The Clinical Course of Patients with Preschool Manifestation of Type 1 Diabetes Is Independent of the HLA DR-DQ Genotype
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Inclusion Criteria
2.3. Variable Assessment
2.4. Control Population
2.5. Human Leukocyte Antigen Typing
2.6. Neonatal Diabetes Genes
2.7. C-peptide
2.8. Statistics
3. Results
3.1. Patient Characteristics
3.2. Comparison of Human Leukocyte Antigen Haplotypes between the Study Group and the Control Population
3.2.1. Prevalence of Human Leukocyte Antigen Haplotype Combinations
3.2.2. Prevalence of HLA Haplotypes
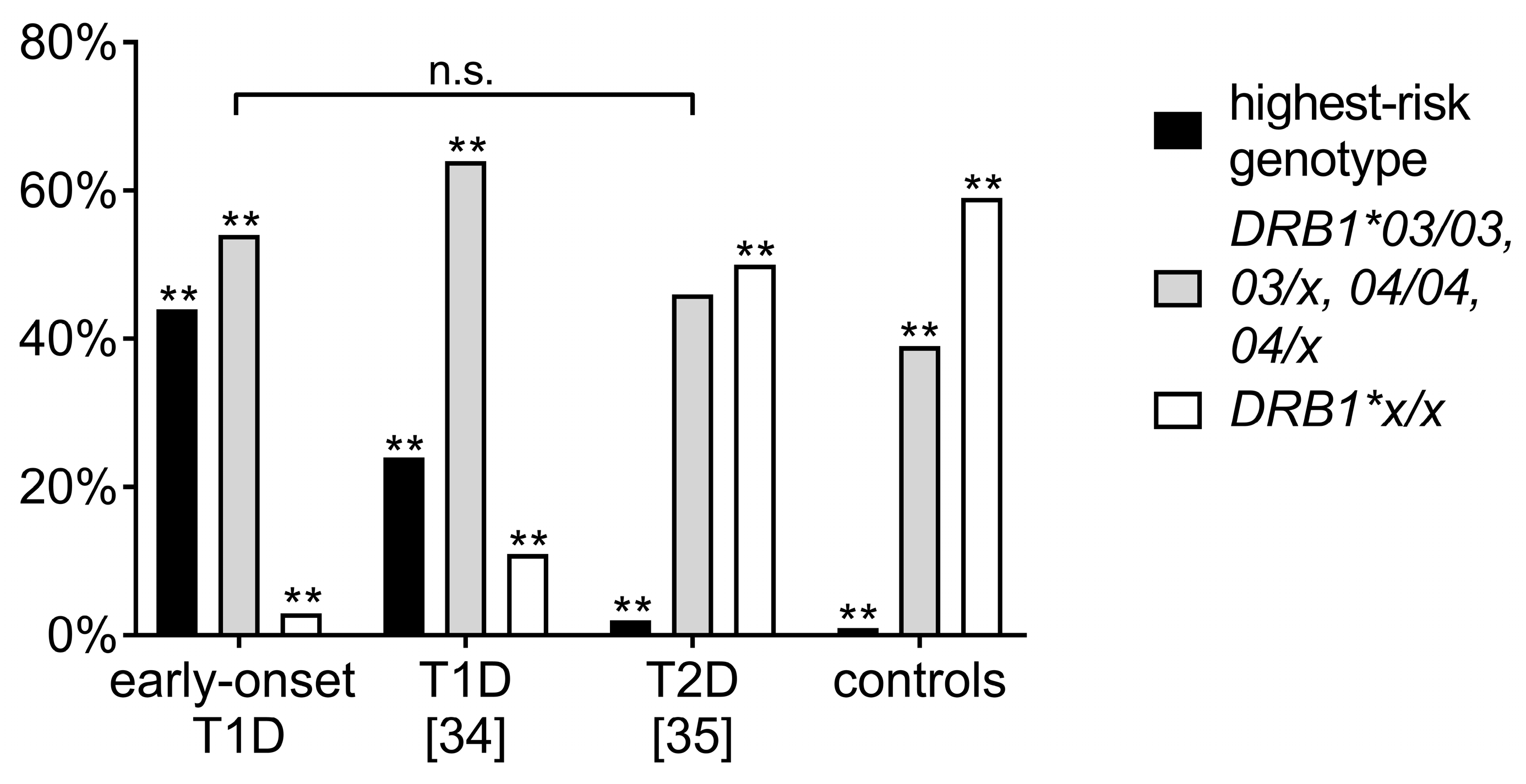
3.3. Comparison of Human Leukocyte Antigen Haplotypes between the Study Cohort and Other Adolescent-Onset T1D and T2D Cohorts
3.4. Comparison of Clinical Data of the Highest-Risk Haplotype Combination vs. Other Haplotypes
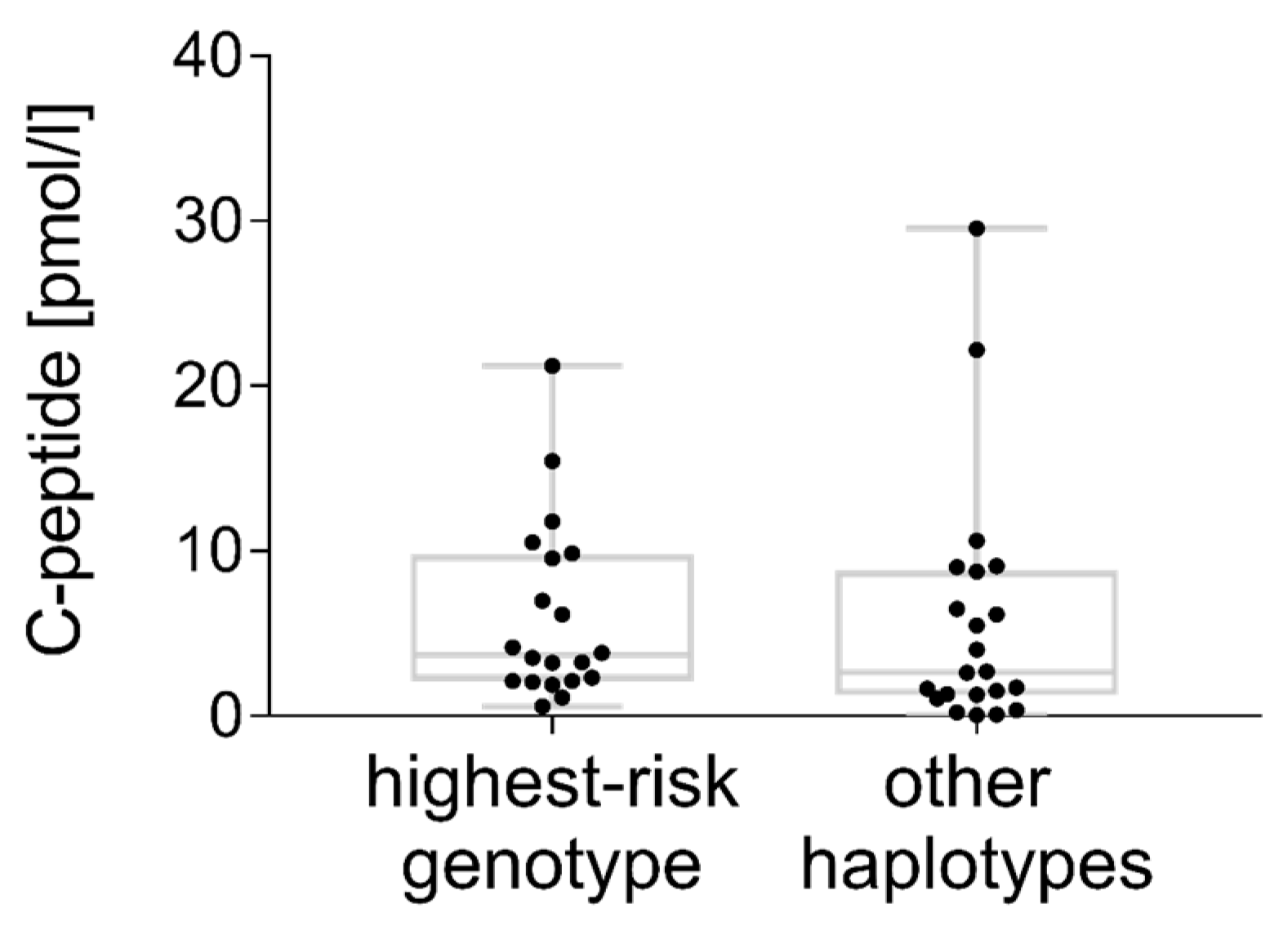
3.5. C-peptide
4. Discussion
4.1. Aim and Main Findings
4.2. Limitations
4.3. Highest-Risk Genotype
4.4. Other Haplotypes Conferring Risk for T1D
4.5. Protection
4.6. Peptide Binding to the MHC Antigen
4.7. The Combination of Alleles Determines the T1D Risk
4.8. Clinical Data
4.9. C-peptide
4.10. Clinical Implications
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patterson, C.C.; Dahlquist, G.G.; Gyürüs, E.; Green, A.; Soltész, G.; EURODIAB Study Group. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: A multicentre prospective registration study. Lancet 2009, 373, 2027–2033. [Google Scholar] [CrossRef]
- Patterson, C.C.; Gyürüs, E.; Rosenbauer, J.; Cinek, O.; Neu, A.; Schober, E.; Parslow, R.C.; Joner, G.; Svensson, J.; Castell, C.; et al. Trends in childhood type 1 diabetes incidence in Europe during 1989–2008: Evidence of non-uniformity over time in rates of increase. Diabetologia 2012, 55, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Vehik, K.; Hamman, R.F.; Lezotte, D.; Norris, J.M.; Klingensmith, G.J.; Dabelea, D. Childhood growth and age at diagnosis with Type 1 diabetes in Colorado young people. Diabet. Med. 2009, 26, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Knerr, I.; Wolf, J.; Reinehr, T.; Stachow, R.; Grabert, M.; Schober, E.; Rascher, W.; Holl, R.W.; DPV Scientific Initiative of Germany and Austria. The ‘accelerator hypothesis’: Relationship between weight, height, body mass index and age at diagnosis in a large cohort of 9248 German and Austrian children with type 1 diabetes mellitus. Diabetologia 2005, 48, 2501–2504. [Google Scholar] [CrossRef] [PubMed]
- Bendas, A.; Rothe, U.; Kiess, W.; Kapellen, T.M.; Stange, T.; Manuwald, U.; Salzsieder, E.; Holl, R.W.; Schoffer, O.; Stahl-Pehe, A.; et al. Trends in Incidence Rates during 1999–2008 and Prevalence in 2008 of Childhood Type 1 Diabetes Mellitus in Germany—Model-Based National Estimates. PLoS ONE 2015, 10, e0132716. [Google Scholar] [CrossRef] [PubMed]
- Morran, M.P.; Vonberg, A.; Khadra, A.; Pietropaolo, M. Immunogenetics of type 1 diabetes mellitus. Mol. Asp. Med. 2015, 42, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Erlich, H.; Valdes, A.M.; Noble, J.; Carlson, J.A.; Varney, M.; Concannon, P.; Mychaleckyj, J.C.; Todd, J.A.; Bonella, P.; Fear, A.L.; Lavant, E.; et al. Type Diabetes Genetics Consortium. HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: Analysis of the type 1 diabetes genetics consortium families. Diabetes 2008, 57, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.A.; Valdes, A.M.; Cook, M.; Klitz, W.; Thomson, G.; Erlich, H.A. The role of HLA class II genes in insulin-dependent diabetes mellitus: Molecular analysis of 180 Caucasian, multiplex families. Am. J. Hum. Genet. 1996, 59, 1134–1148. [Google Scholar] [PubMed]
- Lambert, A.P.; Gillespie, K.M.; Thomson, G.; Cordell, H.J.; Todd, J.A.; Gale, E.A.; Bingley, P.J. Absolute risk of childhood-onset type 1 diabetes defined by human leukocyte antigen class II genotype: A population-based study in the United Kingdom. J. Clin. Endocrinol. Metab. 2004, 89, 4037–4043. [Google Scholar] [CrossRef] [PubMed]
- Roep, B.O.; Peakman, M. Antigen targets of type 1 diabetes autoimmunity. Cold Spring Harb. Perspect. Med. 2012, 2, a007781. [Google Scholar] [CrossRef] [PubMed]
