Identification, Classification, and Transcriptional Analysis of Rab GTPase Genes from Tomato (Solanum lycopersicum) Reveals Salt Stress Response Genes
Abstract
1. Introduction
2. Materials and Methods
2.1. Identification of Rab GTPases in the S. lycopersicum Genome
2.2. Structure of Rab Genes and Conserved Motifs in Rab Proteins
2.3. Rab GTPase Expression Profiles in Databases
2.4. Plant Material and Growth Conditions
2.5. RNA Extraction and cDNA Synthesis
2.6. Analysis of Gene Expression
2.7. Statistical Analysis
3. Results
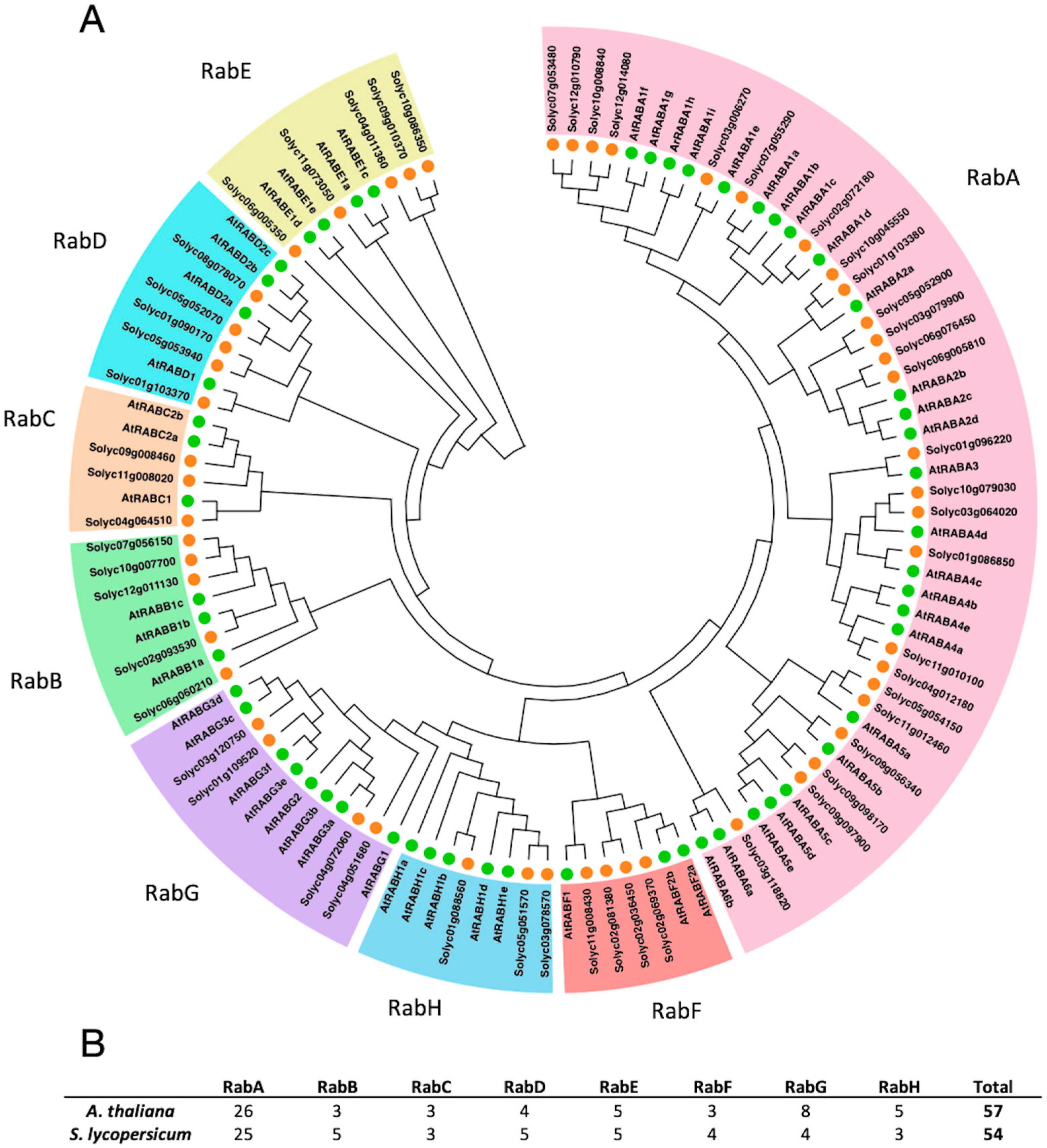
3.1. Identification and Phylogenetic Analysis of Rab GTPase Genes in S. lycopersicum
3.2. Duplication between Rab Genes from S. lycopersicum
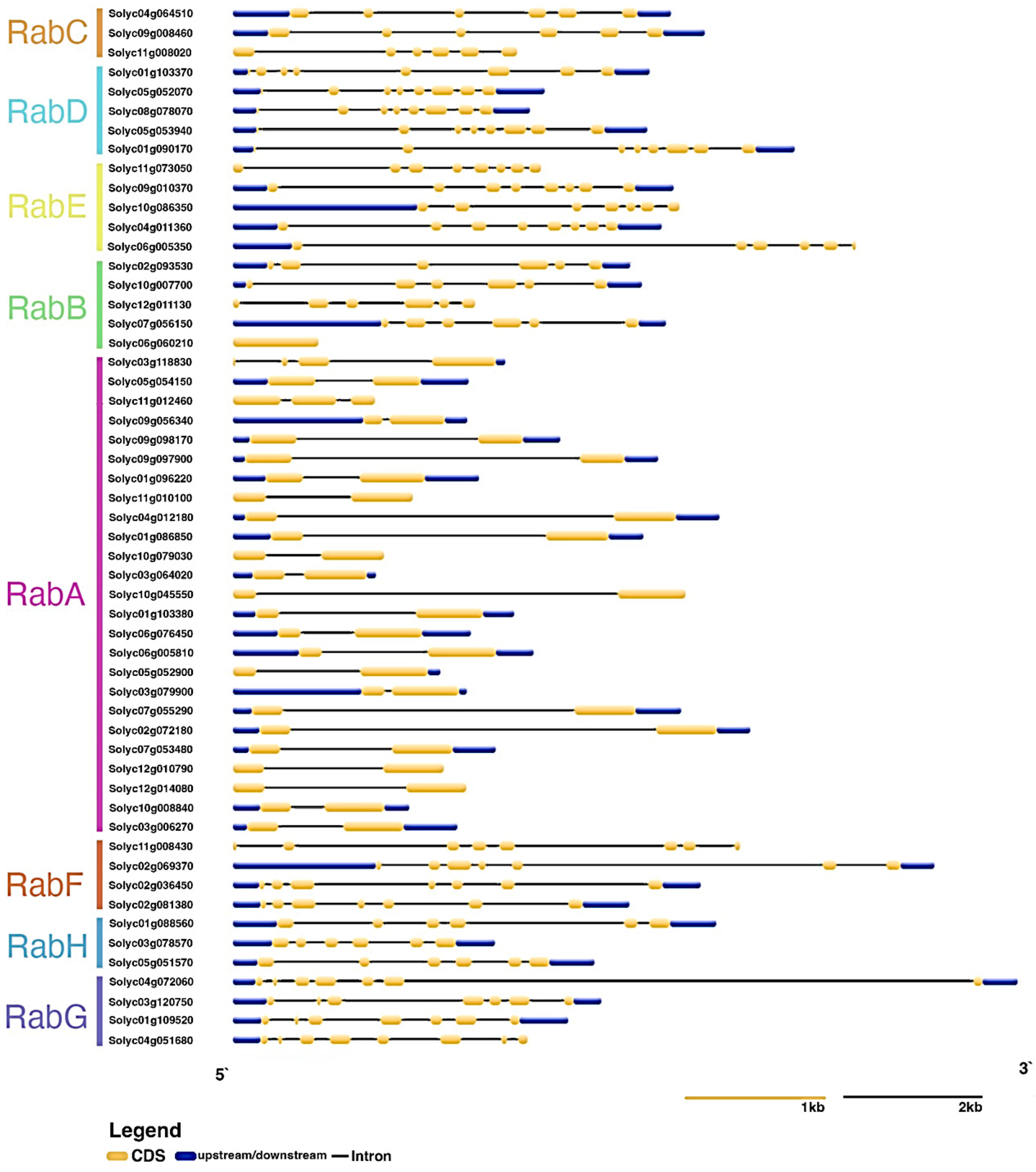
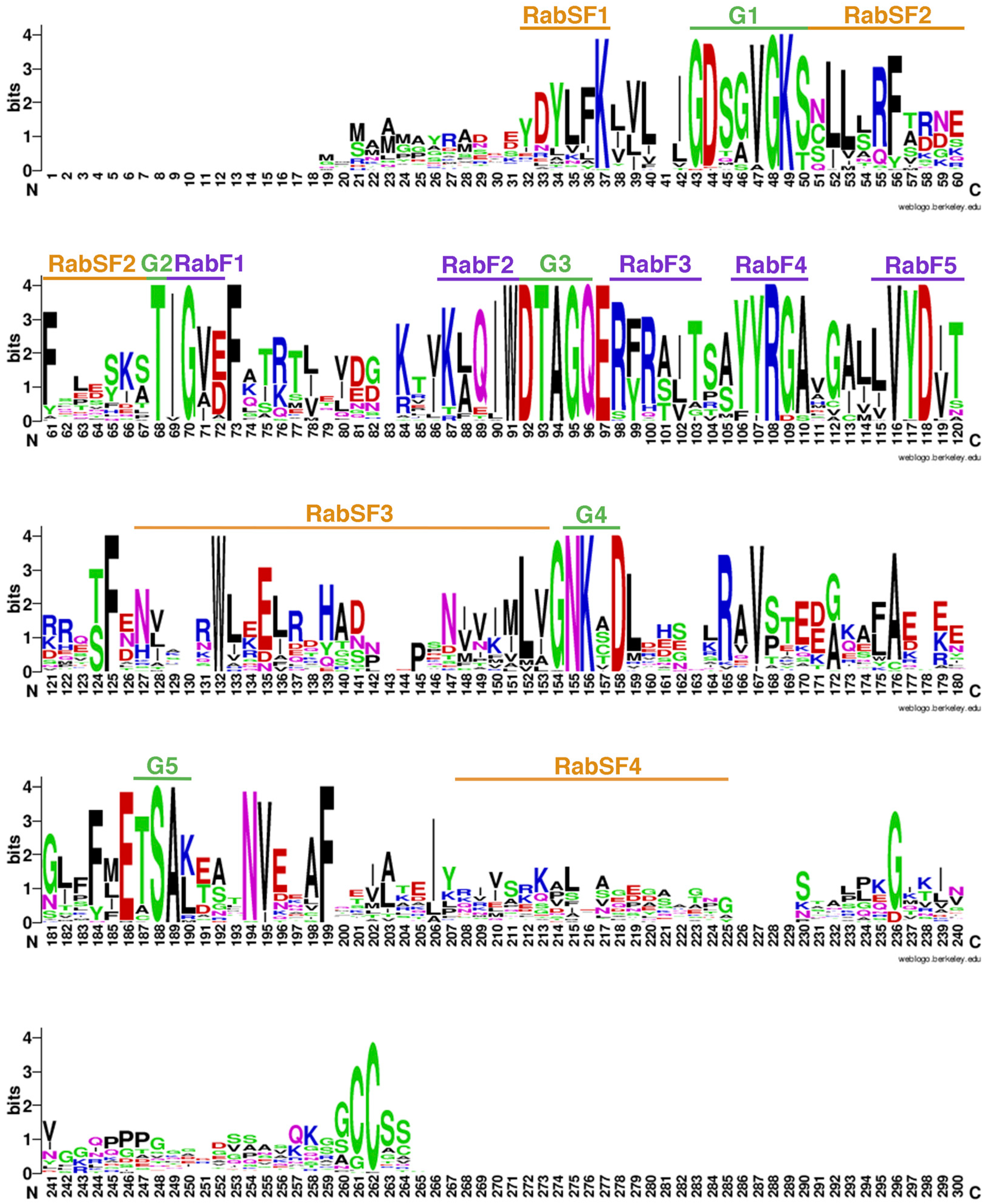
3.3. Structure of Rab Genes and Conserved Motifs of Rab Proteins in S. lycopersicum
3.4. Expression of Rab GTPases in Different Tissues of Cultivated and Wild Tomato
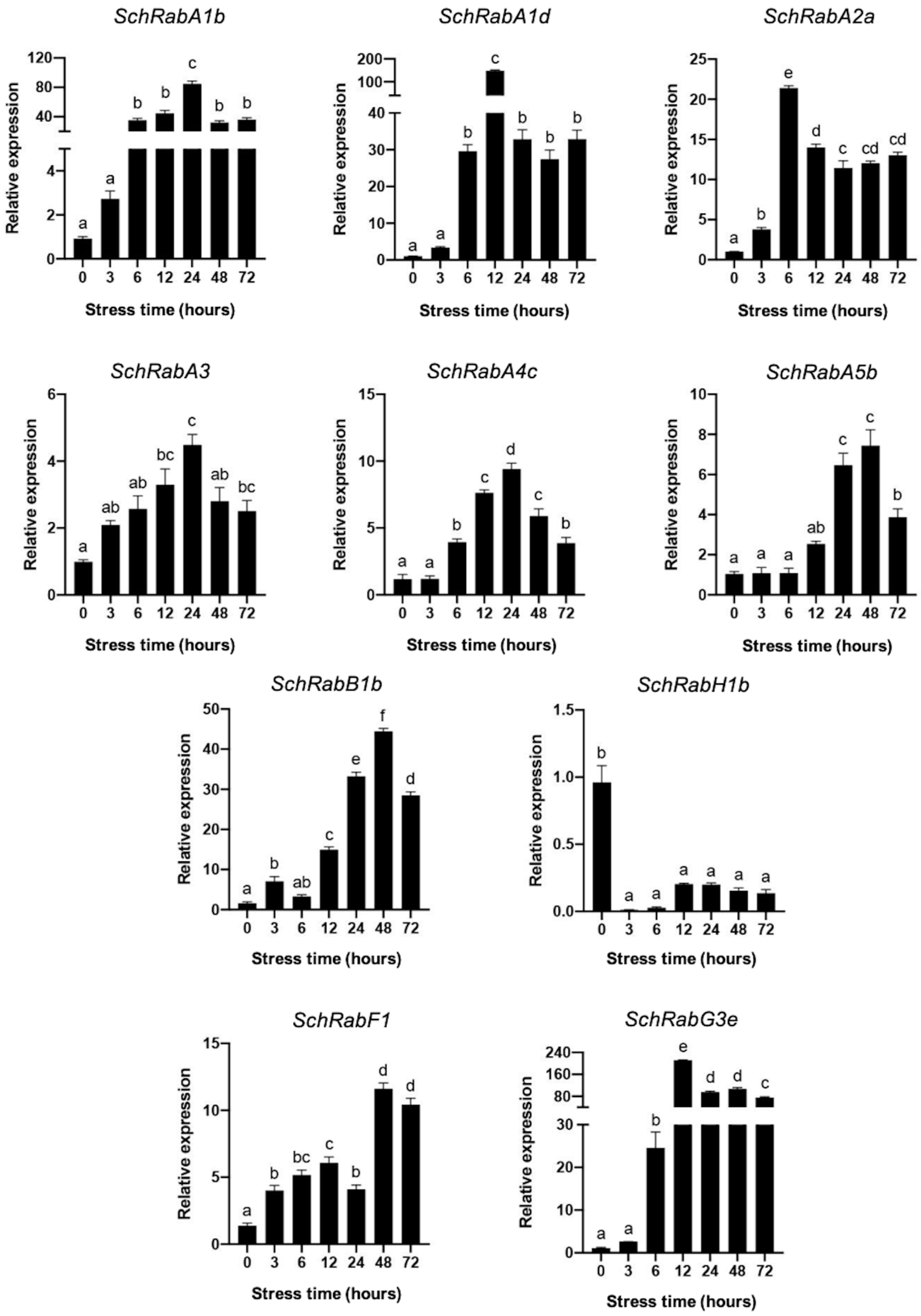
3.5. Differential Expression of Rab GTPase Genes from S. chilense in Leaves and Roots under Saline Stress
4. Discussion
4.1. The Rab GTPase Superfamily Is Represented in S. lycopersicum with 54 Members
4.2. High Functional Diversity of Rab GTPases in Solanaceae
4.3. Differential Expression of Rab GTPases of the Endocytic and TGN Pathway in S. chilense under Stress Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, S.; Wang, Y.; Yan, Y.; Liu, Y.; Wang, J.; Chen, S. A Review on Plant Responses to Salt Stress and Their Mechanisms of Salt Resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Hassani, A.; Azapagic, A.; Shokri, N. Global predictions of primary soil salinization under changing climate in the 21st century. Nat. Commun. 2021, 12, 6663. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shi, H.; Yang, Y.; Feng, X.; Chen, X.; Xiao, F.; Lin, H.; Guo, Y. Insights into plant salt stress signaling and tolerance. J. Genet. Genom. 2023, 51, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, X.S.; Guo, H.D.; Bai, S.Y.; Khan, A.; Wang, X.M.; Gao, Y.M.; Li, J.S. Tomato salt tolerance mechanisms and their potential applications for fighting salinity: A review. Front. Plant Sci. 2022, 13, 949541. [Google Scholar] [CrossRef] [PubMed]
- Žižková, E.; Dobrev, P.I.; Muhovski, Y.; Hošek, P.; Hoyerová, K.; Haisel, D.; Procházková, D.; Lutts, S.; Motyka, V.; Hichri, I. Tomato (Solanum lycopersicum L.) SlIPT3 and SlIPT4 isopentenyltransferases mediate salt stress response in tomato. BMC Plant Biol. 2015, 15, 85. [Google Scholar] [CrossRef] [PubMed]
