AAC as a Potential Target Gene to Control Verticillium dahliae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains, Plants and Inoculation with V. dahliae
2.2. Disease Index
2.3. Bioinformatics Analysis
2.4. siRNA Design and Transformation of V. dahliae Protoplasts
2.5. Plasmid Construction and Fungal Transformation
2.6. Stress Treatments of V. dahliae Strains
2.7. Confocal Microscopy
2.8. Plasmid Construction and Plant Transformation
2.9. Analysis of Fungal Biomass
2.10. qRT-PCR Analysis of the Expression Level of Targeted Genes
2.11. Statistical Analysis
3. Results
3.1. Bioinformatics Analysis of VdAAC
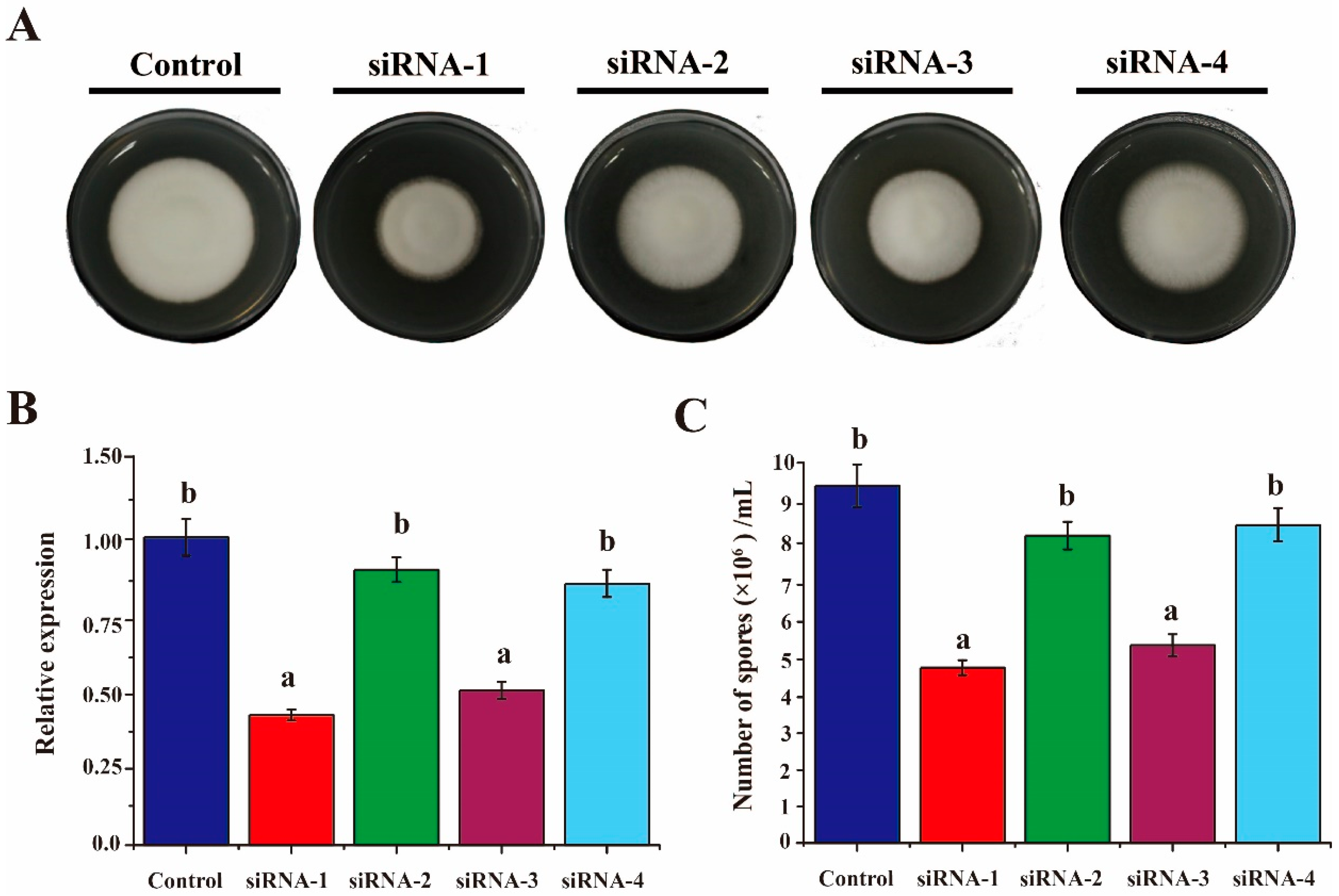
3.2. Silencing of VdAAC Effectively Inhibited Fungal Growth and Sporulation
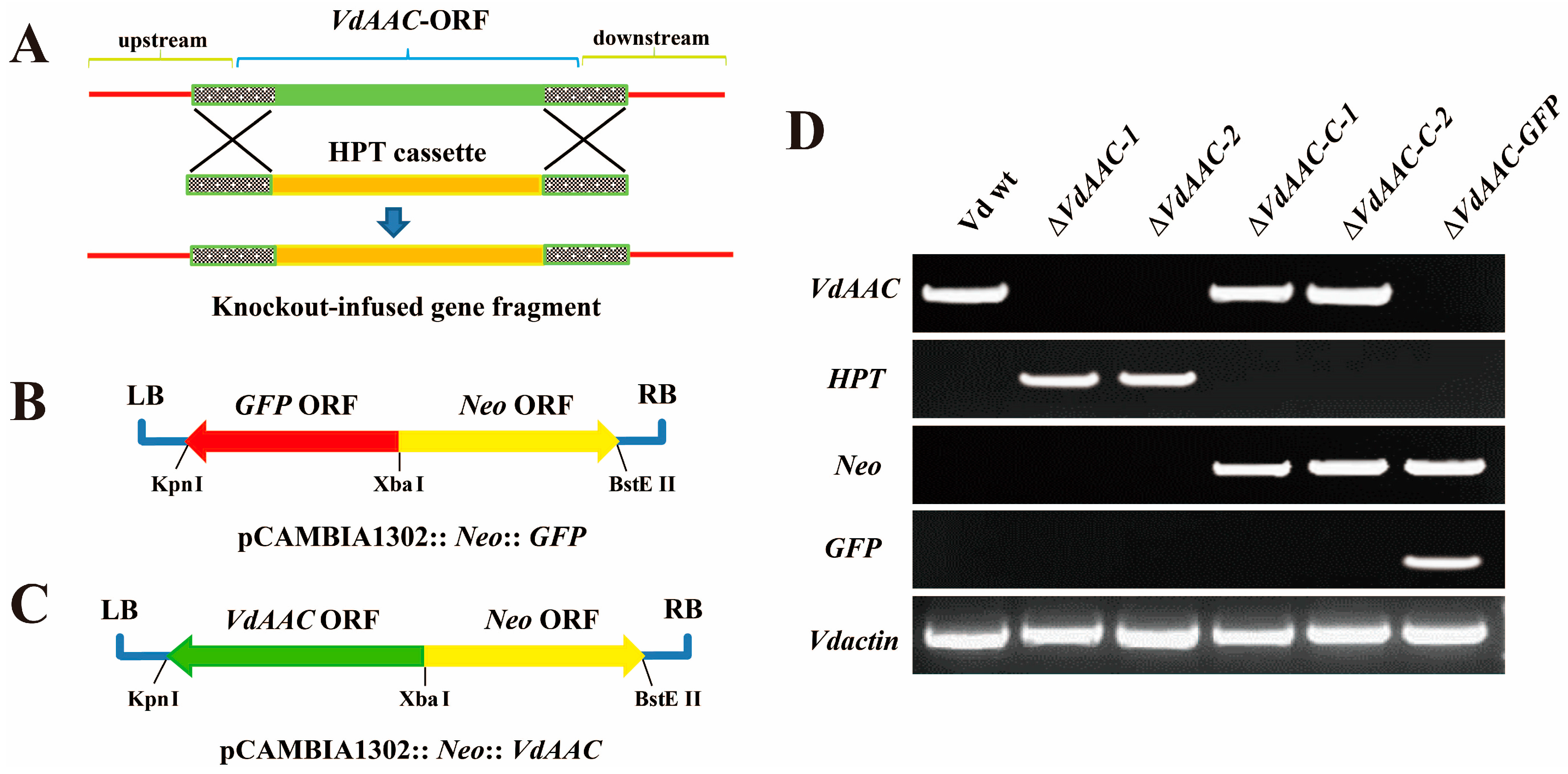
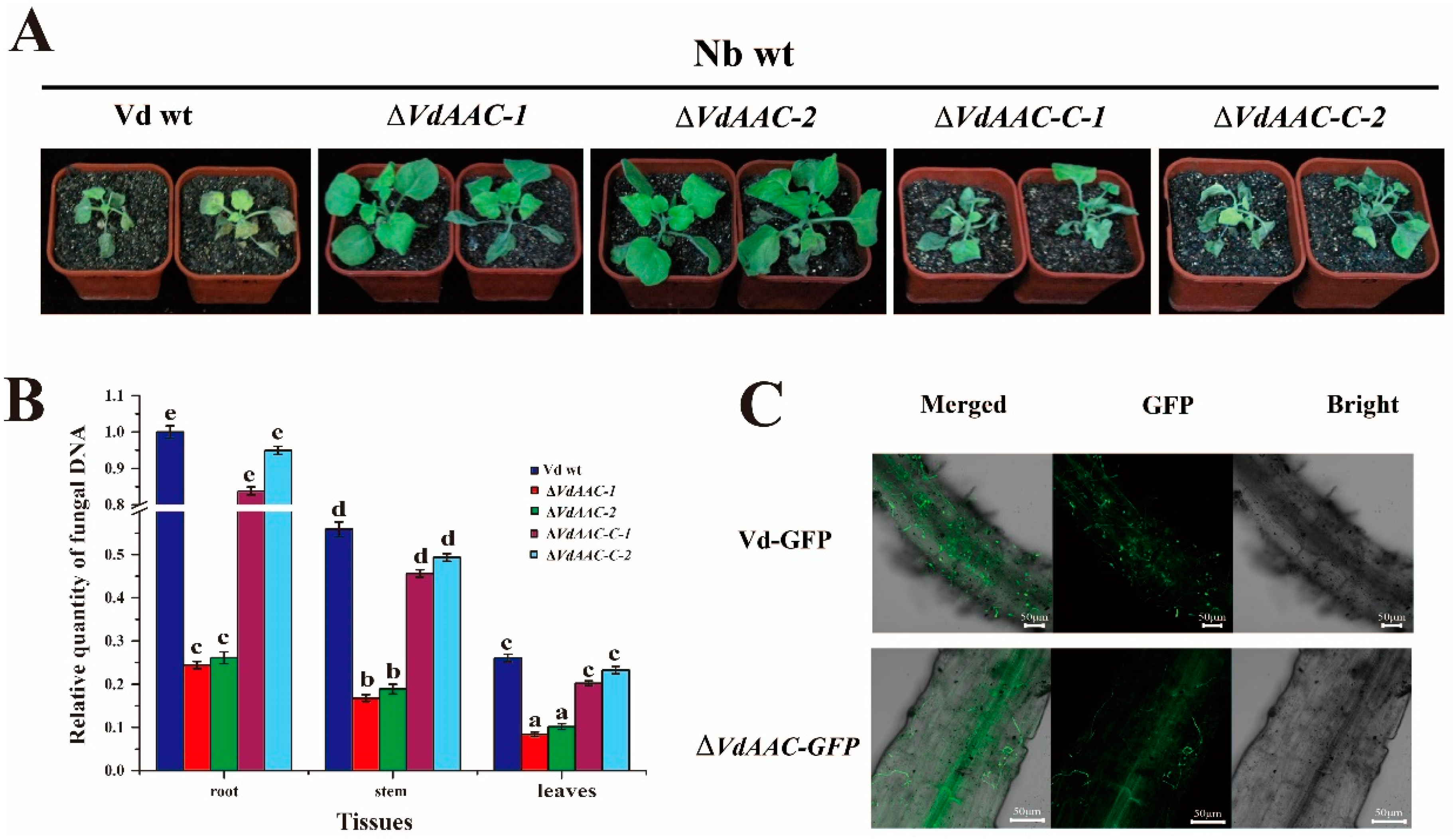
3.3. Generation of the VdAAC Mutant
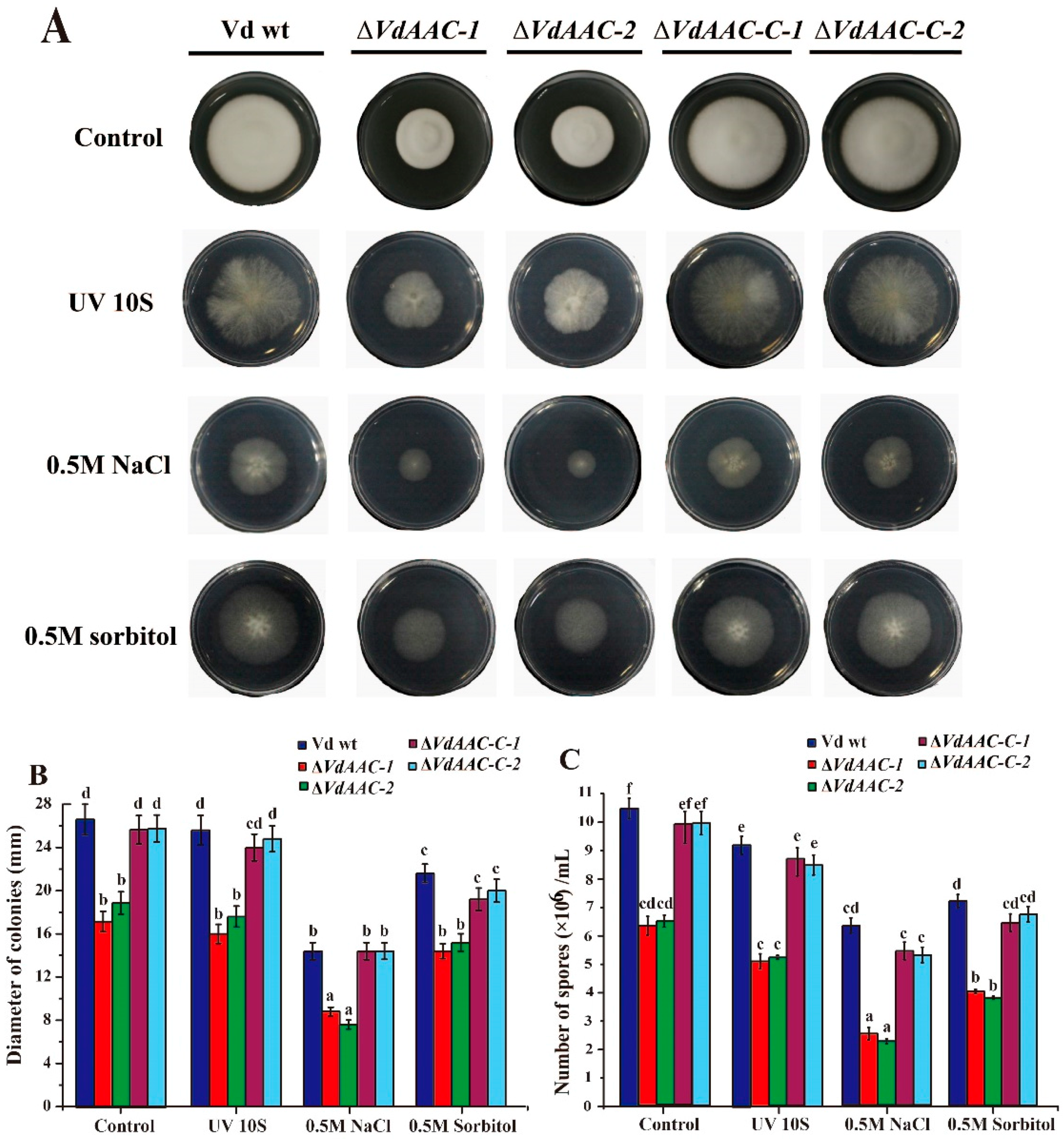
3.4. Stress Response Assay
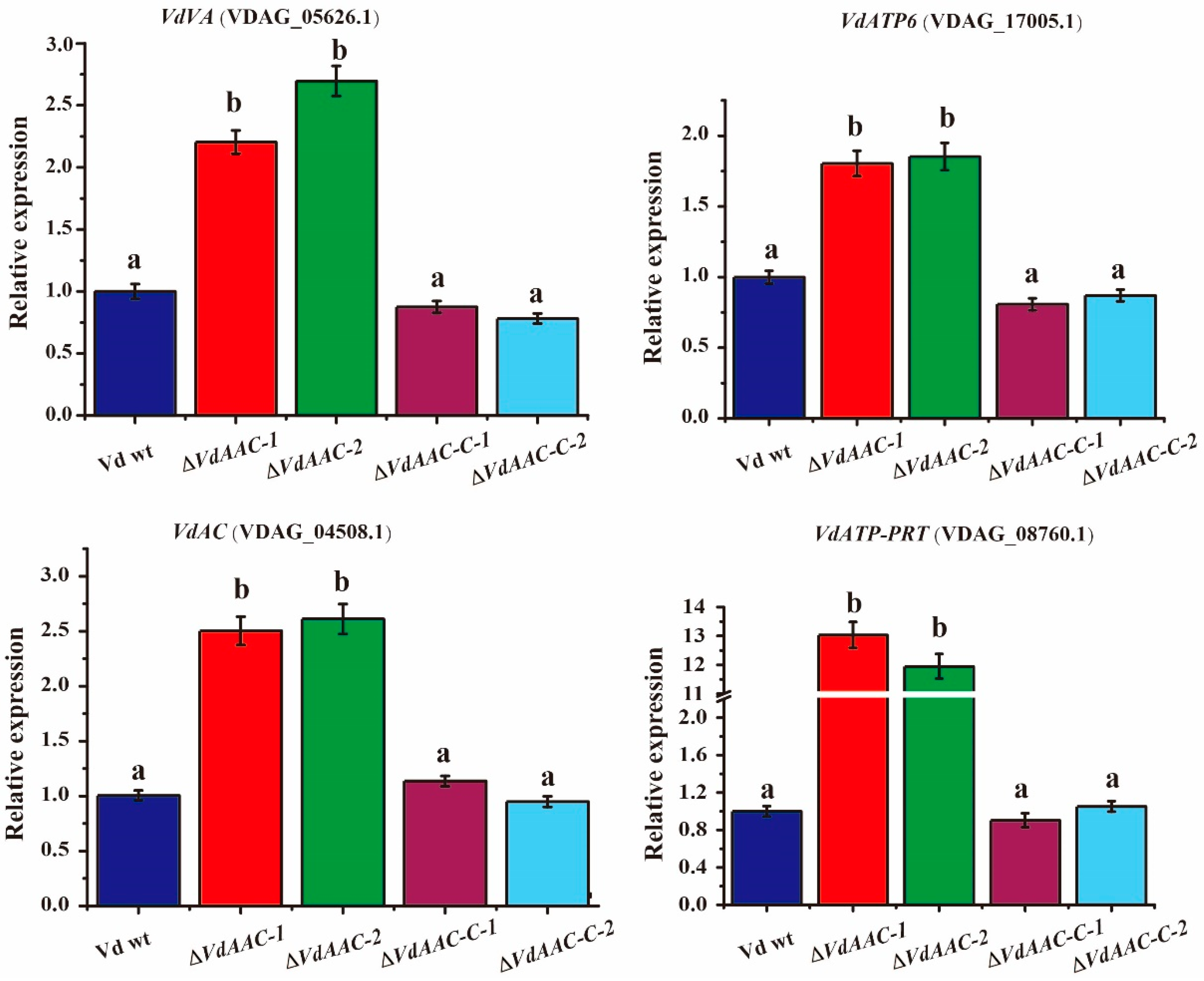
3.5. Expression of Genes Involved in Energy Metabolism in ΔVdAAC
3.6. VdAAC Is Involved in Fungal Virulence
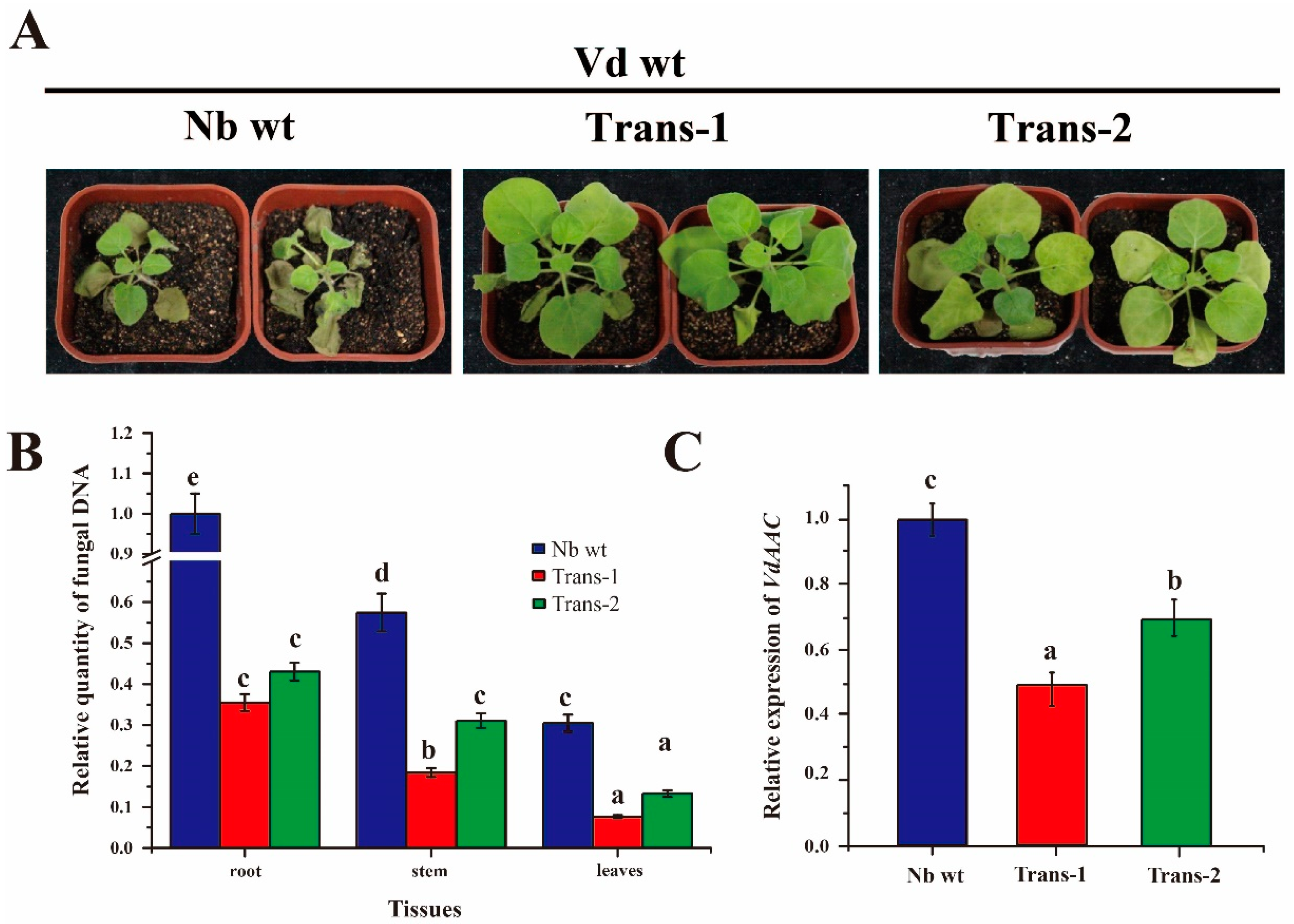
3.7. DsRNA of VdAAC Confers Resistance against Vd in Transgenic Lines
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAC | ADP, ATP carrier |
| siRNAs | short interfering RNAs |
| RNAi | RNA interference |
| CM | complete medium broth |
| MS | Murashige-Skoog |
| dpi | days post inoculation |
| DI | disease index |
| ORF | open reading frame |
References
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, pathogenicity, and management of Verticillium species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Zhu, Y.; Li, Q.; Liu, J.; Tian, Y.; Liu, Y.; Wu, J. Development of Agrobacterium-mediated virus-induced gene silencing and performance evaluation of four marker genes in Gossypium barbadense. PLoS ONE 2013, 8, e73211. [Google Scholar] [CrossRef] [PubMed]
- Pegg, G.F.; Brady, B.L. Verticillium Wilts; CABI Publishing: Oxford, UK, 2002. [Google Scholar]
- Wang, Y.; Liang, C.; Wu, S.; Zhang, X.; Tang, J.; Jian, G.; Jiao, G.; Li, F.; Chu, C. Significant improvement of cotton Verticillium wilt resistance by manipulating the expression of gastrodia antifungal proteins. Mol. Plant 2016, 9, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Tsror, L.; Levin, A.G. Vegetative compatibility and pathogenicity of Verticillium dahliae Kleb. Isolates from Olive in Israel. J. Phytopathol. 2003, 151, 451–455. [Google Scholar] [CrossRef]
