Radiobiology and Reproduction—What Can We Learn from Mammalian Females?
Abstract
:1. Introduction
2. Female Gametogenesis: Its Complexity and Uniqueness
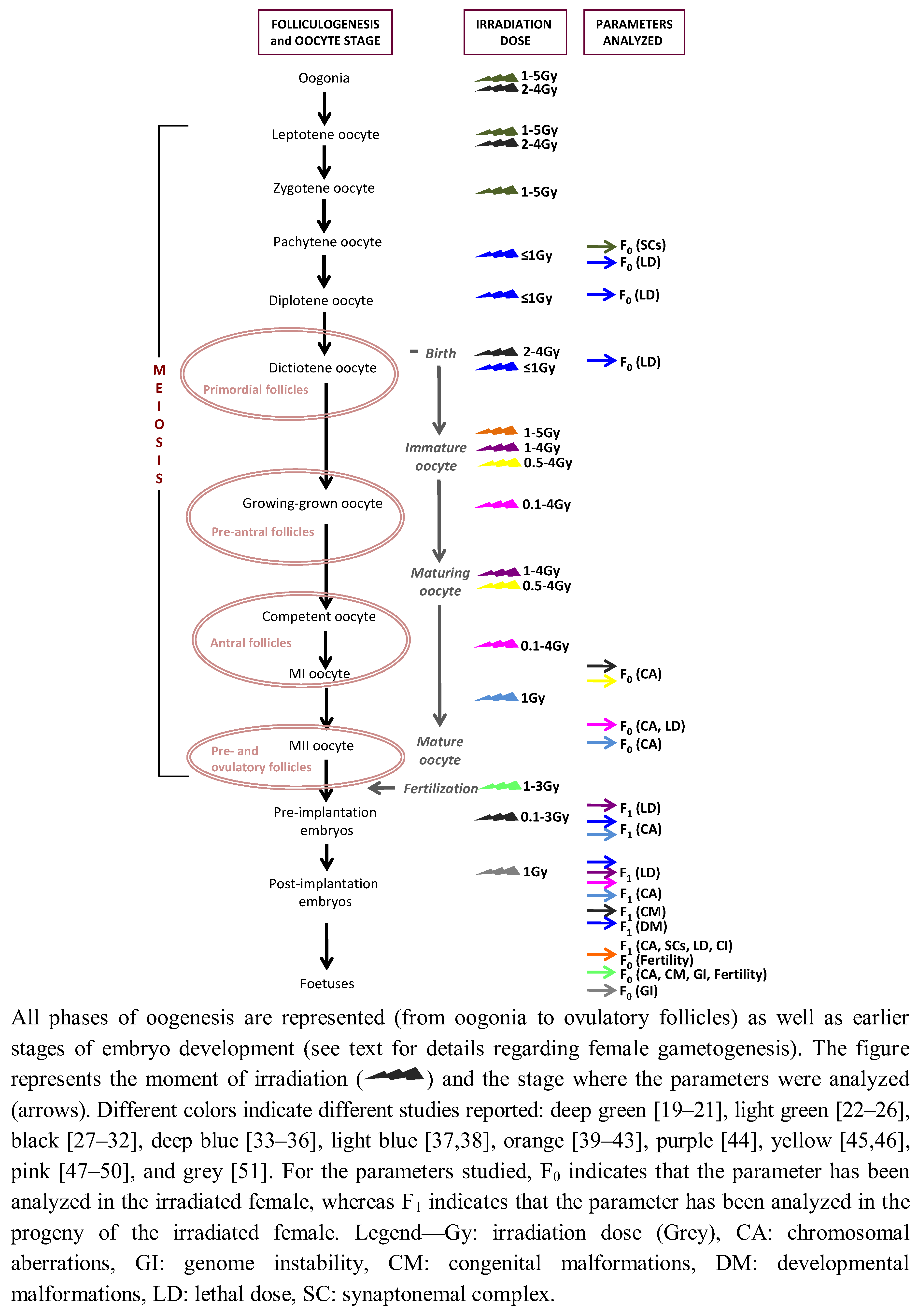
| Species studied | Meiotic/Oocyte stage and irradiation dose | Parameters analyzed | Summary of results | Reference |
|---|---|---|---|---|
| mouse | Adult females | Locus-specific mutation at F1 | (−) | [54] |
| 63 rads | ||||
| mouse | Ovaries | Cell killing | LD50: 0.15 Gy | [16] |
| 0.15 Gy | Fertility | Maximum 4 litters/female; | ||
| Early follicles RS > Larger follicles RS | ||||
| mouse | Mature and immature oocytes | Dominant lethality (pre and post-implantation mortality) | RS species and oocyte stage-dependent | [44] |
| 100-400 rads | ||||
| mouse | Pre-ovulatory oocytes | Chromosomal aberrations at MII | (+) | [37,38] |
| 22-600 rads | Mature oocyte RS > Immature oocyte RS | |||
| Adult females | Chromosomal aberrations at: | |||
| 0.22, 0.66, 2 and 6 Gy | • MII | (+) | ||
| • 2-cell embryo | (+) | |||
| • 13.5-day embryo | (−) | |||
| mouse | Mature and immature oocytes | Chromosomal aberrations at MI | Mature oocyte RS >Immature oocyte RS | [46] |
| 50-400 rads | ||||
| mouse | Immature oocytes | Chromosomal aberrations at dictionema | (−) | [55] |
| 400 rads | ||||
| mouse | Pre-ovulatory oocytes | Chromosomal non-disjunction at MI | (+) At higher dose | [56] |
| 0.05-0.80 Gy | ||||
| Structural chromosome aberrations at MII | (+) | |||
| mouse | Adult females | Dominant lethality | (+) | [57] |
| 108-504 rads | Developmental malformations | (+) | ||
| Immature oocyte RS > Mature oocyte RS | ||||
| mouse | Juvenile mice | Primordial oocyte killing | LD50: 6-7 rads | [58] |
| 6 and 7 rads | ||||
| mouse | Oocytes at dictionema | Chromosomal aberrations at one-cell embryos | (+) | [47] |
| 100-600 cGy | ||||
| mouse | Pre-ovulatory oocytes | Chromosomal aberrations at MI | (+) | [48] |
| ≤100 cGy | ||||
| mouse | Zygote stage | F1 chromosomal aberrations and micronuclei | (+/−) | [25] |
| 2Gy | ||||
| mouse | Immature oocytes | Chromosomal aberrations at MII | (+) | [50] |
| 0.1 and 0.2 Gy | ||||
| mouse | Fetal oocytes at 14, 16, and 17 days of gestation | SC anomalies at pachynema | Fragmentation stage-dependent | [21] |
| 2 Gy | ||||
| mouse | Pre-implantation stage | Developmental malformations | (+) | [31] |
| ≤3 Gy | LD100: 0.5 Gy | |||
| Oocytes within 1-4 weeks before ovulation | Developmental malformations | (+) | ||
| 2 and 3 Gy | ||||
| mouse | Pre-ovulatory oocytes | Chromosomal aberrations at: | [49] | |
| 1-4 Gy | • MII | (+) | ||
| • pre-implantation stages | (+) | |||
| • post-implantation stages | (−) | |||
| mouse | Pre-implantation stage (2 h, 48 h, 72 and 96 h post-conception) | Developmental malformations and mortality | (+) | [32] |
| RS irradiation stage-dependent | ||||
| 0.1-2.5 Gy | ||||
| mouse | Female zygote stage | Fertility alterations | (+) | [22] |
| 1 Gy | F1 developmental malformations | (+) | ||
| mouse | Female zygote stage | Fertility alterations | (−) | [23,24] |
| 0.2 and 0.4 Gy | F1 developmental malformations | (−) | ||
| Trans-generational genomic instability (chromosomal aberrations) | ||||
| (−) | ||||
| mouse | Pre-conception stage | Developmental malformations | (+) dose-dependent | [26] |
| 1, 2.8, and 3 Gy | ||||
| Zygote stage | Developmental malformations | (+) dose-dependent | ||
| 1, 2.8, and 3 Gy | ||||
| Pre-implantation stage | Developmental malformations | (+) dose-dependent | ||
| 1, 2.8, and 3 Gy | ||||
| Zygote | Trans-generational genome instability (chromosomal aberrations) | (+) | ||
| 500 mGy, 1,000 mGy and 2,000 mGy | ||||
| mouse | Post-implantation stage | Trans-generational genome instability (ESTR mutation frequencies) | (−) | [51] |
| 1 Gy | ||||
