The Immune Microenvironment Landscape of Pituitary NeuroEndocrine Tumors, a Transcriptomic Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. RNA Purification
2.3. Microarray GeneChip Clariom D Assay
2.4. Bioinformatic Analysis of PitNET Transcriptome
2.5. Deconvolution Analysis for Immune Cell Infiltration Inference
2.6. Identification of Determinants of Tumor Immunogenicity and Prediction of Response to ICi
2.7. Immunofluorescence Assays and Confocal Microscopy
2.8. Reverse Transcription and qPCR
2.9. Statistical Analysis
3. Results
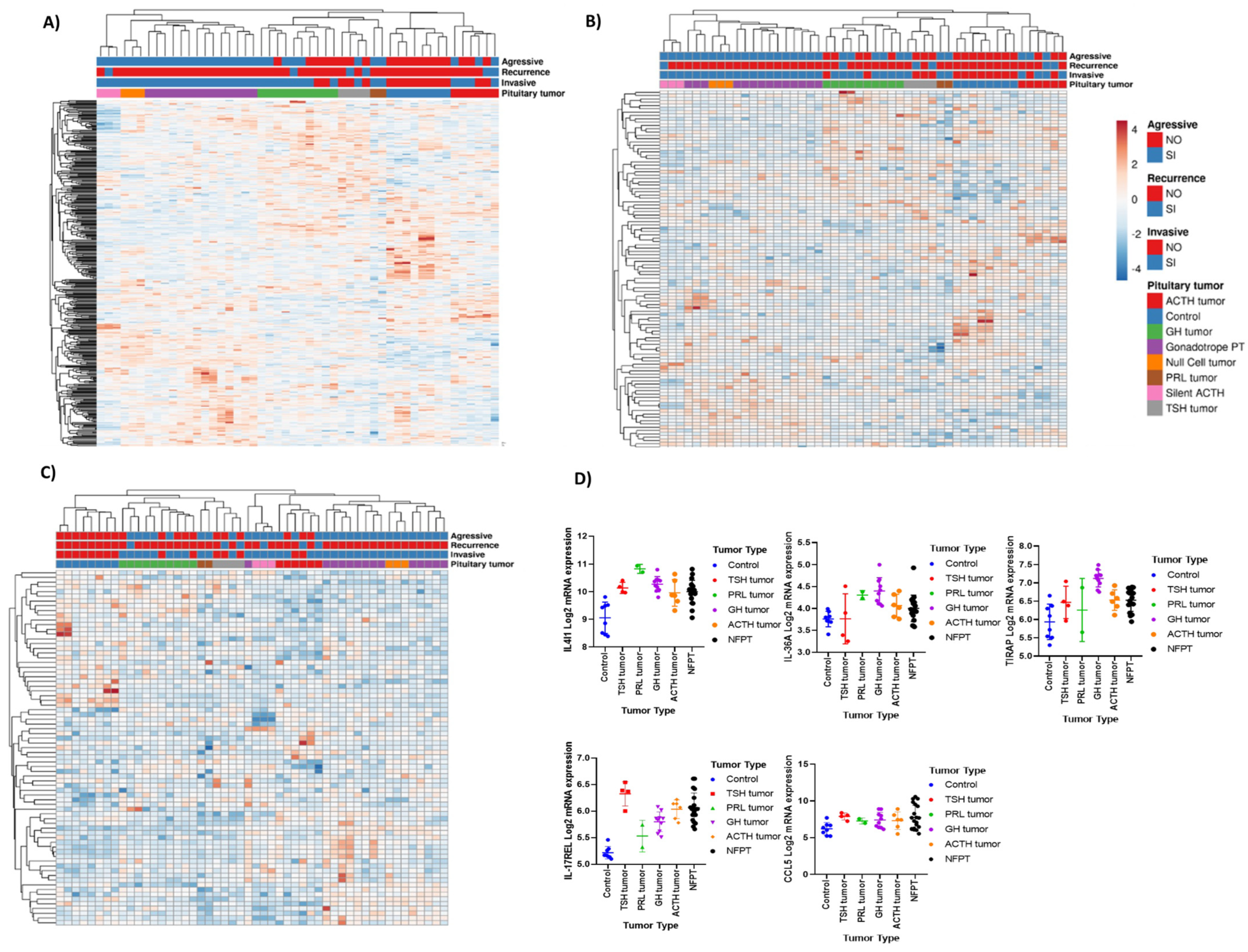
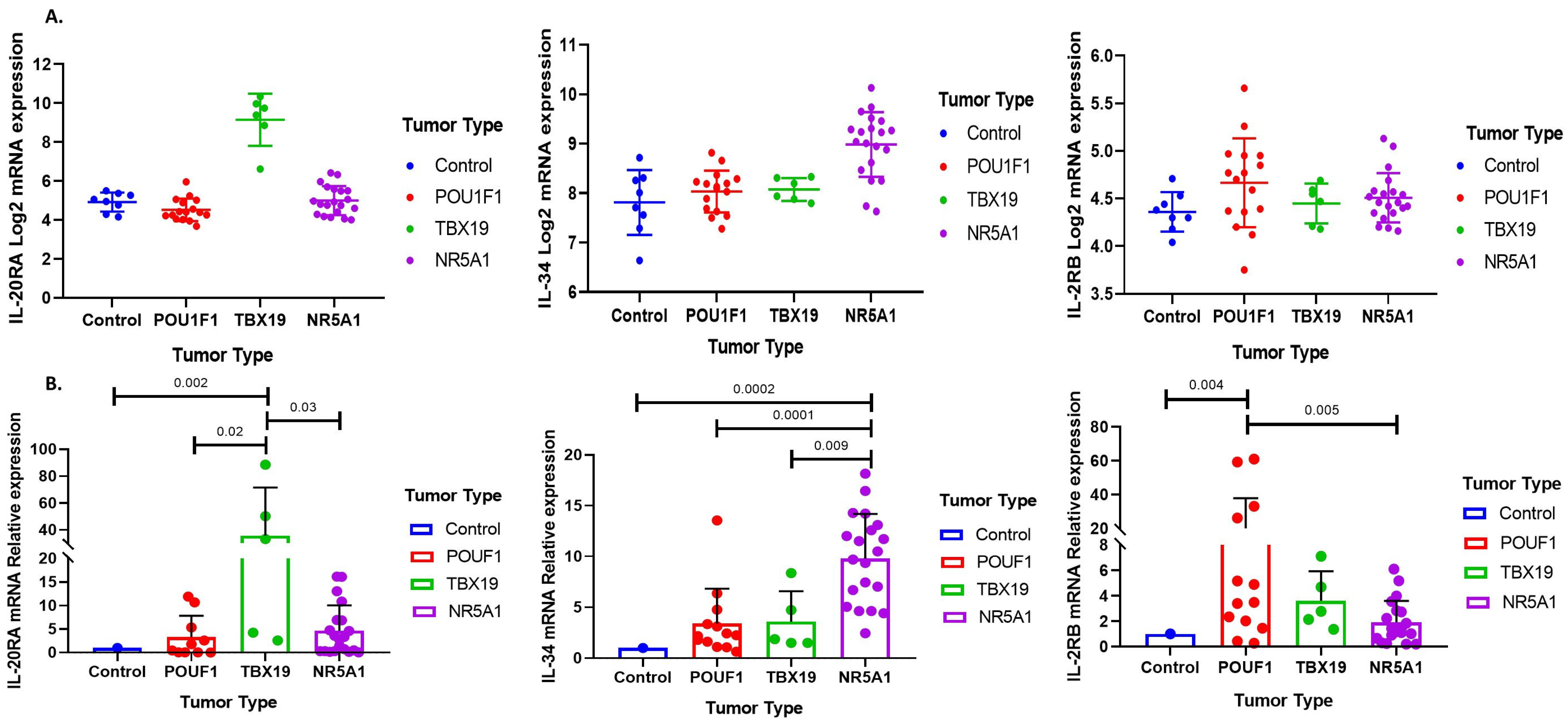
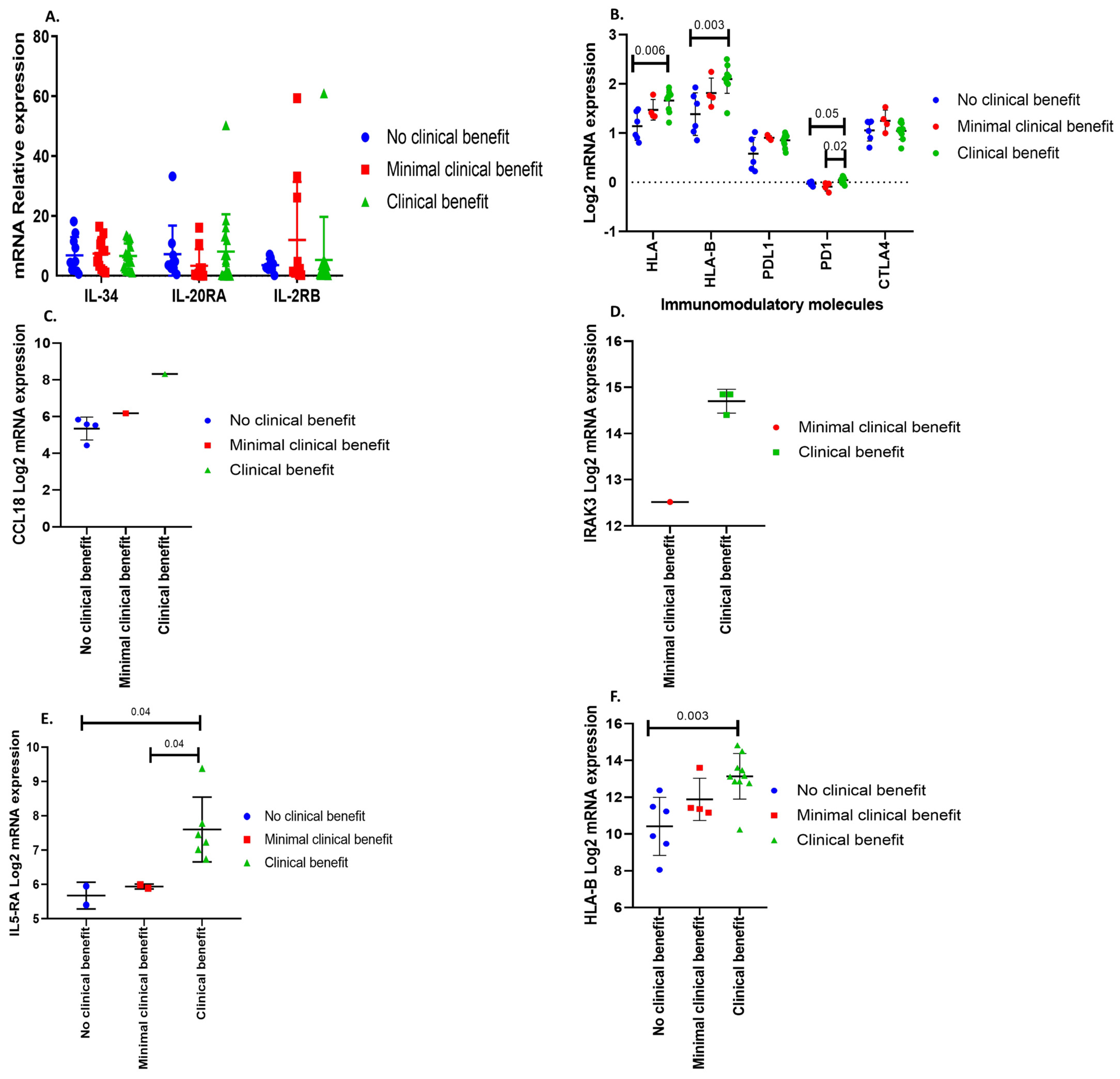
3.1. Immune Response Gene Profiling in Pituitary Neuroendocrine Tumor
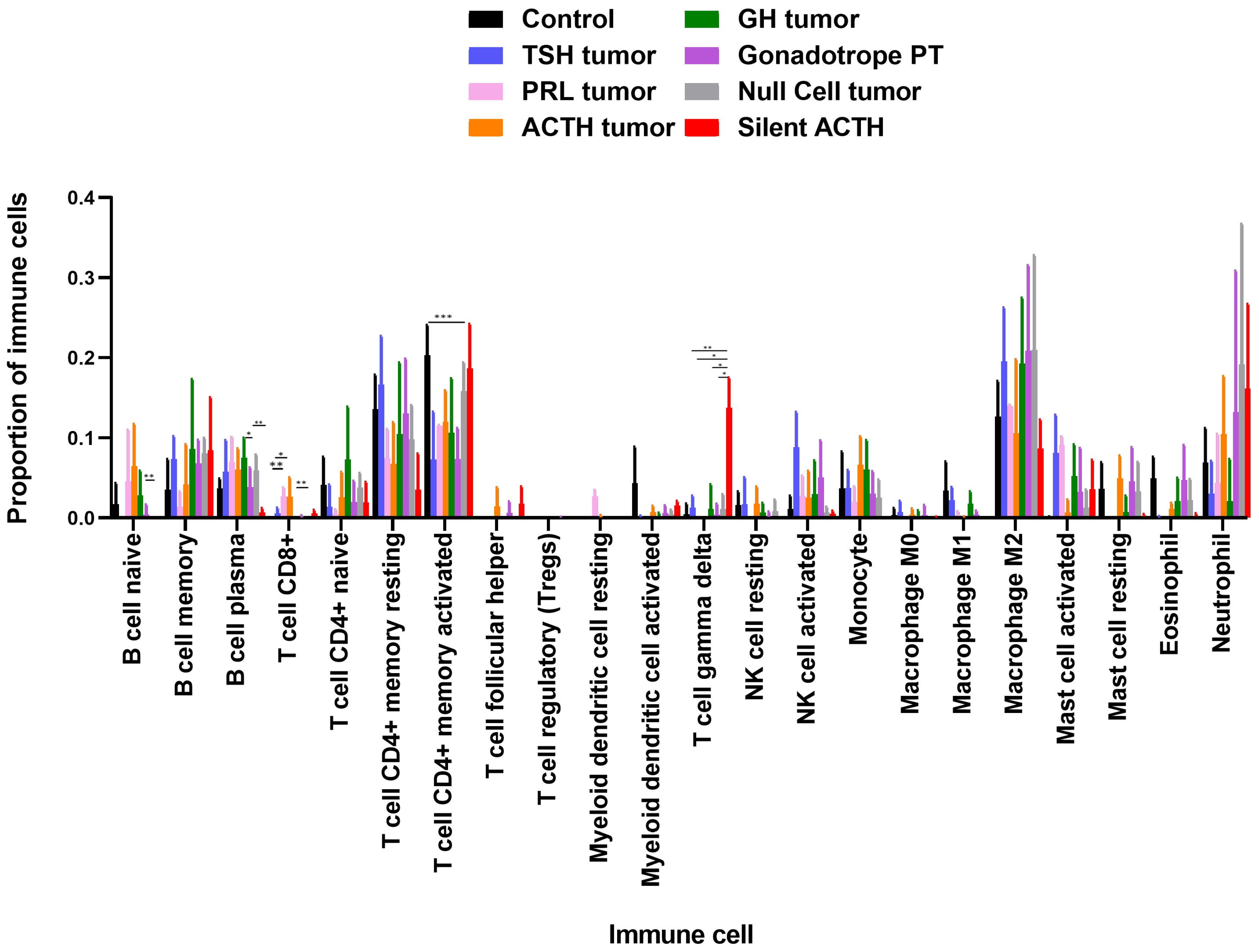
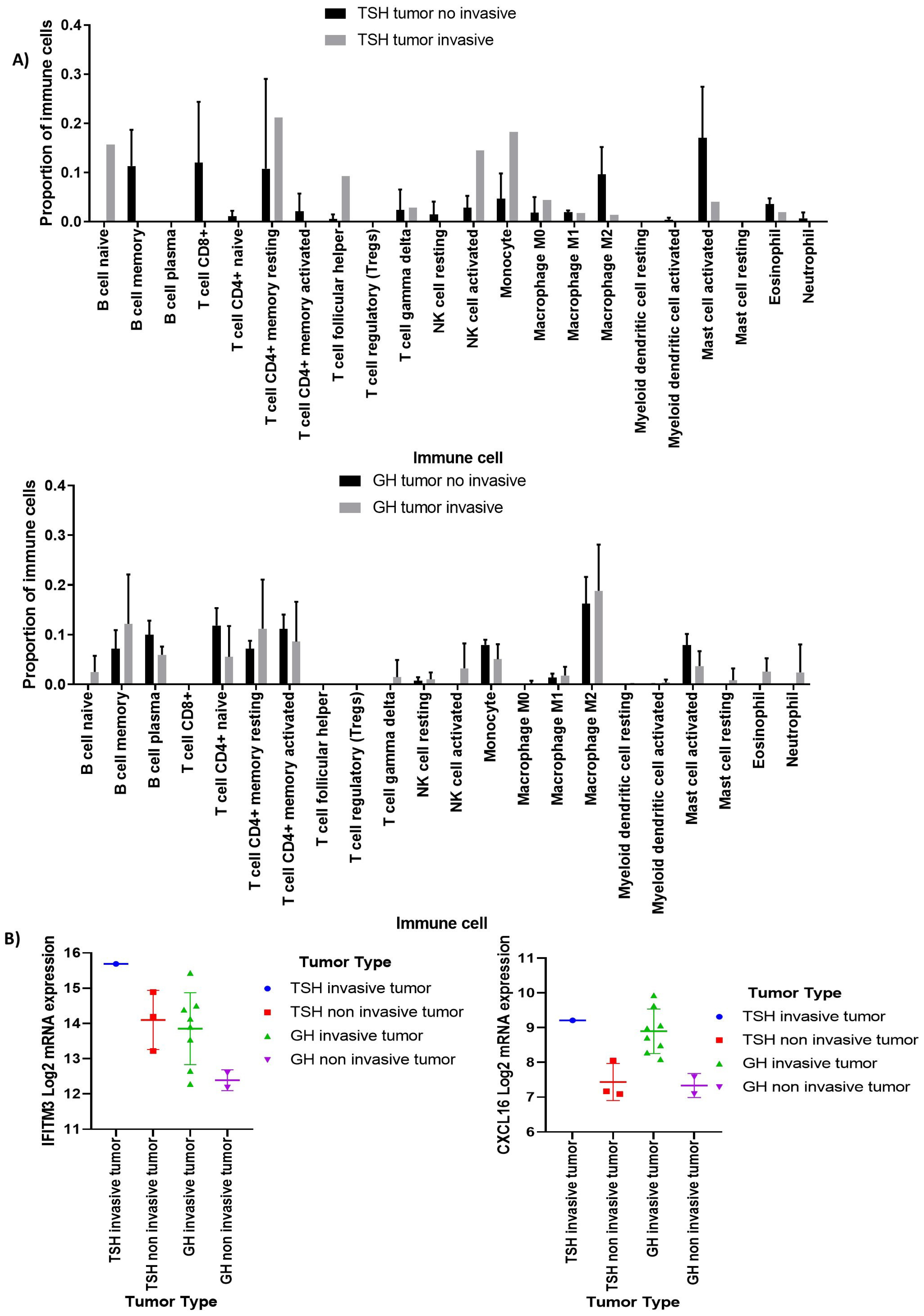
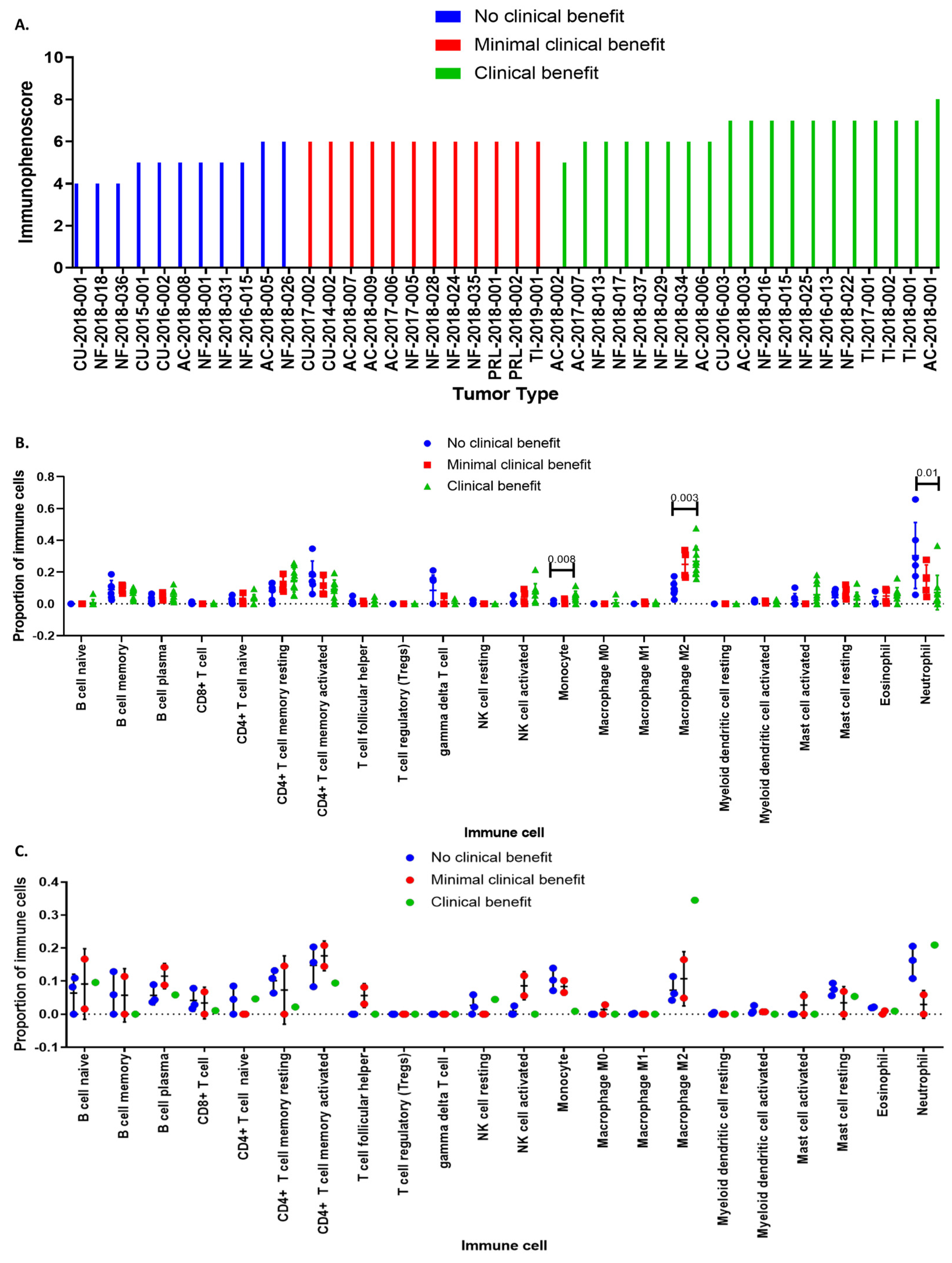
3.2. Deconvolution Analysis for Identification of Immune Cellular Infiltrates in PitNET
3.3. Identification of Immune Cell Infiltrates by Immunofluorescence
3.4. Immunophenoscore Predicts Potential Response to Immunotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes. Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Heshmati, H.M.; Kujas, M.; Casanova, S.; Wollan, P.C.; Racadot, J.; Van Effenterre, R.; Derome, P.J.; Turpin, G. Prevalence of lymphocytic infiltrate in 1400 pituitary adenomas. Endocr. J. 1998, 45, 357–361. [Google Scholar] [CrossRef]
