In Vitro Low-Bortezomib Doses Induce Apoptosis and Independently Decrease the Activities of Glutathione S-Transferase and Glutathione Peroxidase in Multiple Myeloma, Taking into Account the GSTT1 and GSTM1 Gene Variants
Abstract
:1. Introduction
2. Materials and Methods
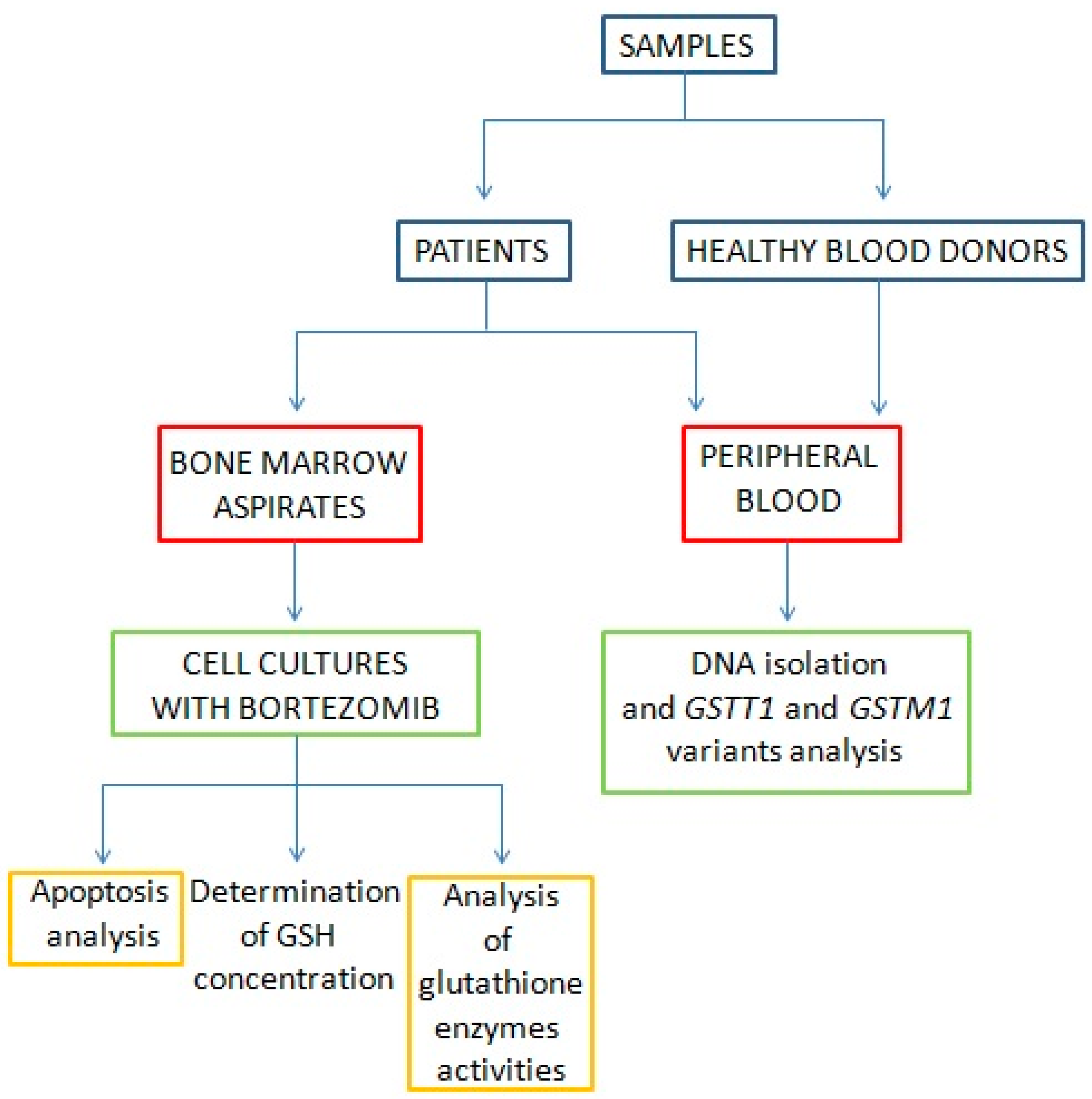
2.1. Patients and Samples
2.2. Multiple Myeloma Cell Culture and In Vitro Bortezomib Treatment
2.3. In Vitro Determination of Apoptotic, Necrotic, and Viable Cells
2.4. Trypan Blue Exclusion Test of Cell Viability
2.5. Analysis of Glutathione Enzyme Activities
2.5.1. Glutathione Peroxidase (GPx) Activity
2.5.2. Glutathione Reductase (GR) Activity
2.5.3. Glutathione S-transferase (GST) Activity
2.6. Determination of Reduced Glutathione (GSH) Concentration
2.7. DNA Isolation
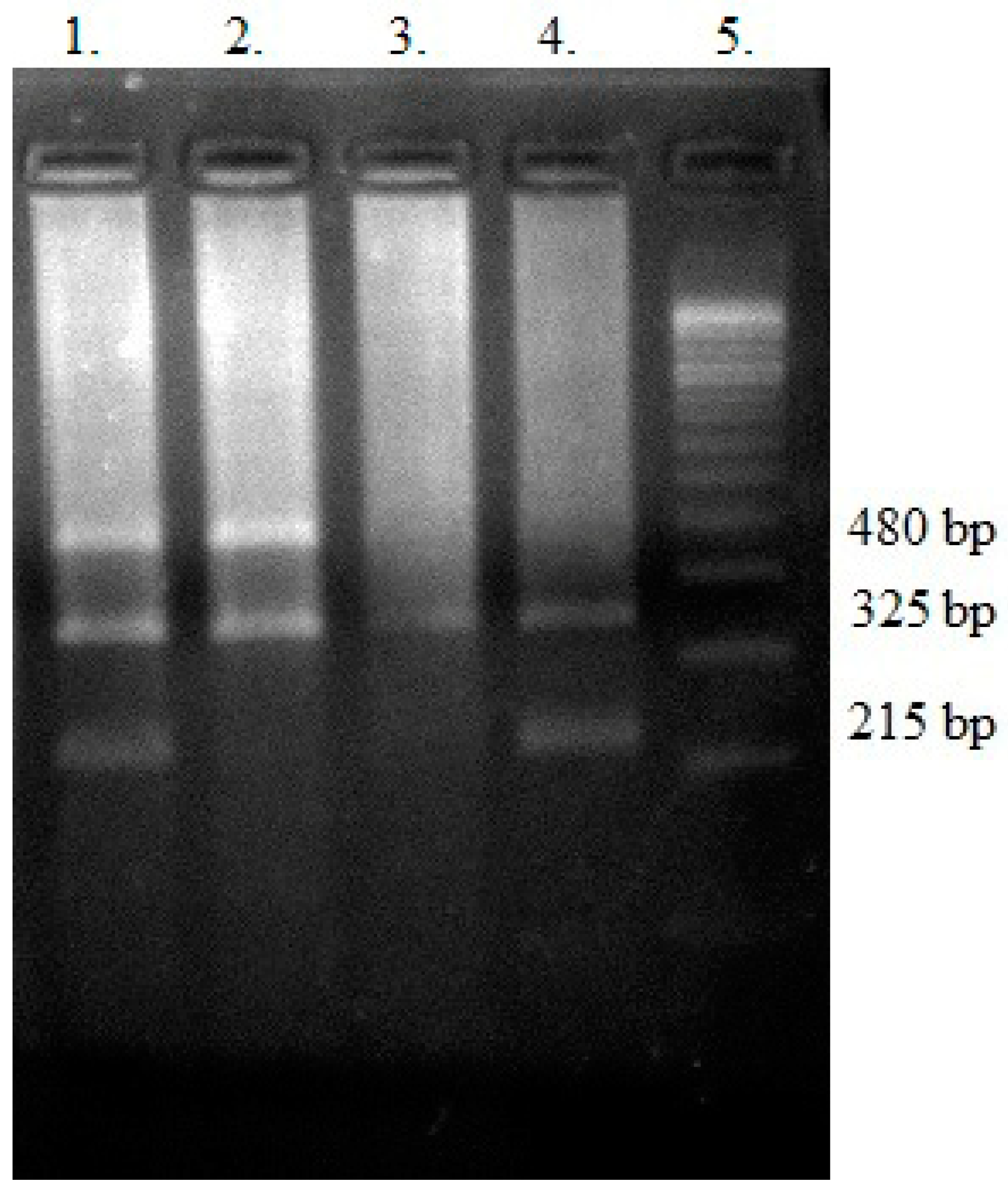
2.8. Genotyping—Polymerase Chain Reaction (PCR) Multiplex
2.9. Statistical Analysis
3. Results
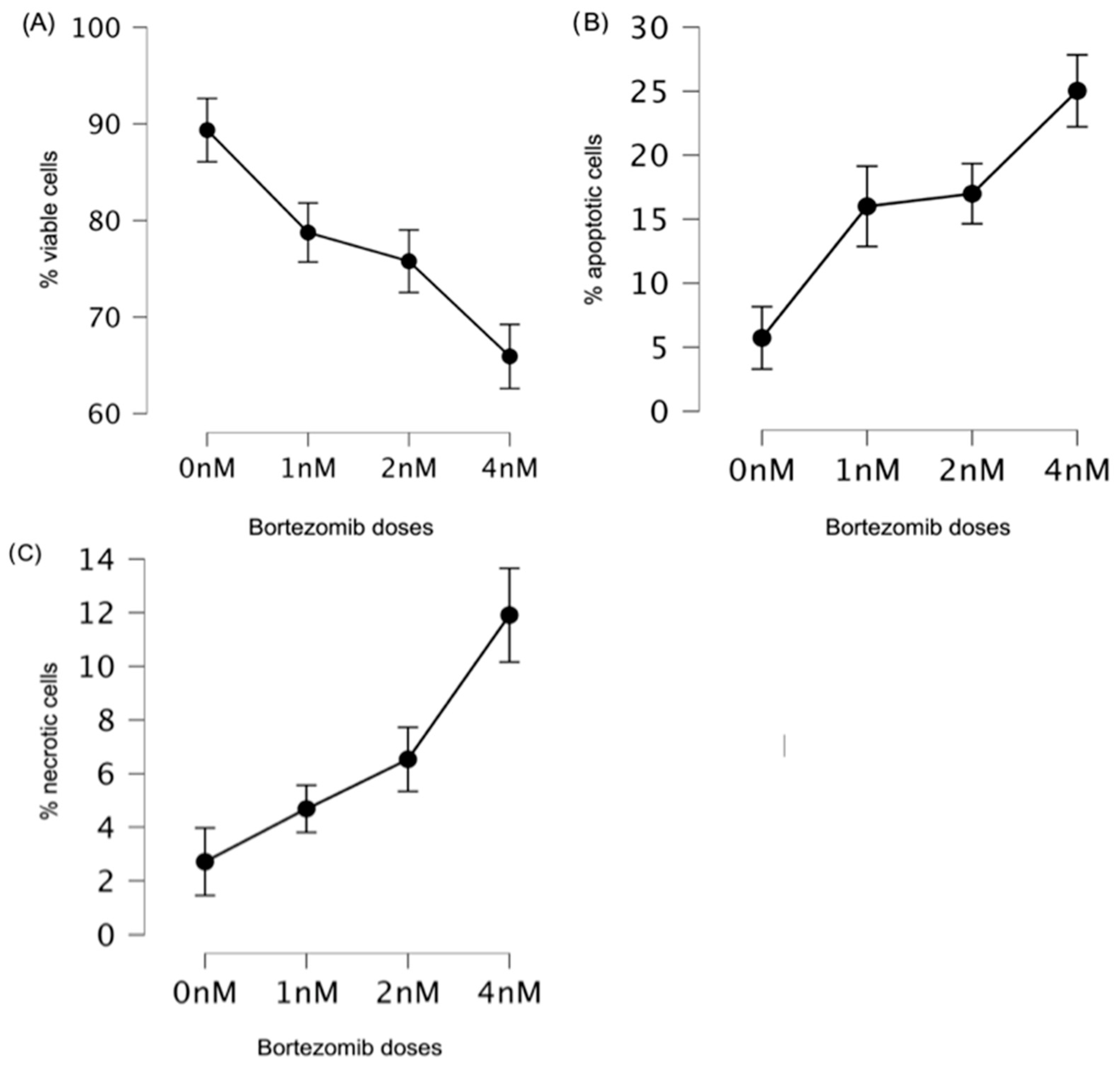
3.1. Low Doses of Bortezomib Decreased the Number of Viable Cells and Induced Apoptosis in Multiple Myeloma