| mouse | Pre-conception stage | Trans-generational genome instability (polymorphism of DNA fragments) | (+) tissue-dependent | [59] |
| 0.5, 1 and 2 Gy | ||||
| mouse | Adult female | Transgenerational genome instability (ESTR mutation frequencies) | (−) | [60] |
| 1 Gy | ||||
| golden hamster | Mature and immature oocytes | Dominant lethality (pre and post-implantation mortality) | RS species and oocyte stage-dependent | [44] |
| 100-400 rads | ||||
| guinea pig | Mature and immature oocytes | Dominant lethality (pre and post-implantation mortality) | RS species and oocyte stage-dependent | [44] |
| 100-400 rads | ||||
| guinea pig | Mature and immature oocytes4 Gy | Dominant lethality (embryo mortality) | Mature oocyte RS > Immature oocyte RS | [45] |
| guinea pig | Oogonia and oocytes at leptonema | Fertility | (−) | [27,28,29,30] |
| 2 and 4 Gy | ||||
| Oocytes at birth and at adulthood | Cell-killing | LD50: 4 Gy | ||
| Fertility | (−) | |||
| 2 and 4 Gy | ||||
| One-cell embryo stage | Developmental malformations | (+) | ||
| 10, 50 and 100 cGy | ||||
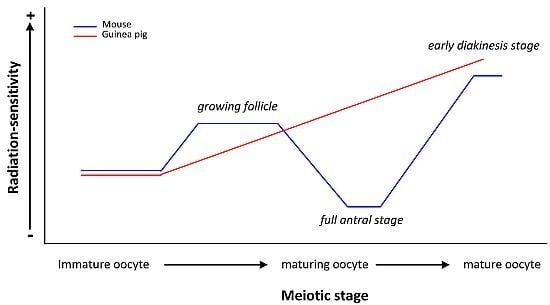
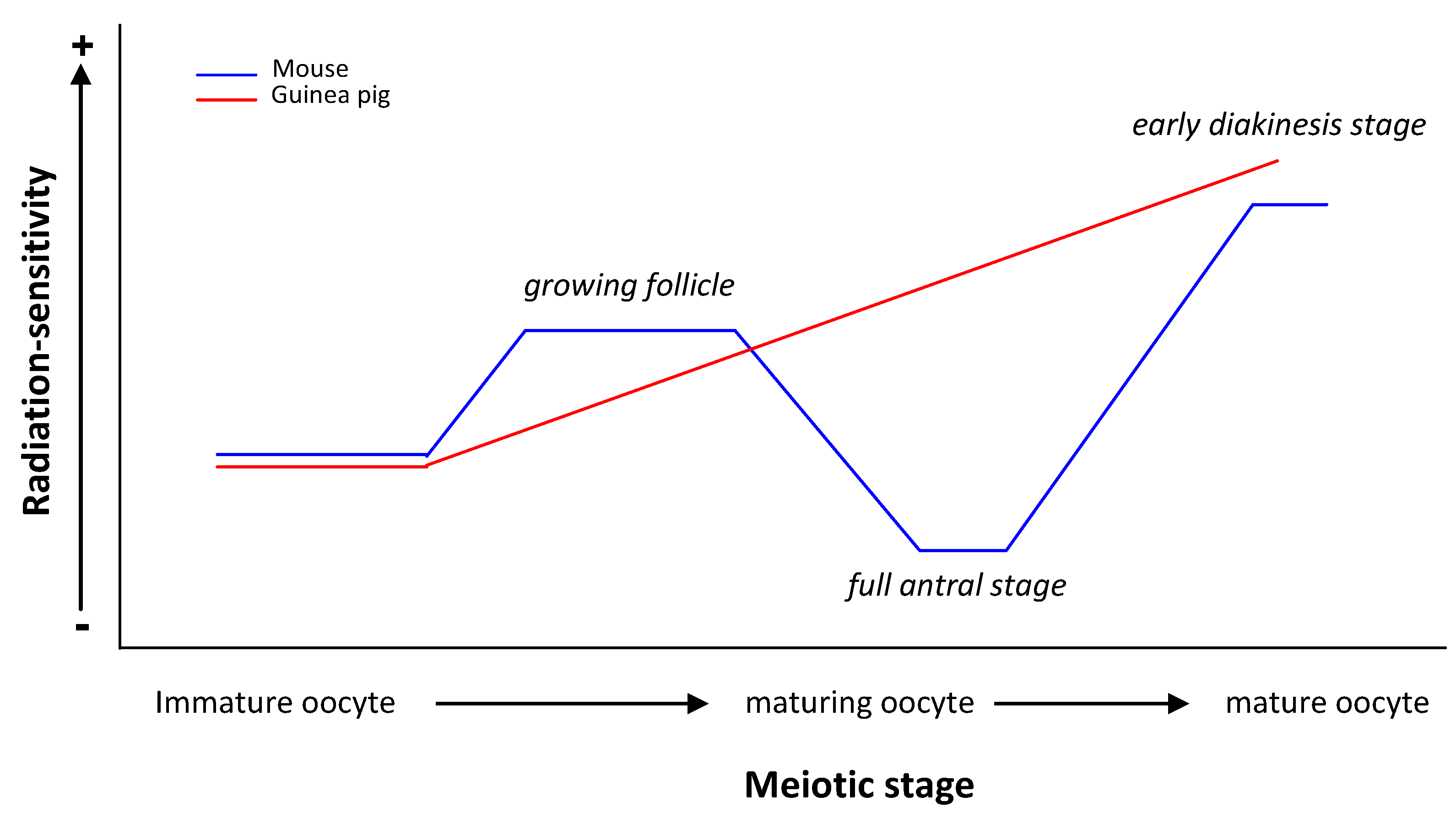
| Oocytes at birth | Chromosomal aberrations at MI | Mature oocyte RS > Immature oocyte RS | ||
| 1 and 2 Gy | Nearly mature guinea pig oocyte RS > Nearly mature mouse oocyte RS | |||
| guinea pig | Oocytes at different stages of folliculogenesis | Chromosomal aberrations | Immature but grown oocyte RS < Maturing oocyte RS < Mature oocyte RS | [23,24] |
| 4.0 Gy | ||||
| RS of guinea pig oocyte ≠ RS of mouse oocyte | ||||
| Chinese hamster | Oocytes around ovulation | Chromosomal aberrations at diakinesi stage | (+) | [61,62,63] |
| 2 Gy | mouse oocyte RS = 2 × Chinese hamster oocyte RS | |||
| Chinese hamster | Oocytes at pachynema | Cell-killing | RS meiotic stage-dependent | [33,34,35,36] |
| 1 Gy | ||||
| Oocytes at diplonema-dictionema | Cell-killing | LD100: 1 Gy | ||
| 1 Gy | ||||
| Oocytes at pachynema and diplonema-dictionema | Developmental malformations | (−) | ||
| Chromosomal aberrations at 1-cell embryos | ||||
| 1 Gy | (−) | |||
| rat | Ovary | Cell-killing | Primordial germ cell reduction (66%) | [64] |
| 1 Gy | ||||
| rat | Ovary | Cell-killing | LD50: 1 Gy | [16] |
| 1 Gy | ||||
| rat | Oogonia, oocytes at leptonema and zygonema | SC anomalies at pachynema | Fragmentation stage-dependent | [19,20] |
| Cell killing | ||||
| 1, 2 and 5 Gy | (+) dose-dependent | |||
| rat | Immature oocytes of pre-pubertal and post-pubertal females | Fertility alterations | Pre-pubertal oocyte RS < Post-pubertal oocyte RS (−) | [39,40] |
| SC alterations of F1 female fetuses | ||||
| 1, 2 and 5 Gy | ||||
| rat | Primordial follicle oocytes | Fertility alterations | (−) | [41,42,43] |
| 5 Gy | F1 constitutional chromosomal aberrations | (−) | ||
| Trans-generational genome instability | (+) | |||
| Trans-generational sensitivity to chemical mutagen | Increased |
3. Indicators of Radiation-Induced Genotoxic Effects
3.1. Cell Killing (Lethal Dose, LD)
3.2. Fertility Alterations
3.3. Developmental Malformations
3.4. Genetic Mutations
4. Radiation-Induced Genotoxic Effects in Mammalian Female Germ-Cells
4.1. Analyzing the F0
4.2. Trans-Generational Studies
4.3. The Rat as a Model Species
5. Human Females: An Approachable Model? Future and Prospects
6. Conclusions
Acknowledgments
References and Notes
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar]
- Valerie, K.; Povirk, L.F. Regulation and mechanisms of mammalian double-strand break repair. Oncogene 2003, 22, 5792–5812. [Google Scholar] [CrossRef]
- Jaco, I.; Muñoz, P.; Goytisolo, F.; Wesoly, J.; Bailey, S.; Taccioli, G.; Blasco, M.A. Role of mammalian Rad54 in telomere length maintenance. Mol. Cell Biol. 2003, 23, 5572–5580. [Google Scholar] [CrossRef]