- Roșca, M.; Mihalache, G.; Stoleru, V. Tomato responses to salinity stress: From morphological traits to genetic changes. Front. Plant Sci. 2023, 14, 1118383. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Cornejo, J.; Madrid-Espinoza, J.; Verdugo, I.; Pérez-Díaz, J.; Martín-Davison, A.S.; Norambuena, L.; Ruiz-Lara, S. The Exocytosis Associated SNAP25-Type Protein, SlSNAP33, Increases Salt Stress Tolerance by Modulating Endocytosis in Tomato. Plants 2021, 10, 1322. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Cornejo, J.; Madrid-Espinoza, J.; Verdugo, I.; Norambuena, L.; Ruiz-Lara, S. A SNARE-like protein from Solanum lycopersicum increases salt tolerance by modulating vesicular trafficking in tomato. Front. Plant Sci. 2023, 14, 1212806. [Google Scholar] [CrossRef]
- Nielsen, E.; Cheung, A.Y.; Ueda, T. The Regulatory RAB and ARF GTPases for Vesicular Trafficking. Plant Physiol. 2008, 147, 1516–1526. [Google Scholar] [CrossRef]
- Zerial, M.; McBride, H. Rab Proteins as Membrane Organizers. Nat. Rev. Mol. Cell Biol. 2001, 2, 107–117. [Google Scholar] [CrossRef]
- Hutagalung, A.H.; Novick, P.J. Role of Rab GTPases in membrane traffic and cell physiology. Physiol. Rev. 2011, 91, 119–149. [Google Scholar] [CrossRef]
- Stenmark, H. Rab GTPases as Coordinators of Vesicle Traffic. Nat. Rev. Mol. Cell Biol. 2009, 10, 513–525. [Google Scholar] [CrossRef]
- Khalilova, L.A.; Sergienko, O.V.; Orlova, Y.V.; Myasoedov, N.A.; Karpichev, I.V.; Balnokin, Y.V. Arabidopsis thaliana Mutant with T-DNA Insertion in the Flot1 (At5g25250) Gene Promotor Possesses Increased Resistance to NaCl. Russ. J. Plant Physiol. 2020, 67, 275–284. [Google Scholar] [CrossRef]
- Sergienko, O.; Khalilova, L.A.; Orlova, Y.; Shuvalov, A.; Myasoedov, N.A.; Karpichev, I. Mutation of the ARA7/AtRabF2b Gene, That Increases the Content of the Ara7 Protein Regulating Endocytic Trafficking Pathways, Improves Salt Tolerance of the Arabidopsis thaliana (L.) Heynh Plants. Russ. J. Plant Physiol. 2022, 69, 11. [Google Scholar] [CrossRef]
- Asaoka, R.; Uemura, T.; Ito, J.; Fujimoto, M.; Ito, E.; Ueda, T.; Nakano, A. Arabidopsis RABA1 GTPases Are Involved in Transport between the Trans-Golgi Network and the Plasma Membrane, and Are Required for Salinity Stress Tolerance. Plant J. 2013, 73, 240–249. [Google Scholar] [CrossRef]
- Bolte, S.; Schiene, K.; Dietz, K.J. Characterization of a Small GTP-Binding Protein of the Rab 5 Family in Mesembryanthemum Crystallinum with Increased Level of Expression during Early Salt Stress. Plant Mol. Biol. 2000, 42, 923–936. [Google Scholar] [CrossRef]