3.2. Differences in the Reduced Glutathione (GSH) Concentration between the Control (0 nM) and Bortezomib Treatment Groups (1 nM, 2 nM, and 4 nM)
3.3. Changes in Glutathione Enzyme Activities
3.4. GSTT1 and GSTM1 Variants in the Context of the Analyzed Antioxidant Parameters
3.5. Correlations of the Analyzed Antioxidant Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shapiro-Shelef, M.; Calame, K. Plasma Cell Differentiation and Multiple Myeloma. Curr. Opin. Immunol. 2004, 16, 226–234. [Google Scholar] [CrossRef]
- Corre, J.; Munshi, N.; Avet-Loiseau, H. Genetics of Multiple Myeloma: Another Heterogeneity Level? Blood 2015, 125, 1870–1876. [Google Scholar] [CrossRef] [PubMed]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and Oxidative Stress in Neurodegenerative Diseases. J. Alzheimer’s Dis. 2014, 42, S125–S152. [Google Scholar] [CrossRef] [PubMed]
- Nerini-Molteni, S.; Ferrarini, M.; Cozza, S.; Caligaris-Cappio, F.; Sitia, R. Redox Homeostasis Modulates the Sensitivity of Myeloma Cells to Bortezomib. Br. J. Haematol. 2008, 141, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.K.; Loucks, F.A.; Schroeder, E.K.; Bouchard, R.J.; Tyler, K.L.; Linseman, D.A. Glutathione Binding to the Bcl-2 Homology-3 Domain Groove. J. Biol. Chem. 2007, 282, 29296–29304. [Google Scholar] [CrossRef]
- Circu, M.L.; Yee Aw, T. Glutathione and Apoptosis. Free Radic. Res. 2008, 42, 689–706. [Google Scholar] [CrossRef]
- Allocati, N.; Masulli, M.; Di Ilio, C.; Federici, L. Glutathione Transferases: Substrates, Inihibitors and pro-Drugs in Cancer and Neurodegenerative Diseases. Oncogenesis 2018, 7, 8. [Google Scholar] [CrossRef]
- Su, D.; Wang, X.; Campbell, M.R.; Song, L.; Safi, A.; Crawford, G.E.; Bell, D.A. Interactions of Chromatin Context, Binding Site Sequence Content, and Sequence Evolution in Stress-Induced P53 Occupancy and Transactivation. PLoS Genet. 2015, 11, e1004885. [Google Scholar] [CrossRef]
- Singh, M.S.; Michael, M. Role of Xenobiotic Metabolic Enzymes in Cancer Epidemiology. In Cancer Epidemiology; Humana Press: Totowa, NJ, USA, 2009; pp. 243–264. [Google Scholar]
- Curtin, N.J. DNA Repair Dysregulation from Cancer Driver to Therapeutic Target. Nat. Rev. Cancer 2012, 12, 801–817. [Google Scholar] [CrossRef]