- Lupi, I.; Manetti, L.; Caturegli, P.; Menicagli, M.; Cosottini, M.; Iannelli, A.; Acerbi, G.; Bevilacqua, G.; Bogazzi, F.; Martino, E. Tumor infiltrating lymphocytes but not serum pituitary antibodies are associated with poor clinical outcome after surgery in patients with pituitary adenoma. J. Clin. Endocrinol. Metab. 2010, 95, 289–296. [Google Scholar] [CrossRef]
- Lu, J.Q.; Adam, B.; Jack, A.S.; Lam, A.; Broad, R.W.; Chik, C.L. Immune Cell Infiltrates in Pituitary Adenomas: More Macrophages in Larger Adenomas and More T Cells in Growth Hormone Adenomas. Endocr. Pathol. 2015, 26, 263–272. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, X.; Gao, L.; Deng, K.; Lian, W.; Bao, X.; Feng, M.; Duan, L.; Zhu, H.; Xing, B. The Immune Profile of Pituitary Adenomas and a Novel Immune Classification for Predicting Immunotherapy Responsiveness. J. Clin. Endocrinol. Metab. 2020, 105, e3207–e3223. [Google Scholar] [CrossRef]
- Marques, P.; Barry, S.; Carlsen, E.; Collier, D.; Ronaldson, A.; Awad, S.; Dorward, N.; Grieve, J.; Mendoza, N.; Muquit, S.; et al. Chemokines modulate the tumour microenvironment in pituitary neuroendocrine tumours. Acta Neuropathol. Commun. 2019, 7, 172. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, C.; Zhang, D.; Peng, J.; Ma, S.; Wang, X.; Guan, X.; Li, P.; Li, D.; Jia, G.; et al. Comprehensive analysis of the immunological landscape of pituitary adenomas: Implications of immunotherapy for pituitary adenomas. J. Neurooncol. 2020, 149, 473–487. [Google Scholar] [CrossRef]