- Duressa, D.; Anchieta, A.; Chen, D.; Klimes, A.; Garcia-Pedrajas, M.D.; Dobinson, K.F.; Klosterman, S.J. RNA-seq analyses of gene expression in the microsclerotia of Verticillium dahliae. BMC Genom. 2013, 14, 607. [Google Scholar] [CrossRef] [PubMed]
- Fradin, E.F.; Thomma, B.P. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Wang, X.Y.; Chen, J.Y.; Kong, Z.Q.; Gui, Y.J.; Li, N.Y.; Bao, Y.M.; Dai, X.F. Identification and characterization of a pathogenicity-related gene VdCYP1 from Verticillium dahliae. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Wang, Y.; Tang, C.; Fang, Y.; Zou, J.; Tian, C. VdCrz1 is involved in microsclerotia formation and required for full virulence in Verticillium dahliae. Fungal Genet. Biol. 2015, 82, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Xu, J.; Zhou, L.; Guo, W. VdMsb regulates virulence and microsclerotia production in the fungal plant pathogen Verticillium dahliae. Gene 2014, 550, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Nakayashiki, H.; Hanada, S.; Nguyen, B.Q.; Kadotani, N.; Tosa, Y.; Mayama, S. RNA silencing as a tool for exploring gene function in ascomycete fungi. Fungal Genet. Biol. 2005, 42, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, R.; Purohit, H.J. siRNA mediated gene silencing in Fusarium sp. HKF15 for overproduction of bikaverin. Bioresour. Technol. 2014, 157, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Mumbanza, F.M.; Kiggundu, A.; Tusiime, G.; Tushemereirwe, W.K.; Niblett, C.; Bailey, A. In vitro antifungal activity of synthetic dsRNA molecules against two pathogens of banana, Fusarium oxysporum f. sp. cubense and Mycosphaerella fijiensis. Pest Manag. Sci. 2013, 69, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Braus-Stromeyer, S.A.; Timpner, C.; Tran, V.T.; Lohaus, G.; Reusche, M.; Knufer, J.; Teichmann, T.; von Tiedemann, A.; Braus, G.H. Silencing of Vlaro2 for chorismate synthase revealed that the phytopathogen Verticillium longisporum induces the cross-pathway control in the xylem. Appl. Microbiol. Biotechnol. 2010, 85, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Kumar, N.; Weber, L.; Keller, H.; Imani, J.; Kogel, K.H. Host-induced gene silencing of cytochrome P450 lanosterol C14alpha-demethylase-encoding genes confers strong resistance to Fusarium species. Proc. Natl. Acad. Sci. USA 2013, 110, 19324–19329. [Google Scholar] [CrossRef] [PubMed]