- George, S.; Parida, A. Over-Expression of a Rab Family GTPase from Phreatophyte Prosopis Juliflora Confers Tolerance to Salt Stress on Transgenic Tobacco. Mol. Biol. Rep. 2011, 38, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Jha, Y.; Sablok, G.; Subbarao, N.; Sudhakar, R.; Fazil, M.H.; Subramanian, R.B.; Squartini, A.; Kumar, S. Bacterial-Induced Expression of RAB18 Protein in Oryza Sativa Salinity Stress and Insights into Molecular Interaction with GTP Ligand. J. Mol. Recognit. 2014, 27, 521–527. [Google Scholar] [CrossRef]
- Zhu, J.K. Plant Salt Tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Slesak, I.; Miszalski, Z.; Karpinska, B.; Niewiadomska, E.; Ratajczak, R.; Karpinski, S. Redox Control of Oxidative Stress Responses in the C3-CAM Intermediate Plant Mesembryanthemum Crystallinum. Plant Physiol. Biochem. 2002, 40, 669–677. [Google Scholar] [CrossRef]
- Tuteja, N. Cold, Salinity, and Drought Stress. In Plant Stress Biology: From Genomics to Systems Biology; Hirt, H., Ed.; Wiley-Blackwell: Weinheim, Germany, 2010; Volume 444, pp. 137–159. [Google Scholar] [CrossRef]
- Levine, A. Regulation of Stress Responses by Intracellular Vesicle Trafficking? Plant Physiol. Biochem. 2002, 40, 531–535. [Google Scholar] [CrossRef]
- Ebine, K.; Fujimoto, M.; Okatani, Y.; Nishiyama, T.; Goh, T.; Ito, E.; Dainobu, T.; Nishitani, A.; Uemura, T.; Sato, M.H.; et al. A Membrane Trafficking Pathway Regulated by the Plant-Specific RAB GTPase ARA6. Nat. Cell Biol. 2011, 13, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Ebine, K.; Miyakawa, N.; Fujimoto, M.; Uemura, T.; Nakano, A.; Ueda, T. Endosomal Trafficking Pathway Regulated by ARA6, a RAB5 GTPase Unique to Plants. Small GTPases 2012, 3, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Karim, S.; Zhang, H.; Aronsson, H. Arabidopsis RabF1 (ARA6) Is Involved in Salt Stress and Dark-Induced Senescence (DIS). Int. J. Mol. Sci. 2017, 18, 309. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Luo, C.; Dong, L.; Van Toan, C.; Wei, P.; He, X. Molecular characterization and expression analysis of a GTP-binding protein (MiRab5) in Mangifera indica. Gene 2014, 540, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.K.; Agarwal, P.; Jain, P.; Jha, B.; Reddy, M.K.; Sopory, S.K. Constitutive overexpression of a stress-inducible small GTP-binding protein PgRab7 from Pennisetum glaucum enhances abiotic stress tolerance in transgenic tobacco. Plant Cell Rep. 2008, 27, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Vernoud, V.; Horton, A.C.; Yang, Z.; Nielsen, E. Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol. 2003, 131, 1191–1208. [Google Scholar] [CrossRef] [PubMed]