- Salzano, A.; Kochiashvili, N.; Nergadze, S.G.; Khoriauli, L.; Smirnova, A.; Ruiz-Herrera, A.; Mondello, C.; Giulotto, E. Enhanced gene amplification in human cells knocked down for DNA-Kcs. DNA Repair 2009, 8, 19–28. [Google Scholar] [CrossRef]
- Ruiz-Herrera, A.; Smirnova, A.; Khouriauli, L.; Nergadze, S.G.; Mondello, C.; Giulotto, E. Gene amplification in human cells knocked down for RAD54. Genome Integr. 2011, 2. [Google Scholar]
- Keeney, S.; Giroux, C.N.; Kleckner, N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 1997, 88, 375–384. [Google Scholar] [CrossRef]
- Roeder, G.S. Meiotic chromosomes: It takes two to tango. Genes Dev. 1997, 11, 2600–2621. [Google Scholar] [CrossRef]
- Moens, P.B.; Kolas, N.K.; Tarsounas, M.; Marcon, E.; Cohen, P.E.; Spyropoulos, B. The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA-DNA interactions without reciprocal recombination. J. Cell Sci. 2002, 115, 1611–1622. [Google Scholar]
- Baker, S.M.; Plug, A.W.; Prolla, T.A.; Bronner, C.E.; Harris, A.C.; Yao, X.; Christie, D.M.; Monell, C.; Arnheim, N.; Bradley, A.; et al. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat. Genet. 1996, 13, 336–342. [Google Scholar] [CrossRef]
- Turner, J.M.; Aprelikova, O.; Xu, X.; Wang, R.; Kim, S.; Chandramouli, G.V.; Barrett, J.C.; Burgoyne, P.S.; Deng, C.X. BRCA1, histone H2AX phosphorylation, and male meiotic sex chromosome inactivation. Curr. Biol. 2004, 14, 2135–2142. [Google Scholar] [CrossRef]
- Wallace, W.H.; Thomson, A.B.; Kelsey, T.W. The radiosensitivity of the human oocyte. Hum. Reprod. 2003, 18, 117–121. [Google Scholar]
- Wallace, W.H.; Thomson, A.B.; Saran, F.; Kelsey, T.W. Predicting age of ovarian failure after radiation to a field that includes the ovaries. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 738–744. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Clarke, M.F. Therapeutic implications of the cancer stem cell hypothesis. Semin. Radiat. Oncol. 2009, 19, 78–86. [Google Scholar]
- Russell, L.B.; Russell, W.L. Frequency and nature of specific-locus mutations induced in female mice by radiations and chemicals: A review. Mutat. Res. 1992, 296, 107–127. [Google Scholar] [CrossRef]
- Adler, I.D.; Carere, A.; Eichenlaub-Ritter, U.; Pacchierotti, F. Gender differences in the induction of chromosomal aberrations and gene mutations in rodent germ cells. Environ. Res. 2007, 104, 37–45. [Google Scholar] [CrossRef]
- Baker, T.G. Comparative aspects of the effects of radiation during oogenesis. Mutat. Res. 1971, 11, 9–22. [Google Scholar] [CrossRef]
- Pepling, M.E.; Spradling, A.C. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 2001, 234, 339–351. [Google Scholar] [CrossRef]
- Kerr, J.B.; Duckett, R.; Myers, M.; Britt, K.L.; Mladenovska, T.; Findlay, J.K. Quantification of healthy follicles in the neonatal and adult mouse ovary: Evidence for maintenance of primordial follicle supply. Reproduction 2006, 132, 95–109. [Google Scholar] [CrossRef]
- Pujol, R.; Cusido, L.; Rubio, A.; Egozcue, J.; Garcia, M. Effect of X-rays on germ cells in female fetuses of Rattus norvegicus irradiated at three different times of gestation. Mutat. Res. 1996, 356, 247–253. [Google Scholar] [CrossRef]
- Pujol, R.; Cusido, L.; Rubio, A.; Egozcue, J.; Garcia, M. X-ray-induced synaptonemal complex damage during meiotic prophase in female fetuses of Rattus norvegicus. Mutat. Res. 1997, 379, 127–134. [Google Scholar] [CrossRef]
- Johannisson, R.; Mormel, R.; Brandenburg, B. Synaptonemal complex damage in fetal mouse oocytes induced by ionizing irradiation. Mutat. Res. 1994, 311, 319–328. [Google Scholar] [CrossRef]
- Pils, S.; Muller, W.U.; Streffer, C. Lethal and teratogenic effects in two successive generations of the hlg mouse strain after radiation exposure of zygotes—Association with genomic instability? Mutat. Res. 1999, 429, 85–92. [Google Scholar]