- Dai, C.; Liang, S.; Sun, B.; Kang, J. The Progress of Immunotherapy in Refractory Pituitary Adenomas and Pituitary Carcinomas. Front. Endocrinol. 2020, 11, 608422. [Google Scholar] [CrossRef]
- Feola, T.; Carbonara, F.; Verrico, M.; Di Crescenzo, R.M.; Gianno, F.; Colonnese, C.; Arcella, A.; de Alcubierre, D.; Tomao, S.; Esposito, V.; et al. Immunotherapy for Aggressive and Metastatic Pituitary Neuroendocrine Tumors (PitNETs): State-of-the Art. Cancers 2022, 14, 4093. [Google Scholar] [CrossRef]
- Nie, D.; Xue, Y.; Fang, Q.; Cheng, J.; Li, B.; Wang, D.; Li, C.; Gui, S.; Zhang, Y.; Zhao, P. Immune Checkpoints: Therapeutic Targets for Pituitary Tumors. Dis. Markers 2021, 2021, 5300381. [Google Scholar] [CrossRef]
- Taniguchi-Ponciano, K.; Portocarrero-Ortiz, L.A.; Guinto, G.; Moreno-Jimenez, S.; Gomez-Apo, E.; Chavez-Macias, L.; Peña-Martínez, E.; Silva-Román, G.; Vela-Patiño, S.; Ordoñez-García, J.; et al. The kinome, cyclins and cyclin-dependent kinases of pituitary adenomas, a look into the gene expression profile among tumors from different lineages. BMC Med. Genom. 2022, 15, 52. [Google Scholar] [CrossRef]