- Minamino, N.; Ueda, T. RAB GTPases and their effectors in plant endosomal transport. Curr. Opin. Plant Biol. 2019, 52, 61–68. [Google Scholar] [CrossRef]
- Koenig, D.; Jiménez-Gómez, J.M.; Kimura, S.; Fulop, D.; Chitwood, D.H.; Headland, L.R.; Kumar, R.; Covington, M.F.; Devisetty, U.K.; Tat, A.V.; et al. Comparative transcriptomics reveals patterns of selection in domesticated and wild tomato. Proc. Natl. Acad. Sci. USA 2013, 110, E2655–E2662. [Google Scholar] [CrossRef]
- Hoffman, N.E.; Ko, K.; Milkowski, D.; Pichersky, E. Isolation and characterization of tomato cDNA and genomic clones encoding the ubiquitin gene ubi3. Plant Mol. Biol. 1991, 17, 1189–1201. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Leal, J.B.; Seabra, M.C. The mammalian Rab family of small GTPases: Definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J. Mol. Biol. 2000, 301, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Merithew, E.; Hatherly, S.; Dumas, J.J.; Lawe, D.C.; Heller-Harrison, R.; Lambright, D.G. Structural plasticity of an invariant hydrophobic triad in the switch regions of Rab GTPases is a determinant of effector recognition. J. Biol. Chem. 2001, 276, 13982–13988. [Google Scholar] [CrossRef]
- Pfeffer, S.R. Rab GTPases: Specifying and deciphering organelle identity and function. Trends Cell Biol. 2001, 11, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Ménétrey, J. Structural biology of Arf and Rab GTPases’ effector recruitment and specificity. Structure 2013, 21, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Torres-Schumann, S.; Godoy, J.A.; Pintor-Toro, J.A. A probable lipid transfer protein gene is induced by NaCl in stems of tomato plants. Plant Mol. Biol. 1992, 18, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Hiragi, S.; Fukuda, M. Rab family of small GTPases: An updated view on their regulation and functions. FEBS J. 2021, 288, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Leal, J.B.; Seabra, M.C. Evolution of the Rab family of small GTP-binding proteins. J. Mol. Biol. 2001, 313, 889–901. [Google Scholar] [CrossRef]
- Zhang, J.; Hill, D.H.; Sylvester, A.W. Diversification of the RAB Guanosine Triphosphatase Family in Dicots and Monocots. J. Integr. Plant Biol. 2007, 49, 1129–1141. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.; Liu, B.; Wang, L.; Zhang, L.; Hu, J.; Chen, J.; Zheng, H.; Lu, M. Characterization of the Populus Rab family genes and the function of PtRabE1b in salt tolerance. BMC Plant Biol. 2018, 18, 124. [Google Scholar] [CrossRef]
- Peng, X.; Ding, X.; Chang, T.; Wang, Z.; Liu, R.; Zeng, X.; Cai, Y.; Zhu, Y. Overexpression of a Vesicle Trafficking Gene, OsRab7, enhances salt tolerance in rice. Sci. World J. 2014, 2014, 483526. [Google Scholar] [CrossRef]