- Chen, H.; Hu, W.; Liu, Q.; Li, W.; Chen, F.; Rao, Z.; Liu, X.; Luo, Y.; Cao, Y. Genetic Polymorphisms of Metabolic Enzymes CYP1A1, CYP2D6, GSTM1 and GSTT1 and Leukemia Susceptibility. Eur. J. Cancer Prev. 2008, 17, 251–258. [Google Scholar] [CrossRef]
- Lowry, R.W.; Rosebrough, G.H.; Furr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Zmorzyński, S.; Popek-Marciniec, S.; Szudy-Szczyrek, A.; Wojcierowska-Litwin, M.; Korszeń-Pilecka, I.; Chocholska, S.; Styk, W.; Hus, M.; Filip, A.A. The Association of GSTT1, GSTM1, and TNF-α Polymorphisms with the Risk and Outcome in Multiple Myeloma. Front. Oncol. 2019, 9, 1056. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, S.Z.; El-Zein, R.A.; Anwar, W.A.; Au, W.W. A Multiplex PCR Procedure for Polymorphic Analysis of GSTM1 and GSTT1 Genes in Population Studies. Cancer Lett. 1996, 107, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Preacher, K.J. Calculation for the Chi-Square Test: An Interactive Calculation Tool for Chi-Square Tests of Goodness of Fit and Independence. Available online: http://quantpsy.org/ (accessed on 15 December 2023).