- Ghag, S.B.; Shekhawat, U.K.; Ganapathi, T.R. Host-induced post-transcriptional hairpin RNA-mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnol. J. 2014, 12, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Jin, Y.; Zhao, J.H.; Gao, F.; Zhou, B.J.; Fang, Y.Y.; Guo, H.S. Host-induced gene silencing of target gene in fungal cells confers effective resistance to cotton wilt disease pathogen Verticillium dahliae. Mol. Plant 2016, 9, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Nury, H.; Dahout-Gonzalez, C.; Trezeguet, V.; Lauquin, G.; Brandolin, G.; Pebay-Peyroula, E. Structural basis for lipid-mediated interactions between mitochondrial ADP/ATP carrier monomers. FEBS Lett. 2005, 579, 6031–6036. [Google Scholar] [CrossRef] [PubMed]
- Klingenberg, M. Molecular aspects of the adenine nucleotide carrier from mitochondria. Arch. Biochem. Biophys. 1989, 270, 1–14. [Google Scholar] [CrossRef]
- Fiore, C.; Le, S.A.; Roux, P.; Schwimmer, C.; Dianoux, A.N.F.; Gjm, L.; Brandolin, G.; Pv, V.; Trezeguet, V. The mitochondrial ADP/ATP carrier: Structural, physiological and pathological aspects. Biochimie 1998, 80, 137–150. [Google Scholar] [CrossRef]
- Pebay-Peyroula, E.; Dahout-Gonzalez, C.; Kahn, R.; Trezeguet, V.; Lauquin, G.J.; Brandolin, G. Structure of mitochondrial ADP/ATP carrier in complex with carboxyatractyloside. Nature 2003, 426, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, T.; Kihira, Y.; Shinohara, Y.; Majima, E.; Terada, H. Characterization of loops of the yeast mitochondrial ADP/ATP carrier facing the cytosol by site-directed mutagenesis. Biochem. Biophys. Res. Commun. 2001, 286, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, K.; Hori, H.; Shinohara, Y. Role of C-terminal region of yeast ADP/ATP carrier 2 protein: Dynamics of flexible C-terminal arm. Anticancer Res. 2009, 29, 4897–4900. [Google Scholar] [PubMed]
- Nowara, D.; Gay, A.; Lacomme, C.; Shaw, J.; Ridout, C.; Douchkov, D.; Hensel, G.; Kumlehn, J.; Schweizer, P. HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 2010, 22, 3130–3141. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Chaves, S.; Alves, S.; Salin, B.; Camougrand, N.; Manon, S.; Sousa, M.J.; Corte-Real, M. Mitochondrial degradation in acetic acid-induced yeast apoptosis: The role of Pep4 and the ADP/ATP carrier. Mol. Microbiol. 2010, 76, 1398–1410. [Google Scholar] [CrossRef] [PubMed]