- Taniguchi-Ponciano, K.; Andonegui-Elguera, S.; Peña-Martínez, E.; Silva-Román, G.; Vela-Patiño, S.; Gomez-Apo, E.; Chavez-Macias, L.; Vargas-Ortega, G.; Espinosa-de-Los-Monteros, L.; Gonzalez-Virla, B.; et al. Transcriptome and methylome analysis reveals three cellular origins of pituitary tumors. Sci. Rep. 2020, 10, 1937. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Principe, M.; Chanal, M.; Ilie, M.D.; Ziverec, A.; Vasiljevic, A.; Jouanneau, E.; Hennino, A.; Raverot, G.; Bartolino, P. Immune Landscape of Pituitary Tumors Reveals Association Between Macrophages and Gonadotroph Tumor Invasion. J. Clin. Endocrinol. Metab. 2020, 105, 3473. [Google Scholar] [CrossRef]
- Luo, M.; Tang, R.; Wang, H. Tumor immune microenvironment in pituitary neuroendocrine tumors (PitNETs): Increased M2 macrophage infiltration and PD-L1 expression in PIT1-lineage subset. J. Neurooncol. 2023, 163, 663–674. [Google Scholar] [CrossRef]
- Barry, S.; Carlsen, E.; Marques, P.; Stiles, C.E.; Gadaleta, E.; Berney, D.M.; Roncaroli, F.; Chelala, C.; Solomou, A.; Herincs, M.; et al. Tumor microenvironment defines the invasive phenotype of AIP-mutation-positive pituitary tumors. Oncogene 2019, 38, 5381–5395. [Google Scholar] [CrossRef]
- Wang, P.F.; Wang, T.J.; Yang, Y.K.; Yao, K.; Li, Z.; Li, Y.M.; Yan, C.X. The expression profile of PD-L1 and CD8 lymphocyte in pituitary adenomas indicating for immunotherapy. J. Neurooncol. 2018, 139, 89–95. [Google Scholar] [CrossRef]