- Olkkonen, V.M.; Stenmark, H. Role of Rab GTPases in Membrane Traffic. Physiol. Rev. 1997, 91, 119–149. [Google Scholar] [CrossRef]
- de Freitas, S.T.; Martinelli, F.; Feng, B.; Reitz, N.F.; Mitcham, E.J. Transcriptome Approach to Understand the Potential Mechanisms Inhibiting or Triggering Blossom-End Rot Development in Tomato Fruit in Response to Plant Growth Regulators. J. Plant Growth Regul. 2018, 37, 183–198. [Google Scholar] [CrossRef]
- Fleming, A.J.; Mandel, T.; Roth, I.; Kuhlemeier, C. The patterns of gene expression in the tomato shoot apical meristem. Plant Cell 1993, 5, 297–309. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adachi, R.; Nigam, R.; Tuvim, M.J.; Demayo, F.; Dickey, B.F. Genomic organization, chromosomal localization, and expression of the murine RAB3D gene. Biochem. Biophys. Res. Commun. 2000, 273, 877–883. [Google Scholar] [CrossRef]
- Tolmachova, T.; Ramalho, J.S.; Anant, J.S.; Schultz, R.A.; Huxley, C.M.; Seabra, M.C. Cloning, mapping and characterization of the human RAB27A gene. Gene 1999, 239, 109–116. [Google Scholar] [CrossRef]
- Hu, R.; Qi, G.; Kong, Y.; Kong, D.; Gao, Q.; Zhou, G. Comprehensive analysis of NAC domain transcription factor gene family in Populus trichocarpa. BMC Plant Biol. 2010, 10, 145. [Google Scholar] [CrossRef] [PubMed]
- Nosenko, T.; Böndel, K.B.; Kumpfmüller, G.; Stephan, W. Adaptation to low temperatures in the wild tomato species Solanum chilense. Mol. Ecol. 2016, 25, 2853–2869. [Google Scholar] [CrossRef]
- Tapia, G.; Méndez, J.; Inostroza, L. Different combinations of morpho-physiological traits are responsible for tolerance to drought in wild tomatoes Solanum chilense and Solanum peruvianum. Plant Biol. 2016, 18, 406–416. [Google Scholar] [CrossRef]
- Kashyap, S.P.; Kumari, N.; Mishra, P.; Prasad Moharana, D.; Aamir, M.; Singh, B.; Prasanna, H.C. Transcriptional regulation-mediating ROS homeostasis and physio-biochemical changes in wild tomato (Solanum chilense) and cultivated tomato (Solanum lycopersicum) under high salinity. Saudi J. Biol. Sci. 2020, 27, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, A.J.; Ruperti, B.; Palme, K. Small GTPases in Vesicle Trafficking. Curr. Opin. Plant Biol. 2004, 7, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Lawson, T.; Lycett, G.W.; Mayes, S.; Ho, W.K.; Chin, C.F. Transcriptome-wide identification and characterization of the Rab GTPase family in mango. Mol. Biol. Rep. 2020, 47, 4183–4197. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Fang, P.; Peng, Y.; Zeng, W.; Zhao, X.; Ding, Y.; Zhuang, Z.; Gao, Q.; Ren, B. Comparative Proteomics of Salt-Tolerant and Salt-Sensitive Maize Inbred Lines to Reveal the Molecular Mechanism of Salt Tolerance. Int. J. Mol. Sci. 2019, 20, 4725. [Google Scholar] [CrossRef] [PubMed]