- Gigolashvili, T.; Geier, M.; Ashykhmina, N.; Frerigmann, H.; Wulfert, S.; Krueger, S.; Mugford, S.G.; Kopriva, S.; Haferkamp, I.; Flugge, U.I. The Arabidopsis thylakoid ADP/ATP carrier TAAC has an additional role in supplying plastidic phosphoadenosine 5’-phosphosulfate to the cytosol. Plant Cell 2012, 24, 4187–4204. [Google Scholar] [CrossRef] [PubMed]
- Talbot, D.A.; Duchamp, C.; Rey, B.; Hanuise, N.; Rouanet, J.L.; Sibille, B.; Brand, M.D. Uncoupling protein and ATP/ADP carrier increase mitochondrial proton conductance after cold adaptation of king penguins. J. Physiol. 2004, 558, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Gnipova, A.; Subrtova, K.; Panicucci, B.; Horvath, A.; Lukes, J.; Zikova, A. The ADP/ATP carrier and its relationship to oxidative phosphorylation in ancestral protist Trypanosoma brucei. Eukaryot. Cell 2015, 14, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Rehman, L.; Su, X.; Guo, H.; Qi, X.; Cheng, H. Protoplast transformation as a potential platform for exploring gene function in Verticillium dahliae. BMC Biotechnol. 2016, 16, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.; Lin, Z.X.; Zhang, X.L.; Chen, W.; Guo, X.P.; Nie, Y.C.; Li, Y.H. Mapping and quantitative trait loci analysis of Verticillium wilt resistance genes in cotton. J. Integr. Plant Biol. 2008, 50, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhang, X.; Liang, B.; Li, S.; Wu, Z.; Wang, Q.; Leng, C.; Dong, J.; Wang, T. Expression of baculovirus anti-apoptotic genes p35 and op-iap in cotton (Gossypium hirsutum L.) enhances tolerance to Verticillium wilt. PLoS ONE 2010, 5, e14218. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ben, S.; Sun, Y.; Fan, X.; Tian, C.; Wang, Y. Genome-wide identification, phylogeny and expression profile of vesicle fusion components in Verticillium dahliae. PLoS ONE 2013, 8, e68681. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Biochem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Hoppenau, C.E.; Tran, V.-T.; Kusch, H.; Aßhauer, K.P.; Landesfeind, M.; Meinicke, P.; Popova, B.; Braus-Stromeyer, S.A.; Braus, G.H. Verticillium dahliae VdTHI4, involved in thiazole biosynthesis, stress response and DNA repair functions, is required for vascular disease induction in tomato. Environ. Exp. Bot. 2014, 108, 14–22. [Google Scholar] [CrossRef]