- Starheim, K.K.; Holien, T.; Misund, K.; Johansson, I.; Baranowska, K.A.; Sponaas, A.-M.; Hella, H.; Buene, G.; Waage, A.; Sundan, A.; et al. Intracellular Glutathione Determines Bortezomib Cytotoxicity in Multiple Myeloma Cells. Blood Cancer J. 2016, 6, e446. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.C.; Farrell, A.T.; Sridhara, R.; Pazdur, R. United States Food and Drug Administration Approval Summary: Bortezomib for the Treatment of Progressive Multiple Myeloma after One Prior Therapy. Clin. Cancer Res. 2006, 12, 2955–2960. [Google Scholar] [CrossRef]
- Sharma, A.; Tripathi, M.; Satyam, A.; Kumar, L. Study of Antioxidant Levels in Patients with Multiple Myeloma. Leuk. Lymphoma 2009, 50, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Obeng, E.A. Proteasome Inhibitors Induce a Terminal Unfolded Protein Response in Multiple Myeloma Cells. Blood 2006, 107, 4907–4916. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular Mechanisms and Physiological Consequences of Redox-Dependent Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Perez-Galan, P. The Proteasome Inhibitor Bortezomib Induces Apoptosis in Mantle-Cell Lymphoma through Generation of ROS and Noxa Activation Independent of P53 Status. Blood 2006, 107, 257–264. [Google Scholar] [CrossRef]
- Cong, H.; Xu, L.; Wu, Y.; Qu, Z.; Bian, T.; Zhang, W.; Xing, C.; Zhuang, C. Inhibitor of Apoptosis Protein (IAP) Antagonists in Anticancer Agent Discovery: Current Status and Perspectives. J. Med. Chem. 2019, 62, 5750–5772. [Google Scholar] [CrossRef]
- Reece, D.E.; Sullivan, D.; Lonial, S.; Mohrbacher, A.F.; Chatta, G.; Shustik, C.; Burris, H.; Venkatakrishnan, K.; Neuwirth, R.; Riordan, W.J.; et al. Pharmacokinetic and Pharmacodynamic Study of Two Doses of Bortezomib in Patients with Relapsed Multiple Myeloma. Cancer Chemother. Pharmacol. 2011, 67, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Aykin-Burns, N.; Ahmad, I.M.; Zhu, Y.; Oberley, L.W.; Spitz, D.R. Increased Levels of Superoxide and H2O2 Mediate the Differential Susceptibility of Cancer Cells versus Normal Cells to Glucose Deprivation. Biochem. J. 2009, 418, 29–37. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting Cancer Cells by ROS-Mediated Mechanisms: A Radical Therapeutic Approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Salem, K.; McCormick, M.L.; Wendlandt, E.; Zhan, F.; Goel, A. Copper–Zinc Superoxide Dismutase-Mediated Redox Regulation of Bortezomib Resistance in Multiple Myeloma. Redox Biol. 2015, 4, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Schafer, F.Q.; Buettner, G.R. Redox Environment of the Cell as Viewed through the Redox State of the Glutathione Disulfide/Glutathione Couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Akhigbe, R.E.; Oluwole, D.T.; Adegoke, T.E.; Hamed, M.A.; Anyogu, D.C.; Ajayi, A.F. Suppression of Glutathione System and Upregulation of Caspase 3-Dependent Apoptosis Mediate Rohypnol-Induced Gastric Injury. Redox Rep. 2022, 27, 111–118. [Google Scholar] [CrossRef]