- Huang, X.; Xu, J.; Wu, Y.; Sheng, L.; Li, Y.; Zha, B.; Sun, T.; Yang, J.; Zang, S.; Liu, J. Alterations in CD8+ Tregs, CD56+ Natural Killer Cells and IL-10 Are Associated with Invasiveness of Nonfunctioning Pituitary Adenomas (NFPAs). Pathol. Oncol. Res. 2021, 27, 598887. [Google Scholar] [CrossRef]
- Molinier-Frenkel, V.; Prévost-Blondel, A.; Castellano, F. The IL4I1 Enzyme: A New Player in the Immunosuppressive Tumor Microenvironment. Cells 2019, 8, 757. [Google Scholar] [CrossRef]
- Sadik, A.; Somarribas Patterson, L.F.; Öztürk, S.; Mohapatra, S.R.; Panitz, V.; Secker, P.F.; Pfänder, P.; Loth, S.; Salem, H.; Prentzell, M.T.; et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell 2020, 182, 1252–1270.e34. [Google Scholar] [CrossRef]
- Yuan, Z.C.; Xu, W.D.; Liu, X.Y.; Liu, X.Y.; Huang, A.F.; Su, L.C. Biology of IL-36 Signaling and Its Role in Systemic Inflammatory Diseases. Front. Immunol. 2019, 10, 2532. [Google Scholar] [CrossRef]
- Baker, K.J.; Buskiewicz, E.; Finucane, M.; Chelliah, A.; Burke, L.; Houston, A.; Brint, E. IL-36 expression is increased in NSCLC with IL-36 stimulation of lung cancer cells promoting a pro-tumorigenic phenotype. Cytokine 2023, 165, 156170. [Google Scholar] [CrossRef]
- Aldinucci, D.; Borghese, C.; Casagrande, N. The CCL5/CCR5 Axis in Cancer Progression. Cancers 2020, 12, 1765. [Google Scholar] [CrossRef]