- Jacquet, P.; Buset, J.; Vankerkom, J.; Baatout, S.; de Saint-Georges, L.; Baugnet-Mahieu, L.; Desaintes, C. Radiation-Induced chromosome aberrations in guinea-pig growing oocytes, and their relation to follicular atresia. Mutat. Res. 2001, 473, 249–254. [Google Scholar] [CrossRef]
- Jacquet, P.; Buset, J.; Neefs, M.; Vankerkom, J.; Benotmane, M.A.; Derradji, H.; Hildebrandt, G.; Baatout, S. Transgenerational developmental effects and genomic instability after x-irradiation of preimplantation embryos: Studies on two mouse strains. Mutat. Res. 2010, 687, 54–62. [Google Scholar] [CrossRef]
- Pampfer, S.; Streffer, C. Increased chromosome aberration levels in cells from mouse fetuses after zygote x-irradiation. Int. J. Radiat. Biol. 1989, 55, 85–92. [Google Scholar] [CrossRef]
- Streffer, C. Transgenerational transmission of radiation damage: Genomic instability and congenital malformation. J. Radiat. Res. 2006, 47, B19–B24. [Google Scholar]
- Jacquet, P.; Vankerkom, J.; Lambiet-Collier, M. The female guinea pig, a useful model for the genetic hazard of radiation in man; preliminary results on germ cell radiosensitivity in foetal, neonatal and adult animals. Int. J. Radiat. Biol. 1994, 65, 357–367. [Google Scholar] [CrossRef]
- Jacquet, P.; de Saint-Georges, L.; Vankerkom, J.; Baugnet-Mahieu, L. Embryonic death, dwarfism and fetal malformations after irradiation of embryos at the zygote stage: Studies on two mouse strains. Mutat. Res. 1995, 332, 73–87. [Google Scholar]
- Jacquet, P.; de Saint-Georges, L.; Buset, J.; Baatout, S.; van kerkom, J.; Baugnet-Mahieu, L. Cytogenetic effects of x-rays in the guinea pig female germ cells. I. The immature oocyte. Mutat. Res. 1997, 391, 189–192. [Google Scholar] [CrossRef]
- Jacquet, P.; de Saint-Georges, L.; Buset, J.; Baatout, S.; Vankerkom, J.; Baugnet-Mahieu, L. Cytogenetic effects of x-rays in the guinea pig female germ cells. II. The maturing oocyte. Mutat. Res. 1997, 391, 193–199. [Google Scholar] [CrossRef]
- Muller, W.U.; Schotten, H. Induction of malformations by x-ray exposure of various stages of the oogenesis of mice. Mutat. Res. 1995, 331, 119–125. [Google Scholar] [CrossRef]
- Gu, Y.; Kai, M.; Kusama, T. The embryonic and fetal effects in ICR mice irradiated in the various stages of the preimplantation period. Radiat. Res. 1997, 147, 735–740. [Google Scholar] [CrossRef]
- Tateno, H.; Mikamo, K. Neonatal oocyte development and selective oocyte-killing by X-rays in the chinese hamster, Cricetulus griseus. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1984, 45, 139–149. [Google Scholar] [CrossRef]
- Tateno, H.; Mikamo, K. Effects of neonatal ovarian x-irradiation in the chinese hamster. I. Correlation between the age of irradiation and the fertility span. J. Radiat. Res. 1989, 30, 185–190. [Google Scholar] [CrossRef]
- Tateno, H.; Mikamo, K. Effects of neonatal ovarian x-irradiation in the chinese hamster. II. Absence of chromosomal and developmental damages in surviving oocytes irradiated at the pachytene and resting dictyate stages. J. Radiat. Res. 1989, 30, 209–217. [Google Scholar]
- Tateno, H.; Mikamo, K. Absence of late effects on survival and developmental abilities of pachytene oocytes x-irradiated during neonatal stages in the chinese hamster. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1986, 49, 121–130. [Google Scholar] [CrossRef]
- Reichert, W.; Hansmann, I.; Rohrborn, G. Chromosome anomalies in mouse oocytes after irradiation. Humangenetik 1975, 28, 25–38. [Google Scholar]
- Reichert, W.; Buselmaier, W.; Vogel, F. Elimination of X-ray-induced chromosomal aberrations in the progeny of female mice. Mutat. Res. 1984, 139, 87–94. [Google Scholar] [CrossRef]
- Martinez-Flores, I.; Egozcue, J.; Garcia, M. Effects on female fertility and germinal cells in prepubertal and adult rats (Rattus norvegicus) after X-ray irradiation. Adv. Exp. Med. Biol. 1998, 444, 215–219. [Google Scholar]