- Robaczewska, J.; Kedziora-Kornatowska, K.; Kozakiewicz, M.; Zary-Sikorska, E.; Pawluk, H.; Pawliszak, W.; Kedziora, J. Role of Glutathione Metabolism and Glutathione-Related Antioxidant Defense Systems in Hypertension. J. Physiol. Pharmacol. 2016, 67, 331–337. [Google Scholar]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and Thioredoxin Antioxidant Pathways Synergize to Drive Cancer Initiation and Progression. Cancer Cell 2015, 27, 211–222. [Google Scholar] [CrossRef]
- Lv, H.; Zhen, C.; Liu, J.; Yang, P.; Hu, L.; Shang, P. Unraveling the Potential Role of Glutathione in Multiple Forms of Cell Death in Cancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 3150145. [Google Scholar] [CrossRef]
- Hentze, H.; Schmitz, I.; Latta, M.; Krueger, A.; Krammer, P.H.; Wendel, A. Glutathione Dependence of Caspase-8 Activation at the Death-Inducing Signaling Complex. J. Biol. Chem. 2002, 277, 5588–5595. [Google Scholar] [CrossRef]
- Takanashi, M.; Morimoto, A.; Yagi, T.; Kuriyama, K.; Kano, G.; Imamura, T.; Hibi, S.; Todo, S.; Imashuku, S. Impact of Glutathione S-Transferase Gene Deletion on Early Relapse in Childhood B-Precursor Acute Lymphoblastic Leukemia. Haematologica 2003, 88, 1238–1244. [Google Scholar]
- Faber, E.W.; Lourenço, G.J.; Ortega, M.M.; Lorand-Metze, I.; De Souza, C.A.; Lima, C.S.P. Polymorphisms of VEGF, GSTM1 and GSTT1 Genes in Multiple Myeloma Risk. Hematol. Oncol. 2012, 30, 105–107. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, Y.-Y.; Zhang, X.-D.; Li, Y.; Liu, Z.-G. Risk Effects of GST Gene Polymorphisms in Patients with Acute Myeloid Leukemia: A Prospective Study. Asian Pac. J. Cancer Prev. 2013, 14, 3861–3864. [Google Scholar] [CrossRef]
- Kim, H.N.; Kim, N.Y.; Yu, L.; Tran, H.T.T.; Kim, Y.-K.; Lee, I.-K.; Shin, M.-H.; Park, K.-S.; Choi, J.-S.; Kim, H.-J. Association of GSTT1 Polymorphism with Acute Myeloid Leukemia Risk Is Dependent on Smoking Status. Leuk. Lymphoma 2012, 53, 681–687. [Google Scholar] [CrossRef]
- Yuille, M.; Condie, A.; Hudson, C.; Kote-Jarai, Z.; Stone, E.; Eeles, R.; Matutes, E.; Catovsky, D.; Houlston, R. Relationship between Glutathione S-Transferase M1, T1, and P1 Polymorphisms and Chronic Lymphocytic Leukemia. Blood 2002, 99, 4216–4218. [Google Scholar] [CrossRef]
- Kalivendi, S.V.; Cunningham, S.; Kotamraju, S.; Joseph, J.; Hillard, C.J.; Kalyanaraman, B. α-Synuclein Up-Regulation and Aggregation during MPP+-Induced Apoptosis in Neuroblastoma Cells. J. Biol. Chem. 2004, 279, 15240–15247. [Google Scholar] [CrossRef]
- Michiels, C.; Raes, M.; Toussaint, O.; Remacle, J. Importance of SE-Glutathione Peroxidase, Catalase, and CU/ZN-SOD for Cell Survival against Oxidative Stress. Free Radic. Biol. Med. 1994, 17, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione Peroxidases. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R. Tissue-Specific Functions of Individual Glutathione Peroxidases. Free Radic. Biol. Med. 1999, 27, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Boysen, J.; Nelson, M.; Secreto, C.; Warner, S.L.; Bearss, D.J.; Lesnick, C.; Shanafelt, T.D.; Kay, N.E.; Ghosh, A.K. Targeted Axl Inhibition Primes Chronic Lymphocytic Leukemia B Cells to Apoptosis and Shows Synergistic/Additive Effects in Combination with BTK Inhibitors. Clin. Cancer Res. 2015, 21, 2115–2126. [Google Scholar] [CrossRef] [PubMed]





| Variables | MM Patients, n = 80 |
|---|---|
| Sex | |
| Male | 46 |
| Female | 34 |
| Age * | |
| Mean age (years) | 66.95 |
| Type of MM * | |