- Mikkola, T.; Almahmoudi, R.; Salo, T.; Al-Samadi, A. Variable roles of interleukin-17F in different cancers. BMC Cancer 2022, 22, 54. [Google Scholar] [CrossRef]
- Baud’huin, M.; Renault, R.; Charrier, C.; Riet, A.; Moreau, A.; Brion, R.; Gouin, F.; Duplomb, L.; Heymann, D. Interleukin-34 is expressed by giant cell tumours of bone and plays a key role in RANKL-induced osteoclastogenesis. J. Pathol. 2010, 221, 77–86. [Google Scholar] [CrossRef]
- Guillonneau, C.; Bézie, S.; Anegon, I. Immunoregulatory properties of the cytokine IL-34. CMLF 2017, 74, 2569–2586. [Google Scholar] [CrossRef]
- Gao, W.; Wen, H.; Liang, L.; Dong, X.; Du, R.; Zhou, W.; Zhang, X.; Zhang, C.; Xiang, R.; Li, N. IL20RA signaling enhances stemness and promotes the formation of an immunosuppressive microenvironment in breast cancer. Theranostics 2021, 11, 2564–2580. [Google Scholar] [CrossRef]
- Alderdice, M.; Craig, S.G.; Humphries, M.P.; Gilmore, A.; Johnston, N.; Bingham, V.; Coyle, V.; Senevirathne, S.; Longley, D.B.; Loughrey, M.B.; et al. Evolutionary genetic algorithm identifies IL2RB as a potential predictive biomarker for immune-checkpoint therapy in colorectal cancer. NAR Genom. Bioinform. 2021, 3, lqab016. [Google Scholar] [CrossRef]
- Franzè, E.; Stolfi, C.; Troncone, E.; Scarozza, P.; Monteleone, G. Role of Interleukin-34 in Cancer. Cancers 2020, 12, 252. [Google Scholar] [CrossRef]