- Tzima, A.K.; Paplomatas, E.J.; Tsitsigiannis, D.I.; Kang, S. The G protein beta subunit controls virulence and multiple growth- and development-related traits in Verticillium dahliae. Fungal Genet. Biol. 2012, 49, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; McCallum, B.; Bakkeren, G. Endogenous silencing of Puccinia triticina pathogenicity genes through in planta-expressed sequences leads to the suppression of rust diseases on wheat. Plant J. 2013, 73, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Ellendorff, U.; Fradin, E.F.; de Jonge, R.; Thomma, B.P. RNA silencing is required for Arabidopsis defence against Verticillium wilt disease. J. Exp. Bot. 2009, 60, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Song, Y.; Liu, C.M.; Thomma, B.P. Mutational analysis of the Ve1 immune receptor that mediates Verticillium resistance in tomato. PLoS ONE 2014, 9, e99511. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Cai, W.; Wang, J.; Hong, G.; Tao, X.; Wang, L.; Huang, Y.; Chen, X. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Obrepalska-Steplowska, A.; Wieczorek, P.; Budziszewska, M.; Jeszke, A.; Renaut, J. How can plant virus satellite RNAs alter the effects of plant virus infection? A study of the changes in the Nicotiana benthamiana proteome after infection by peanut stunt virus in the presence or absence of its satellite RNA. Proteomics 2013, 13, 2162–2175. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Nelson, H.; Nelson, N.; Yaver, K. Mutations in the yeast vacuolar ATPase result in the mislocalization of vacuolar proteins. J. Exp. Biol. 1992, 172, 83–92. [Google Scholar] [PubMed]
- Clague, M.J.; Urbe, S.; Aniento, F.; Gruenberg, J. Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J. Biol. Chem. 1994, 269, 21–24. [Google Scholar] [PubMed]
- Klionsky, D.J.; Herman, P.K.; Emr, S.D. The fungal vacuole: Composition, function, and biogenesis. Microbiol. Rev. 1990, 54, 266–292. [Google Scholar] [PubMed]
- Deckers-Hebestreit, G.; Schmid, R.; Kiltz, H.H.; Altendorf, K. F0 portion of Escherichia coli ATP synthase: Orientation of subunit c in the membrane. Biochemistry 1987, 26, 5486–5492. [Google Scholar] [CrossRef] [PubMed]
- Van Walraven, H.S.; Scholts, M.J.; Lill, H.; Matthijs, H.C.; Dilley, R.A.; Kraayenhof, R. Introduction of a carboxyl group in the loop of the F0 c-subunit affects the H+/ATP coupling ratio of the ATP synthase from Synechocystis 6803. J. Bioenerg. Biomembr. 2002, 34, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Salomon, Y.; Londos, C.; Rodbell, M. A highly sensitive adenylate cyclase assay. Anal. Biochem. 1974, 58, 541–548. [Google Scholar] [CrossRef]