- Martinez-Flores, I.; Saez, C.; Egozcue, J.; Garcia, M. Effects of ionizing radiation on oocytes of prepubertally irradiated rats. Int. J. Radiat. Biol. 2000, 76, 1403–1407. [Google Scholar] [CrossRef]
- Camats, N.; Garcia, F.; Parrilla, J.J.; Calaf, J.; Martin-Mateo, M.; Caldes, M.G. The GnRH analogue triptorelin confers ovarian radio-protection to adult female rats. Mutat.Res. 2009, 669, 67–79. [Google Scholar] [CrossRef]
- Camats, N.; Ruiz-Herrera, A.; Parrilla, J.J.; Acien, M.; Paya, P.; Giulotto, E.; Egozcue, J.; Garcia, F.; Garcia, M. Genomic instability in rat: Breakpoints induced by ionising radiation and interstitial telomeric-like sequences. Mutat. Res. 2006, 595, 156–166. [Google Scholar]
- Camats, N.; Garcia, F.; Parrilla, J.J.; Calaf, J.; Martin, M.; Caldes, M.G. Trans-generational radiation-induced chromosomal instability in the female enhances the action of chemical mutagens. Mutat. Res. 2008, 640, 16–26. [Google Scholar] [CrossRef]
- Cox, B.D.; Lyon, M.F. X-ray induced dominant lethal mutations in mature and immature oocytes of guinea-pigs and golden hamsters. Mutat. Res. 1975, 28, 421–436. [Google Scholar] [CrossRef]
- Caine, A.; Lyon, M.F. The induction of chromosome aberrations in mouse dictyate oocytes by X-rays and chemical mutagens. Mutat. Res. 1977, 45, 325–331. [Google Scholar] [CrossRef]
- Brewen, J.G.; Payne, H.S.; Preston, R.J. X-ray-induced chromosome aberrations in mouse dictyate oocytes. I. Time and dose relationships. Mutat. Res. 1976, 35, 111–120. [Google Scholar] [CrossRef]
- Tease, C. Dose-related chromosome non-disjunction in female mice after x-irradiation of dictyate oocytes. Mutat. Res. 1985, 151, 109–119. [Google Scholar] [CrossRef]
- Tease, C.; Fisher, G. X-ray-induced chromosome aberrations in immediately preovulatory oocytes. Mutat. Res. 1986, 173, 211–215. [Google Scholar] [CrossRef]
- Tease, C.; Fisher, G. Cytogenetic and genetic studies of radiation-induced chromosome damage in mouse oocytes. I. Numerical and structural chromosome anomalies in metaphase II oocytes, pre- and post-implantation embryos. Mutat. Res. 1996, 349, 145–153. [Google Scholar] [CrossRef]
- Griffin, C.S.; Tease, C.; Fisher, G. The effect of low-dose x-irradiation on numerical and structural chromosome anomaly induction in mouse immature oocytes. Mutat. Res. 1990, 231, 137–142. [Google Scholar] [CrossRef]
- Barber, R.C.; Hardwick, R.J.; Shanks, M.E.; Glen, C.D.; Mughal, S.K.; Voutounou, M.; Dubrova, Y.E. The effects of in utero irradiation on mutation induction and transgenerational instability in mice. Mutat. Res. 2009, 664, 6–12. [Google Scholar] [CrossRef]
- Garcia-Caldés, M.; Camats, N.; Pujol, R. Efectos Hereditarios de las Radiaciones Ionizantes en Hembras de mamíferos. In Genética Toxicológica, 1st; Mudry, M.D., Carballo, M.A., Eds.; De los Cuatro Vientos: Buenos Aires, Argentina, 2006; pp. 359–392. [Google Scholar]
- Adriaens, I.; Smitz, J.; Jacquet, P. The current knowledge on radiosensitivity of ovarian follicle development stages. Hum. Reprod. Update 2009, 15, 359–377. [Google Scholar] [CrossRef]
- Russell, W.L. Effect of the interval between irradiation and conception on mutation frequency in female mice. Proc. Natl. Acad. Sci. USA 1965, 54, 1552–1557. [Google Scholar] [CrossRef]
- Brewen, J.G.; Payne, H.S. X-ray-induced chromosome aberrations in mouse dictyate oocytes. II. Fractionation and dose rate effects. Genetics 1977, 87, 699–708. [Google Scholar]
- Hansmann, I.; Jenderny, J.; Probeck, H.D. Nondisjunction and chromosome breakage in mouse oocytes after various X-ray doses. Hum. Genet. 1982, 61, 190–192. [Google Scholar]
- Kirk, M.; Lyon, M.F. Induction of congenital anomalies in offspring of female mice exposed to varying doses of X-rays. Mutat. Res. 1982, 106, 73–83. [Google Scholar]
- Dobson, R.L.; Felton, J.S. Female germ cell loss from radiation and chemical exposures. Am. J. Ind. Med. 1983, 4, 175–190. [Google Scholar] [CrossRef]