- Zhang, A.; Xu, Y.; Xu, H.; Ren, J.; Meng, T.; Ni, Y.; Zhu, Q.; Zhang, W.B.; Pan, Y.B.; Jin, J.; et al. Lactate-induced M2 polarization of tumor-associated macrophages promotes the invasion of pituitary adenoma by secreting CCL17. Theranostics 2021, 11, 3839–3852. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.; Korbonits, M. Tumor microenvironment and pituitary tumour behaivour. J. Endocrinol. Investig. 2023, 46, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Serioli, S.; Agostini, L.; Pietrantoni, A.; Valeri, F.; Costanza, F.; Chiloiro, S.; Buffoli, B.; Piazza, A.; Poliani, P.L.; Peris-Celda, M.; et al. Aggressive PitNETs and Potential Target Therapies: A Systematic Review of Molecular and Genetic Pathways. Int. J. Mol. Sci. 2023, 24, 15719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Q.; Zhu, J.; Yao, B.; Ma, C.; Qiao, N.; He, S.; Ye, Z.; Wang, Y.; Han, R.; et al. Integrated proteogenomic characterization across major histological types of pituitary neuroendocrine tu-mors. Cell Res. 2022, 32, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; La Rosa, S.; Brouland, J.P.; Pitteloud, N.; Harel, E.; Santoni, F.; Brunner, M.; Daniel, R.T.; Messerer, M. PD-L1 Expression in Pituitary Neuroendocrine Tumors/Pituitary Adenomas. Cancers 2023, 15, 4471. [Google Scholar] [CrossRef]