- Mazel, A.; Leshem, Y.; Tiwari, B.S.; Levine, A. Induction of salt and osmotic stress tolerance by overexpression of an intracellular vesicle trafficking protein AtRab7 (AtRabG3e). Plant Physiol. 2004, 134, 118–128. [Google Scholar] [CrossRef]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of Rice Rab7 Gene Improves Drought and Heat Tolerance and Increases Grain Yield in Rice (Oryza sativa L.). Genes 2019, 10, 56. [Google Scholar] [CrossRef]




| GeneName | ChrName | Chromosome Location | Length (aa) | Isoelectric Point | Mw (kDa) | GRAVY | Subcellular Location | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | Strand | WPS | CELLO | ||||||
| Solyc01g086850.2 | 1 | 81,771,059 | 81,775,731 | - | 226 | 6.76 | 24.78 | −0.192 | Cyt | Cyt |
| Solyc01g088560.2 | 1 | 83,336,020 | 83,341,655 | - | 209 | 7.67 | 23.01 | −0.162 | Chl | Chl |
| Solyc01g090170.2 | 1 | 83,802,202 | 83,809,193 | + | 204 | 5.1 | 22.45 | −0.254 | Cyt | Gol |
| Solyc01g096220.2 | 1 | 87,314,716 | 87,316,882 | + | 244 | 6 | 27.1 | −0.199 | Nuc | Cyt |
| Solyc01g103370.2 | 1 | 91,989,852 | 91,994,839 | + | 203 | 5.33 | 22.5 | −0.277 | Nuc | Cyt |
| Solyc01g103380.2 | 1 | 91,995,157 | 91,998,149 | - | 215 | 6.22 | 23.8 | −0.291 | Chl | Chl |
| Solyc01g109520.2 | 1 | 96,449,221 | 96,452,841 | - | 207 | 5.1 | 23.01 | −0.397 | Chl | Chl |
| Solyc02g036450.2 | 2 | 30,788,590 | 30,794,213 | - | 201 | 7.75 | 21.96 | −0.181 | Chl | Chl |
| Solyc02g069370.2 | 2 | 39,281,375 | 39,289,536 | - | 201 | 5.89 | 21.66 | −0.155 | Chl | Chl |
| Solyc02g072180.2 | 2 | 41,496,822 | 41,503,133 | + | 218 | 5.5 | 24.09 | −0.375 | Nuc | Cyt |
| Solyc02g081380.2 | 2 | 45,368,874 | 45,373,407 | - | 201 | 6.15 | 21.82 | −0.216 | Chl | Chl |
| Solyc02g093530.2 | 2 | 54,369,660 | 54,374,256 | - | 212 | 7.72 | 23.29 | −0.293 | Cyt | Cyt |
| Solyc03g006270.2 | 3 | 888,904 | 890,977 | + | 217 | 5.63 | 24.01 | −0.290 | Cyt | Cyt |
| Solyc03g064020.2 | 3 | 37,223,959 | 37,225,122 | + | 225 | 6.12 | 24.96 | −0.204 | Cyt | Cyt |
| Solyc03g078570.2 | 3 | 51,178,678 | 51,181,240 | - | 208 | 6.39 | 23.14 | −0.255 | Chl | Cyt |
| Solyc03g079900.2 | 3 | 51,787,671 | 51,789,397 | + | 216 | 6.97 | 23.96 | −0.264 | Csk | Cyt |
| Solyc03g118820.2 | 3 | 67,626,322 | 67,629,418 | + | 243 | 5.37 | 23.16 | −0.301 | Nuc | Cyt |
| Solyc03g120750.2 | 3 | 69,027,066 | 69,031,274 | - | 206 | 5.21 | 22.97 | −0.338 | Chl | Cyt |