| IgG | 44 |
| IgA | 16 |
| Light chain | 20 |
| Free light chain ratio | 473 |
| Stage according to the International Staging System * | |
| I | 20 |
| II | 16 |
| III | 44 |
| Renal failure * | |
| No | 54 |
| Yes | 26 |
| Stage of kidney disease * | |
| G1 | 18 |
| G2 | 18 |
| G3A | 12 |
| G3B | 14 |
| G4 | 10 |
| G5 | 8 |
| Plasma cells * | |
| Percentage of plasma cells in bone marrow, M ± SD | 31.94 ± 21.21 |
| Cytogenetic changes | |
| No | 68 |
| Yes -including: del17(p13.1) t(4;14) t(14;16) | 12 8 6 2 |
| Clinical values * | |
| Albumins (g/dL), M ± SD | 3.55 ± 0.67 |
| β2-microglobulin (mg/L), M ± SD | 6.93 ± 4.19 |
| Calcium (mM/L), M ± SD | 2.41 ± 0.33 |
| Hemoglobin (g/dL), M ± SD | 9.98 ± 1.96 |
| Creatinine (mg/dL), M ± SD | 2.10 ± 2.26 |
| C-reactive protein (mg/L), M ± SD | 10.03 ± 15.09 |
| Chemotherapy | |
| Cyclophosphamide, Thalidomide, Dexamethasone (CTD) | 60 |
| Velcade, Cyclophosphamide, Dexamethasone (VCD) | 18 |
| Velcade, Thalidomide, Dexamethasone (VTD) | 2 |
| Survival | |
| Progression free survival (months) M ± SD | 20.35 ± 16.57 |
| Overall survival (months) M ± SD | 30.77 ± 20.48 |
| Doses of Bortezomib | Mean Values (%) | SD | SE | |
|---|---|---|---|---|
| Viable cells * | 0 nM | 89.36 | 7.51 | 1.19 |
| 1 nM | 78.75 | 12.28 | 1.94 | |
| 2 nM | 75.78 | 12.64 | 2.00 | |
| 4 nM | 65.92 | 12.78 | 2.02 | |
| Apoptotic cells | 0 nM | 5.73 | 5.23 | 0.83 |
| 1 nM | 16.00 | 12.28 | 1.94 | |
| 2 nM | 16.99 | 6.92 | 1.09 | |
| 4 nM | 25.03 | 11.12 | 1.76 | |
| Necrotic cells | 0 nM | 2.71 | 3.64 | 0.58 |
| 1 nM | 4.69 | 3.04 | 0.48 | |
| 2 nM | 6.54 | 4.49 | 0.71 | |
| 4 nM | 11.90 | 6.78 | 1.07 |
| Bortezomib Doses | Mean Difference (%) | Cohen’s d | p-Value |
|---|---|---|---|
| 0 nM vs. 1 nM | 10.61 | 0.92 | <0.01 |
| 0 nM vs. 2 nM | 13.58 | 1.18 | <0.01 |
| 0 nM vs. 4 nM | 23.44 | 2.04 | <0.01 |
| 1 nM vs. 2 nM | 2.97 | - | 0.68 |
| 1 nM vs. 4 nM | 12.83 | 1.11 | <0.01 |
| 2 nM vs. 4 nM | 9.86 | 0.86 | <0.01 |
| Bortezomib Doses | Mean Difference (%) | Cohen’s d | p-Value |
|---|---|---|---|
| 0 nM vs. 1 nM | −10.28 | −1.10 | <0.01 |
| 0 nM vs. 2 nM | −11.26 | −1.20 | <0.01 |
| 0 nM vs. 4 nM | −19.31 | −2.06 | <0.01 |
| 1 nM vs. 2 nM | −0.99 | −0.11 | 0.79 |
| 1 nM vs. 4 nM | −9.03 | −0.97 | <0.01 |
| 2 nM vs. 4 nM | −8.04 | −0.86 | <0.01 |
| Bortezomib Doses | Mean Difference (%) | Cohen’s d | p-Value |
|---|---|---|---|
| 0 nM vs. 1 nM | −1.98 | −0.42 | 0.20 |
| 0 nM vs. 2 nM | −3.83 | −0.81 | <0.01 |
| 0 nM vs. 4 nM | −9.20 | −1.95 | <0.01 |
| 1 nM vs. 2 nM | −1.85 | −0.39 | 0.27 |
| 1 nM vs. 4 nM | −7.22 | −1.53 | <0.01 |
| 2 nM vs. 4 nM | −5.37 | −1.14 | <0.01 |
| Bortezomib Doses | Mean Difference (µM/mg Protein) | Cohen’s d | p-Value |
|---|---|---|---|
| 0 nM vs. 1 nM | 0.04 | - | 0.90 |
| 0 nM vs. 2 nM | 0.04 | - | 0.91 |
| 0 nM vs. 4 nM | 0.07 | 0.53 | 0.05 |
| 1 nM vs. 2 nM | 0.01 | - | 1.00 |
| 1 nM vs. 4 nM | 0.03 | - | 1.00 |
| 2 nM vs. 4 nM | 0.03 | - | 1.00 |
| Bortezomib Doses | Mean Difference (U·mg−1 Protein) | Cohen’s d | p-Value |
|---|---|---|---|
| 0 nM vs. 1 nM | 0.01 | 0.09 | <0.01 |
| 0 nM vs. 2 nM | 0.03 | 0.24 | <0.01 |
| 0 nM vs. 4 nM | 0.05 | 0.44 | <0.01 |
| 1 nM vs. 2 nM | 0.02 | 0.15 | <0.01 |
| 1 nM vs. 4 nM | 0.04 | 0.35 | <0.01 |