- Wu, C.C.; Wang, Y.A.; Livingston, J.A.; Zhang, J.; Futreal, P.A. Prediction of biomarkers and therapeutic combinations for anti-PD-1 immunotherapy using the global gene network association. Nat. Commun. 2022, 13, 42. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vela-Patiño, S.; Salazar, M.I.; Taniguchi-Ponciano, K.; Vadillo, E.; Gomez-Apo, E.; Escobar-España, A.; Perez-Koldenkova, V.; Bonifaz, L.; Aguilar-Flores, C.; Marrero-Rodríguez, D.; et al. The Immune Microenvironment Landscape of Pituitary NeuroEndocrine Tumors, a Transcriptomic Approach. Genes 2024, 15, 531. https://doi.org/10.3390/genes15050531
Vela-Patiño S, Salazar MI, Taniguchi-Ponciano K, Vadillo E, Gomez-Apo E, Escobar-España A, Perez-Koldenkova V, Bonifaz L, Aguilar-Flores C, Marrero-Rodríguez D, et al. The Immune Microenvironment Landscape of Pituitary NeuroEndocrine Tumors, a Transcriptomic Approach. Genes. 2024; 15(5):531. https://doi.org/10.3390/genes15050531
Chicago/Turabian StyleVela-Patiño, Sandra, Ma. Isabel Salazar, Keiko Taniguchi-Ponciano, Eduardo Vadillo, Erick Gomez-Apo, Aurea Escobar-España, Vadim Perez-Koldenkova, Laura Bonifaz, Cristina Aguilar-Flores, Daniel Marrero-Rodríguez, and et al. 2024. "The Immune Microenvironment Landscape of Pituitary NeuroEndocrine Tumors, a Transcriptomic Approach" Genes 15, no. 5: 531. https://doi.org/10.3390/genes15050531