| Solyc04g011360.2 | 4 | 3,839,267 | 3,844,110 | - | 217 | 7.65 | 23.9 | −0.378 | Nuc | Chl |
| Solyc04g012180.2 | 4 | 4,461,532 | 4,467,418 | - | 223 | 8.44 | 24.64 | −0.266 | Cyt | Cyt |
| Solyc04g051680.2 | 4 | 50,982,372 | 50,985,730 | - | 220 | 4.93 | 24.71 | −0.284 | Cyt | Cyt |
| Solyc04g064510.2 | 4 | 55,670,912 | 55,675,897 | - | 210 | 5.4 | 23.27 | −0.212 | Chl | Chl |
| Solyc04g072060.2 | 4 | 59,116,633 | 59,126,825 | - | 205 | 5.46 | 22.93 | −0.319 | Chl | Cyt |
| Solyc05g051570.2 | 5 | 61,938,794 | 61,942,841 | - | 208 | 6.4 | 23.09 | −0.169 | Chl | Chl |
| Solyc05g052070.2 | 5 | 62,410,368 | 62,413,664 | - | 204 | 5.27 | 22.62 | −0.373 | Cyt | Cyt |
| Solyc05g052900.2 | 5 | 63,076,702 | 63,078,931 | - | 216 | 7.68 | 23.99 | −0.240 | Cyt | Cyt |
| Solyc05g053940.2 | 5 | 63,958,473 | 63,963,310 | - | 204 | 5.26 | 22.41 | −0.264 | Csk | Gol |
| Solyc05g054150.2 | 5 | 64,106,692 | 64,108,791 | + | 227 | 5.84 | 25.05 | −0.388 | Cyt | Cyt |
| Solyc06g005350.2 | 6 | 362,156 | 370,204 | - | 145 | 9.44 | 16.14 | −0.187 | Cyt | Chl |
| Solyc06g005810.2 | 6 | 839,934 | 842,843 | - | 217 | 6.1 | 23.83 | −0.289 | Cyt | Cyt |
| Solyc06g060210.1 | 6 | 38,174,983 | 38,175,594 | + | 204 | 8.8 | 23.06 | −0.357 | Chl | Cyt |
| Solyc06g076450.2 | 6 | 47,498,298 | 47,500,384 | - | 216 | 6.44 | 23.82 | −0.244 | Cyt | Cyt |
| Solyc07g053480.2 | 7 | 61,927,834 | 61,930,515 | + | 218 | 5.66 | 24.09 | −0.254 | Cyt | Cyt |
| Solyc07g055290.2 | 7 | 63,373,504 | 63,378,793 | - | 219 | 5.73 | 24.16 | −0.316 | Cyt | Cyt |
| Solyc07g056150.2 | 7 | 63,994,885 | 63,999,176 | - | 213 | 6.9 | 23.19 | −0.194 | Cyt | Mit |
| Solyc08g078070.2 | 8 | 61,918,544 | 6,1921,746 | + | 204 | 5.48 | 22.5 | −0.302 | Nuc | Gol |
| Solyc09g008460.2 | 9 | 1,905,656 | 1,911,197 | - | 217 | 5.73 | 23.65 | −0.274 | Chl | Chl |
| Solyc09g010370.2 | 9 | 3,753,893 | 3,759,018 | - | 217 | 7.65 | 23.95 | −0.361 | Chl | Chl |
| Solyc09g056340.2 | 9 | 48,781,284 | 48,783,613 | + | 211 | 4.97 | 23.61 | −0.353 | Cyt | Cyt |
| Solyc09g097900.2 | 9 | 71,891,302 | 71,896,403 | - | 217 | 4.96 | 24.26 | −0.337 | Cyt | Cyt |
| Solyc09g098170.2 | 9 | 72,044,889 | 72,048,527 | + | 218 | 5.13 | 24.29 | −0.410 | Cyt | Cyt |
| Solyc10g007700.2 | 10 | 1,953,681 | 1,958,548 | - | 212 | 6.9 | 23.12 | −0.221 | Cyt | Cyt |
| Solyc10g008840.2 | 10 | 2,900,438 | 2,901,941 | + | 216 | 5.53 | 24.29 | −0.256 | Cyt | Cyt |
| Solyc10g045550.1 | 10 | 34,584,594 | 34,590,416 | + | 216 | 8.34 | 23.92 | −0.287 | Cyt | Chl |
| Solyc10g079030.1 | 10 | 60,670,713 | 60,672,194 | - | 227 | 6.32 | 24.94 | −0.200 | Chl | Mit |