- Klimpel, A.; Gronover, C.S.; Williamson, B.; Stewart, J.A.; Tudzynski, B. The adenylate cyclase (BAC) in Botrytis cinerea is required for full pathogenicity. Mol. Plant Pathol. 2002, 3, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Piszkiewicz, D.; Tilley, B.E.; Rand-Meir, T.; Parsons, S.M. Amino acid sequence of ATP phosphoribosyltransferase of Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 1979, 76, 1589–1592. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Sharma, V.; Sacchettini, J.C. Crystal structure of ATP phosphoribosyltransferase from Mycobacterium tuberculosis. J. Biol. Chem. 2003, 278, 8333–8339. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Ames, B.N. The histidine operon and its regulation. In Metabolic Regulation; Vogel, H.J., Ed.; Academic Press: New York, NY, USA, 1971; Volume 5, pp. 349–387. [Google Scholar]
- Goldberger, R.F.; Kovach, J.S. Regulation of histidine biosynthesis in Salmonella typhimurium. Curr. Top. Cell. Regul. 1972, 5, 285–308. [Google Scholar] [PubMed]
- Voncken, F.; Gao, F.; Wadforth, C.; Harley, M.; Colasante, C. The phosphoarginine energy-buffering system of Trypanosoma brucei involves multiple arginine kinase isoforms with different subcellular locations. PLoS ONE 2013, 8, e65908. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A. Arginine kinase: A potential pharmacological target in trypanosomiasis. Infect. Disord. Drug Targets 2014, 14, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.R.; Canepa, G.E.; Bouvier, L.A.; Pereira, C.A. Trypanosoma cruzi: Oxidative stress induces arginine kinase expression. Exp. Parasitol. 2006, 114, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, H.; Kuang, J.; Sun, C.; Shi, J.; Duan, X.; Qu, H.; Jiang, Y. Short-term anaerobic, pure oxygen and refrigerated storage conditions affect the energy status and selective gene expression in litchi fruit. LWT-Food Sci. Technol. 2015, 60, 1254–1261. [Google Scholar] [CrossRef]
- Trezeguet, V.; Zeman, I.; David, C.; Lauquin, G.J.; Kolarov, J. Expression of the ADP/ATP carrier encoding genes in aerobic yeasts; phenotype of an ADP/ATP carrier deletion mutant of Schizosaccharomyces pombe. Biochim. Biophys. Acta 1999, 1410, 229–236. [Google Scholar] [CrossRef]
- Miura, K.; Inouye, S.; Sakai, K.; Takaoka, H.; Kishi, F.; Tabuchi, M.; Tanaka, T.; Matsumoto, H.; Shirai, M.; Nakazawa, T.; Nakazawa, A. Cloning and characterization of adenylate kinase from Chlamydia pneumoniae. J. Biol. Chem. 2001, 276, 13490–13498. [Google Scholar] [CrossRef] [PubMed]