- Lomaeva, M.G.; Vasil’eva, G.V.; Fomenko, L.A.; Antipova, V.N.; Gaziev, A.I.; Bezlepkin, V.G. Increased genomic instability in somatic cells of the progeny of female mice exposed to acute x-radiation in the preconceptional period. Genetika 2011, 47, 1371–1377. [Google Scholar]
- Abouzeid Ali, H.E.; Barber, R.C.; Dubrova, Y.E. The effects of maternal irradiation during adulthood on mutation induction and transgenerational instability in mice. Mutat. Res. 2012, 732, 21–25. [Google Scholar] [CrossRef]
- Mikamo, K. Meiotic chromosomal radiosensitivity in primary oocytes of the chinese hamster. Cytogenet. Cell Genet. 1982, 33, 88–94. [Google Scholar] [CrossRef]
- Kamiguchi, Y.; Mikamo, K. Dose-response relationship for induction of structural chromosome aberrations in chinese hamster oocytes after x-irradiation. Mutat. Res. 1982, 103, 33–37. [Google Scholar] [CrossRef]
- Mikamo, K.; Kamiguchi, Y.; Funaki, K.; Sugawara, S.; Tateno, H. Stage-dependent changes of chromosomal radiosensitivity in primary oocytes of the chinese hamster. Cytogenet. Cell Genet. 1981, 30, 174–178. [Google Scholar] [CrossRef]
- Beaumont, H.M. The effects of acute x-irradiation on primordial germ-cells in the female rat. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1966, 10, 17–28. [Google Scholar] [CrossRef]
- Russell, L.B.; Russell, W.L. The Sensitivity of Different Stages in Oogenesis to the Radiation Induction of Dominant Lethals and other Changes in the Mouse. In Progress in Radiobiology; Mitchell, J.S., Holmes, B.E., Smith, C.C., Eds.; Oliver and Boyd Ltd.: Edinburgh, UK, 1956; pp. 187–192. [Google Scholar]
- Dubrova, Y.E. Radiation-induced transgenerational instability. Oncogene 2003, 22, 7087–7093. [Google Scholar] [CrossRef]
- Dubrova, Y.E.; Plumb, M.A. Ionising radiation and mutation induction at mouse minisatellite loci. The story of the two generations. Mutat. Res. 2002, 499, 143–150. [Google Scholar] [CrossRef]
- Savage, J.R. A brief survey of aberration origin theories. Mutat. Res. 1998, 404, 139–147. [Google Scholar] [CrossRef]
- Morgan, W.F. Is there a common mechanism underlying genomic instability, bystander effects and other nontargeted effects of exposure to ionizing radiation? Oncogene 2003, 22, 7094–7099. [Google Scholar] [CrossRef]
- Smith, L.E.; Nagar, S.; Kim, G.J.; Morgan, W.F. Radiation-induced genomic instability: Radiation quality and dose response. Health Phys. 2003, 85, 23–29. [Google Scholar]
- Kadhim, M.A. Role of genetic background in induced instability. Oncogene 2003, 22, 6994–6999. [Google Scholar] [CrossRef]
- Moses, M.J. Chromosomal structures in crayfish spermatocytes. J. Biophys. Biochem. Cytol. 1956, 2, 215–218. [Google Scholar] [CrossRef]
- Zickler, D.; Kleckner, N. Meiotic chromosomes: Integrating structure and function. Annu. Rev. Genet. 1999, 33, 603–754. [Google Scholar] [CrossRef]
- Yang, F.; Wang, P.J. The mammalian synaptonemal complex: A scaffold and beyond. Genome Dyn. 2009, 5, 69–80. [Google Scholar] [CrossRef]
- Dobson, M.J.; Pearlman, R.E.; Karaiskakis, A.; Spyropoulos, B.; Moens, P.B. Synaptonemal complex proteins: Occurrence, epitope mapping and chromosome disjunction. J. Cell. Sci. 1994, 107, 2749–2760. [Google Scholar]
- Kouznetsova, A.; Benavente, R.; Pastink, A.; Hoog, C. Meiosis in mice without a synaptonemal complex. PLoS One 2011, 6. [Google Scholar]
- Cusido, L.; Pujol, R.; Egozcue, J.; Garcia, M. Cyclophosphamide-induced synaptonemal complex damage during meiotic prophase of female Rattus norvegicus. Mutat. Res. 1995, 329, 131–141. [Google Scholar] [CrossRef]
- Allen, J.W.; de Weese, G.K.; Gibson, J.B.; Poorman, P.A.; Moses, M.J. Synaptonemal complex damage as a measure of chemical mutagen effects on mammalian germ cells. Mutat. Res. 1987, 190, 19–24. [Google Scholar] [CrossRef]