| Solyc10g086350.1 | 10 | 65,205,566 | 65,210,133 | - | 217 | 8.37 | 23.87 | −0.349 | Chl | Chl |
| Solyc11g008020.1 | 11 | 2,238,213 | 2,241,626 | + | 217 | 9.28 | 24.26 | −0.319 | Chl | Ex |
| Solyc11g008430.1 | 11 | 2,614,324 | 2,620,969 | - | 201 | 5.91 | 21.57 | −0.191 | Cyt | Chl |
| Solyc11g010100.1 | 11 | 3,222,459 | 3,224,358 | + | 225 | 6.33 | 24.57 | −0.199 | Cyt | Cyt |
| Solyc11g012460.1 | 11 | 5,297,608 | 5,298,810 | + | 277 | 5.31 | 30.85 | −0.383 | Nuc | Nuc |
| Solyc11g073050.1 | 11 | 56,164,984 | 56,168,738 | + | 217 | 8.37 | 23.8 | −0.313 | Chl | Chl |
| Solyc12g010790.1 | 12 | 3,711,266 | 3,713,629 | - | 218 | 5.48 | 24.05 | −0.253 | Cyt | Cyt |
| Solyc12g011130.1 | 12 | 3,971,227 | 3,974,056 | - | 212 | 6.9 | 23.14 | −0.212 | Cyt | Cyt |
| Solyc12g014080.1 | 12 | 4,894,053 | 4,896,737 | - | 218 | 5.53 | 24.29 | −0.256 | Cyt | Cyt |
| Homologous Sequence in S. lycopersicum | Name Given to Rab GTPases in S. chilense (Reference to Arabidopsis Rab GTPases) |
|---|---|
| Solyc07g055290 | SchRabA1b |
| Solyc02g072180 | SchRabA1d |
| Solyc01g103380 | SchRabA2a |
| Solyc01g096220 | SchRabA3 |
| Solyc01g086850 | SchRabA4c |
| Solyc09g056340 | SchRabA5b |
| Solyc02g093530 | SchRabB1b |
| Solyc01g088560 | SchRabH1b |
| Solyc11g008430 | SchRabF1 |
| Solyc01g109520 | SchRabG3e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, F.; San Martín-Davison, A.; Salinas-Cornejo, J.; Madrid-Espinoza, J.; Ruiz-Lara, S. Identification, Classification, and Transcriptional Analysis of Rab GTPase Genes from Tomato (Solanum lycopersicum) Reveals Salt Stress Response Genes. Genes 2024, 15, 453. https://doi.org/10.3390/genes15040453
Soto F, San Martín-Davison A, Salinas-Cornejo J, Madrid-Espinoza J, Ruiz-Lara S. Identification, Classification, and Transcriptional Analysis of Rab GTPase Genes from Tomato (Solanum lycopersicum) Reveals Salt Stress Response Genes. Genes. 2024; 15(4):453. https://doi.org/10.3390/genes15040453
Chicago/Turabian StyleSoto, Flavia, Alex San Martín-Davison, Josselyn Salinas-Cornejo, José Madrid-Espinoza, and Simón Ruiz-Lara. 2024. "Identification, Classification, and Transcriptional Analysis of Rab GTPase Genes from Tomato (Solanum lycopersicum) Reveals Salt Stress Response Genes" Genes 15, no. 4: 453. https://doi.org/10.3390/genes15040453
APA StyleSoto, F., San Martín-Davison, A., Salinas-Cornejo, J., Madrid-Espinoza, J., & Ruiz-Lara, S. (2024). Identification, Classification, and Transcriptional Analysis of Rab GTPase Genes from Tomato (Solanum lycopersicum) Reveals Salt Stress Response Genes. Genes, 15(4), 453. https://doi.org/10.3390/genes15040453