- Claypool, S.M.; Oktay, Y.; Boontheung, P.; Loo, J.A.; Koehler, C.M. Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J. Cell Biol. 2008, 182, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Hawke, M.A.; Lazarovits, G. Production and manipulation of individual microsclerotia of Verticillium dahliae for use in studies of survival. Phytopathology 1994, 23, 582–584. [Google Scholar]
- Isaac, I. Verticillium wilt of sainfoin. Ann. Appl. Biol. 1946, 33, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Debode, J.; De Maeyer, K.; Perneel, M.; Pannecoucque, J.; De Backer, G.; Hofte, M. Biosurfactants are involved in the biological control of Verticillium microsclerotia by Pseudomonas spp. J. Appl. Microbiol. 2007, 103, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Li, Z.F.; Feng, Z.L.; Feng, H.J.; Zhao, L.H.; Shi, Y.Q.; Hu, X.P.; Zhu, H.Q. Isolation and functional analysis of the pathogenicity-related gene VdPR3 from Verticillium dahliae on cotton. Curr. Genet. 2015, 61, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, P.; Boshoven, J.C.; Salas, O.; Bowler, K.; Islam, T.; Keykha Saber, M.; van den Berg, G.C.; Bar-Peled, M.; Thomma, B.P. Rhamnose synthase activity is required for pathogenicity of the vascular wilt fungus Verticillium dahliae. Mol. Plant Pathol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Novina, C.D.; Sharp, P.A. The RNAi revolution. Nature 2004, 430, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Kalantidis, K.; Psaradakis, S.; Tabler, M.; Tsagris, M. The occurrence of CMV-specific short RNAS in transgenic tobacco expressing virus-derived double-stranded RNA is indicative of resistance to the virus. MPMI 2002, 15, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Allen, R.; Davis, E.L.; Baum, T.J.; Hussey, R.S. Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc. Natl. Acad. Sci. USA 2006, 103, 14302–14306. [Google Scholar] [CrossRef] [PubMed]
- Schwind, N.; Zwiebel, M.; Itaya, A.; Ding, B.; Wang, M.B.; Krczal, G.; Wassenegger, M. RNAi-mediated resistance to Potato spindle tuber viroid in transgenic tomato expressing a viroid hairpin RNA construct. Mol. Plant Pathol. 2009, 10, 459–469. [Google Scholar] [CrossRef] [PubMed]

| Name | Sense Sequence | Antisense Sequence |
|---|---|---|
| Control | UUCUCCGAACGUGUCACGUTT | ACGUGACACGUUCGGAGAATT |