- Jacquet, P.; Adriaens, I.; Buset, J.; Neefs, M.; Vankerkom, J. Cytogenetic studies in mouse oocytes irradiated in vitro at different stages of maturation, by use of an early preantral follicle culture system. Mutat. Res. 2005, 583, 168–177. [Google Scholar]
- Brewen, J.G.; Payne, H.S. X-ray stage sensitivity of mouse oocytes and its bearing on dose-response curves. Genetics 1979, 91, 149–161. [Google Scholar]
- Tease, C. Radiation-induced chromosome non-disjunction in oocytes stimulated by different doses of superovulating hormones. Mutat. Res. 1982, 105, 95–100. [Google Scholar] [CrossRef]
- Edwards, R.G.; Searle, A.G. Genetic radiosensitivity of specific post-dictyate stages in mouse oocytes. Genet. Res. 1963, 4, 389–398. [Google Scholar] [CrossRef]
- Mandl, A.M. The radiosensitivity of oocytes at different stages of maduration. Proc. R. Soc. Lond. Ser. B 1963, 158, 119–141. [Google Scholar] [CrossRef]
- Ashwood-Smith, M.J.; Edwards, R.G. DNA repair by oocytes. Mol. Hum. Reprod. 1996, 2, 46–51. [Google Scholar] [CrossRef]
- Pan, H.; O’brien, M.J.; Wigglesworth, K.; Eppig, J.J.; Schultz, R.M. Transcript profiling during mouse oocyte development and the effect of gonadotropin priming and development in vitro. Dev. Biol. 2005, 286, 493–506. [Google Scholar] [CrossRef]
- Zheng, P.; Schramm, R.D.; Latham, K.E. Developmental regulation and in vitro culture effects on expression of DNA repair and cell cycle checkpoint control genes in rhesus monkey oocytes and embryos. Biol. Reprod. 2005, 72, 1359–1369. [Google Scholar]
- Su, Y.Q.; Sugiura, K.; Woo, Y.; Wigglesworth, K.; Kamdar, S.; Affourtit, J.; Eppig, J.J. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev. Biol. 2007, 302, 104–117. [Google Scholar] [CrossRef]
- Menezo, Y., Jr.; Russo, G.; Tosti, E.; El Mouatassim, S.; Benkhalifa, M. Expression profile of genes coding for DNA repair in human oocytes using pangenomic microarrays, with a special focus on ROS linked decays. J. Assist. Reprod. Genet. 2007, 24, 513–520. [Google Scholar]
- Hamatani, T.; Yamada, M.; Akutsu, H.; Kuji, N.; Mochimaru, Y.; Takano, M.; Toyoda, M.; Miyado, K.; Umezawa, A.; Yoshimura, Y. What can we learn from gene expression profiling of mouse oocytes? Reproduction 2008, 135, 581–592. [Google Scholar] [CrossRef]
- Houmard, B.; Small, C.; Yang, L.; Naluai-Cecchini, T.; Cheng, E.; Hassold, T.; Griswold, M. Global gene expression in the human fetal testis and ovary. Biol. Reprod. 2009, 81, 438–443. [Google Scholar]
- Wang, S.; Kou, Z.; Jing, Z.; Zhang, Y.; Guo, X.; Dong, M.; Wilmut, I.; Gao, S. Proteome of mouse oocytes at different developmental stages. Proc. Natl. Acad. Sci. USA 2010, 107, 17639–17644. [Google Scholar]
- Zeng, F.; Baldwin, D.A.; Schultz, R.M. Transcript profiling during preimplantation mouse development. Dev. Biol. 2004, 272, 483–496. [Google Scholar] [CrossRef]
- Tease, C.; Fisher, G. The influence of maternal age on radiation-induced chromosome aberrations in mouse oocytes. Mutat. Res. 1991, 262, 57–62. [Google Scholar] [CrossRef]
- Barber, R.C.; Dubrova, Y.E. The offspring of irradiated parents, are they stable? Mutat. Res. 2006, 598, 50–60. [Google Scholar] [CrossRef]
- Luning, K.G.; Frolen, H.; Nilsson, A. Genetic effects of 239Pu salt injections in male mice. Mutat. Res. 1976, 34, 539–542. [Google Scholar] [CrossRef]
- Dubrova, Y.E.; Plumb, M.; Brown, J.; Boulton, E.; Goodhead, D.; Jeffreys, A.J. Induction of minisatellite mutations in the mouse germline by low-dose chronic exposure to gamma-radiation and fission neutrons. Mutat. Res. 2000, 453, 17–24. [Google Scholar]
- Vorobtsova, I.E. Irradiation of male rats increases the chromosomal sensitivity of progeny to genotoxic agents. Mutagenesis 2000, 15, 33–38. [Google Scholar] [CrossRef]