| 2 nM vs. 4 nM | 0.02 | 0.20 | <0.01 |
| Bortezomib Doses | Mean Difference (U·mg−1 Protein) | Cohen’s d | p-Value |
|---|---|---|---|
| 0 nM vs. 1 nM | −0.02 | - | 1.00 |
| 0 nM vs. 2 nM | −0.04 | - | 1.00 |
| 0 nM vs. 4 nM | −0.13 | −0.64 | 0.02 |
| 1 nM vs. 2 nM | −0.02 | - | 1.00 |
| 1 nM vs. 4 nM | −0.11 | −0.55 | 0.06 |
| 2 nM vs. 4 nM | −0.09 | - | 0.26 |
| Bortezomib Doses | Mean Difference (U·mg−1 Protein) | Cohen’s d | p-Value |
|---|---|---|---|
| 0 nM vs.1 nM | 0.02 | 0.16 | 0.96 |
| 0 nM vs. 2 nM | 0.05 | 0.43 | <0.01 |
| 0 nM vs. 4 nM | 0.10 | 0.83 | <0.01 |
| 1 nM vs. 2 nM | 0.03 | 0.26 | 0.15 |
| 1 nM vs. 4 nM | 0.08 | 0.67 | <0.01 |
| 2 nM vs. 4 nM | 0.05 | 0.40 | <0.01 |
| Null (Homozygotes) | Present (Heterozygotes) | Present (Homozygotes) | Total | HWE p-Value and χ2 * | |
|---|---|---|---|---|---|
| CONTROL | GSTT1 | ||||
| E | 31.9 | 49.2 | 18.9 | 100 | p = 0.97, χ2 = 0.001 |
| O | 32 | 49 | 19 | 100 | |
| CASE | |||||
| E | 24.2 | 39.6 | 16.2 | 80 | p = 0.92, χ2 = 0.008 |
| O | 24 | 40 | 16 | 80 | |
| CONTROL | GSTM1 | ||||
| E | 39.7 | 46.6 | 13.7 | 100 | p = 0.89, χ2 = 0.017 |
| O | 40 | 46 | 14 | 100 | |
| CASE | |||||
| E | 24.2 | 39.6 | 16.2 | 80 | p = 0.92, χ2 = 0.008 |
| O | 24 | 40 | 16 | 80 | |
| Genotypes | MM Patients n = 80 | Controls n = 100 | OR | 95%CI | p-Value |
|---|---|---|---|---|---|
| GSTT1 | |||||
| present | 56 | 68 | referent | - | - |
| null | 24 | 32 | 1.01 | 0.58–2.08 | 0.77 |
| GSTM1 | |||||
| present | 56 | 60 | referent | - | - |
| null | 24 | 40 | 1.55 | 0.83–2.09 | 0.16 |
| GSTT1 | GSTM1 | MM Patients n | Controls n | OR | 95%CI | p-Value |
|---|---|---|---|---|---|---|
| present | present | 42 | 46 | R | - | - |
| null | present | 14 | 14 | 0.91 | 0.39–2.14 | 0.83 |
| present | null | 14 | 22 | 1.43 | 0.65–3.16 | 0.37 |
| null | null | 10 | 18 | 1.64 | 0.68–3.95 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zmorzynski, S.; Popek-Marciniec, S.; Biernacka, B.; Szudy-Szczyrek, A.; Chocholska, S.; Styk, W.; Czerwik-Marcinkowska, J.; Swiderska-Kolacz, G. In Vitro Low-Bortezomib Doses Induce Apoptosis and Independently Decrease the Activities of Glutathione S-Transferase and Glutathione Peroxidase in Multiple Myeloma, Taking into Account the GSTT1 and GSTM1 Gene Variants. Genes 2024, 15, 387. https://doi.org/10.3390/genes15030387
Zmorzynski S, Popek-Marciniec S, Biernacka B, Szudy-Szczyrek A, Chocholska S, Styk W, Czerwik-Marcinkowska J, Swiderska-Kolacz G. In Vitro Low-Bortezomib Doses Induce Apoptosis and Independently Decrease the Activities of Glutathione S-Transferase and Glutathione Peroxidase in Multiple Myeloma, Taking into Account the GSTT1 and GSTM1 Gene Variants. Genes. 2024; 15(3):387. https://doi.org/10.3390/genes15030387
Chicago/Turabian StyleZmorzynski, Szymon, Sylwia Popek-Marciniec, Beata Biernacka, Aneta Szudy-Szczyrek, Sylwia Chocholska, Wojciech Styk, Joanna Czerwik-Marcinkowska, and Grazyna Swiderska-Kolacz. 2024. "In Vitro Low-Bortezomib Doses Induce Apoptosis and Independently Decrease the Activities of Glutathione S-Transferase and Glutathione Peroxidase in Multiple Myeloma, Taking into Account the GSTT1 and GSTM1 Gene Variants" Genes 15, no. 3: 387. https://doi.org/10.3390/genes15030387






