S.; Ozasa, S.; Nomura, K.; Okada, K.; Fujiyama, N.; Nakamura, K. Newborn Screening for Spinal Muscular Atrophy in Japan: One Year of Experience. Mol. Genet. Metab. Rep. 2022, 32, 100908. [Google Scholar] [CrossRef]
- Serra-Juhe, C.; Tizzano, E.F. Perspectives in Genetic Counseling for Spinal Muscular Atrophy in the New Therapeutic Era: Early Pre-Symptomatic Intervention and Test in Minors. Eur. J. Hum. Genet. EJHG 2019, 27, 1774–1782. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimizu, T.; Nozaki, M.; Okada, Y.; Sawada, A.; Morisaki, M.; Fujita, H.; Irie, A.; Matsuda, K.; Hasegawa, Y.; Nishi, E.; et al. Multiplex Real-Time PCR-Based Newborn Screening for Severe Primary Immunodeficiency and Spinal Muscular Atrophy in Osaka, Japan: Our Results after 3 Years. Genes 2024, 15, 314. https://doi.org/10.3390/genes15030314
Kimizu T, Nozaki M, Okada Y, Sawada A, Morisaki M, Fujita H, Irie A, Matsuda K, Hasegawa Y, Nishi E, et al. Multiplex Real-Time PCR-Based Newborn Screening for Severe Primary Immunodeficiency and Spinal Muscular Atrophy in Osaka, Japan: Our Results after 3 Years. Genes. 2024; 15(3):314. https://doi.org/10.3390/genes15030314
Chicago/Turabian StyleKimizu, Tomokazu, Masatoshi Nozaki, Yousuke Okada, Akihisa Sawada, Misaki Morisaki, Hiroshi Fujita, Akemi Irie, Keiko Matsuda, Yuiko Hasegawa, Eriko Nishi, and et al. 2024. "Multiplex Real-Time PCR-Based Newborn Screening for Severe Primary Immunodeficiency and Spinal Muscular Atrophy in Osaka, Japan: Our Results after 3 Years" Genes 15, no. 3: 314. https://doi.org/10.3390/genes15030314