| siRNA-1 | UCAAGCUCCUCAUCCAGAATT | UUCUGGAUGAGGAGCUUGATT |
| siRNA-2 | GCAACACUGCCAACGUCAUTT | AUGACGUUGGCAGUGUUGCTT |
| siRNA-3 | GCUUUCCGUGACAAGUUCATT | UGAACUUGUCACGGAAAGCTT |
| siRNA-4 | GCAUGUACGACUCCAUCAATT | UUGAUGGAGUCGUACAUGCTT |
| Primers | Sequence |
|---|---|
| qRT-AAC | TTGCCGAGTGCTTCAAGCGTAC |
| GGCGTAGTCGAGGGAGTAGACG | |
| qRT-Vdactin | GGCTTCCTCAAGGTCGGCTATG |
| GCTGCATGTCATCCCACTTCTTC | |
| qRT-VdITS | CCGCCGGTCCATCAGTCTCTCTGTTTATAC |
| CGCCTGCGGGACTCCGATGCGAGCTGTAAC | |
| qRT-Nbactin | GGACCTTTATGGAAACATTGTGCTCAGT |
| CCAAGATAGAACCTCCAATCCAGACAC | |
| qRT-VA | GGGTATTCAGACCCTATTGGACG |
| CGAACTTCTTGTACTCAGCCTCC | |
| qRT-ATP6 | CTAGACCAATTTGAAATAAGA |
| AAAGATTCTTGGCTAATAGAT | |
| qRT-VdAC | TCTCCATCGTCTTCACCGACATCA |
| TCTGCACGGCGAAACACCACA | |
| qRT-VdATP-PRT | CGACGCCAACGTGCGGTCCTACAA |
| GCCCGAGAAGCTCGTGCCAAT | |
| HPT expression cassette | TTGAAGGAGCATTTTTGGGC |
| TTATCTTTGCGAACCCAGGG | |
| ΔAAC | CTTGGTGAAGGAGAGCGTTGAAAGT |
| GCCCAAAAATGCTCCTTCAATGACAAGTTCAAGGCCATGTTCGGC | |
| CCCTGGGTTCGCAAAGATAACTCCGTTGCTGGTATCGTTGTCTAC | |
| GGTTCCTCGTCGCTGTCAATGACC | |
| Neo expression cassette | aatTCTAGAGTTTGCGGGCTGTCTTGACG |
| ataGGTCACCTACCTGTGCATTCTGGGTAA | |
| GFP expression cassette | ggcTCTAGACTTTCGACACTGAAATACGTCG |
| ataGGTACCGCATCAGAGCAGATTGTACTGAGAG | |
| ΔAAC-C | aaaAGTACTATGTCCGTCGAGAAGCAG |
| aaaCTGCAGTTATTTGAAGGCCTTGCC | |
| Trans-AAC | GGGGACAAGTTTGTACAAAAAAGCAGGCTGTGCTTCAAGCGTAC |
| GGGGACCACTTTGTACAAGAAAGCTGGGTCCCTTGAAGAGAGAC | |
| Det-trans | CGTCATCCGTTACTTCCCTACCCA |
| AGACCGGCAATACCGTCAGAGGC | |
| Det-GFP | CGACGTAAACGGCCACAAGTT |
| TCTTTGCTCAGGGCGGACTGG | |
| Det-AAC | GCGCCAGTTCAACGGTCTTGTCG |
| TCACCAGAGGTCATCATCATGCGAC | |
| Det-Neo | GTTGTCACTGAAGCGGGAAGGG |
| GCGATACCGTAAAGCACGAGGAA | |
| Det-HPT | TTCGACAGCGTCTCCGACCTGA |
| AGATGTTGGCGACCTCGTATTGGG |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Rehman, L.; Guo, H.; Li, X.; Zhang, R.; Cheng, H. AAC as a Potential Target Gene to Control Verticillium dahliae. Genes 2017, 8, 25. https://doi.org/10.3390/genes8010025
Su X, Rehman L, Guo H, Li X, Zhang R, Cheng H. AAC as a Potential Target Gene to Control Verticillium dahliae. Genes. 2017; 8(1):25. https://doi.org/10.3390/genes8010025
Chicago/Turabian StyleSu, Xiaofeng, Latifur Rehman, Huiming Guo, Xiaokang Li, Rui Zhang, and Hongmei Cheng. 2017. "AAC as a Potential Target Gene to Control Verticillium dahliae" Genes 8, no. 1: 25. https://doi.org/10.3390/genes8010025