- Shiraishi, K.; Shimura, T.; Taga, M.; Uematsu, N.; Gondo, Y.; Ohtaki, M.; Kominami, R.; Niwa, O. Persistent induction of somatic reversions of the pink-eyed unstable mutation in F1 mice born to fathers irradiated at the spermatozoa stage. Radiat. Res. 2002, 157, 661–667. [Google Scholar]
- Barber, R.; Plumb, M.A.; Boulton, E.; Roux, I.; Dubrova, Y.E. Elevated mutation rates in the germ line of first- and second-generation offspring of irradiated male mice. Proc. Natl. Acad. Sci. USA 2002, 99, 6877–6882. [Google Scholar] [CrossRef]
- Spears, N. In-vitro growth of oocytes. In-vitro growth of ovarian oocytes. Hum. Reprod. 1994, 9, 969–970. [Google Scholar]
- Zhang, L.; Jiang, S.; Wozniak, P.J.; Yang, X.; Godke, R.A. Cumulus cell function during bovine oocyte maturation, fertilization, and embryo development in vitro. Mol. Reprod. Dev. 1995, 40, 338–344. [Google Scholar] [CrossRef]
- Hartshorne, G.M.; Barlow, A.L.; Child, T.J.; Barlow, D.H.; Hulten, M.A. Immunocytogenetic detection of normal and abnormal oocytes in human fetal ovarian tissue in culture. Hum. Reprod. 1999, 14, 172–182. [Google Scholar] [CrossRef]
- Wright, C.S.; Hovatta, O.; Margara, R.; Trew, G.; Winston, R.M.; Franks, S.; Hardy, K. Effects of follicle-stimulating hormone and serum substitution on the in-vitro growth of human ovarian follicles. Hum. Reprod. 1999, 14, 1555–1562. [Google Scholar]
- Roig, I.; Garcia, R.; Robles, P.; Cortvrindt, R.; Egozcue, J.; Smitz, J.; Garcia, M. Human fetal ovarian culture permits meiotic progression and chromosome pairing process. Hum. Reprod. 2006, 21, 1359–1367. [Google Scholar]
- Brieno-Enriquez, M.A.; Robles, P.; Garcia-Cruz, R.; Roig, I.; Cabero, L.; Martinez, F.; Garcia Caldes, M. A new culture technique that allows in vitro meiotic prophase development of fetal human oocytes. Hum. Reprod. 2010, 25, 74–84. [Google Scholar] [CrossRef]
- Brieno-Enriquez, M.A.; Robles, P.; Camats-Tarruella, N.; Garcia-Cruz, R.; Roig, I.; Cabero, L.; Martinez, F.; Caldes, M.G. Human meiotic progression and recombination are affected by bisphenol a exposure during in vitro human oocyte development. Hum. Reprod. 2011, 26, 2807–2818. [Google Scholar]
- Guerquin, M.J.; Duquenne, C.; Coffigny, H.; Rouiller-Fabre, V.; Lambrot, R.; Bakalska, M.; Frydman, R.; Habert, R.; Livera, G. Sex-specific differences in fetal germ cell apoptosis induced by ionizing radiation. Hum. Reprod. 2009, 24, 670–678. [Google Scholar]
- Le Bouffant, R.; Guerquin, M.J.; Duquenne, C.; Frydman, N.; Coffigny, H.; Rouiller-Fabre, V.; Frydman, R.; Habert, R.; Livera, G. Meiosis initiation in the human ovary requires intrinsic retinoic acid synthesis. Hum. Reprod. 2010, 25, 2579–2590. [Google Scholar]
- Brieno-Enriquez, M.A.; Reig-Viader, R.; Cabero, L.; Toran, N.; Martinez, F.; Roig, I.; Garcia Caldes, M. Gene expression is altered after bisphenol a exposure in human fetal oocytes in vitro. Mol. Hum. Reprod. 2012, 18, 171–183. [Google Scholar]
- Brieño-Enriquez, M.A.; Reis, F.; Toran, N.; Cabero, L.; Garcia, F.; Ruiz-Herrera, A.; Garcia-Caldés, M.G. Universitat Autònoma de Barcelona, Barcelona, Spain. Unpublished work, 2012.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ruiz-Herrera, A.; Garcia, F.; Garcia-Caldés, M. Radiobiology and Reproduction—What Can We Learn from Mammalian Females? Genes 2012, 3, 521-544. https://doi.org/10.3390/genes3030521
Ruiz-Herrera A, Garcia F, Garcia-Caldés M. Radiobiology and Reproduction—What Can We Learn from Mammalian Females? Genes. 2012; 3(3):521-544. https://doi.org/10.3390/genes3030521
Chicago/Turabian StyleRuiz-Herrera, Aurora, Francisca Garcia, and Montserrat Garcia-Caldés. 2012. "Radiobiology and Reproduction—What Can We Learn from Mammalian Females?" Genes 3, no. 3: 521-544. https://doi.org/10.3390/genes3030521
APA StyleRuiz-Herrera, A., Garcia, F., & Garcia-Caldés, M. (2012). Radiobiology and Reproduction—What Can We Learn from Mammalian Females? Genes, 3(3), 521-544. https://doi.org/10.3390/genes3030521