- Bakay, M.; Pandey, R.; Hakonarson, H. Genes involved in type 1 diabetes: An update. Genes 2013, 4, 499–521. [Google Scholar] [CrossRef] [PubMed]
- Hathout, E.H.; Hartwick, N.; Fagoaga, O.R.; Colacino, A.R.; Sharkey, J.; Racine, M.; Nelsen-Cannarella, S.; Mace, J.W. Clinical, autoimmune and HLA characteristics of children diagnosed with type 1 diabetes before 5 years of age. Pediatrics 2003, 111, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Caillat-Zucman, S.; Garchon, H.-J.; Timsit, J.; Assan, R.; Boitard, C.; Djilali-Saiah, I.; Bougnères, P.; Bach, J.F. Age-dependent HLA genetic heterogeneity of type 1 insulin-dependent diabetes mellitus. J. Clin. Investig. 1992, 90, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Emery, L.M.; Babu, S.; Bugawan, T.L.; Norris, J.M.; Erlich, H.A.; Eisenbarth, G.S.; Rewers, M. Newborn HLA-DR, DQ genotype screening: Age- and ethnicity-specific type 1 diabetes risk estimates. Pediatr. Diabet. 2005, 6, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, K.M.; Gale, E.A.M.; Bingley, P.J. High familial risk and genetic susceptibility in early-onset childhood diabetes. Diabetes 2002, 51, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Larsson, H.E.; Hansson, G.; Carlsson, A.; Cederwall, E.; Jonsson, B.; Jönsson, B.; Larsson, K.; Lynch, K.; Neiderud, J.; Lernmark, A.; et al. Children developing type 1 diabetes before 6 years of age have increased linear growth independent of HLA genotypes. Diabetologia 2008, 51, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Aly, T.A.; Ide, A.; Jahromi, M.M.; Barker, J.M.; Fernando, M.S.; Babu, S.R.; Yu, L.; Miao, D.; Erlich, H.A.; Fain, P.R.; et al. Extreme genetic risk for type 1A diabetes. Proc. Natl. Acad. Sci. USA 2006, 103, 14074–14079. [Google Scholar] [CrossRef] [PubMed]
- Nejentsev, S.; Howson, J.M.; Walker, N.M.; Szeszko, J.; Field, S.F.; Stevens, H.E.; Reynolds, P.; Hardy, M.; King, E.; Masters, J.; et al. Wellcome Trust Case Control Consortium. Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature 2007, 450, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Howson, J.M.; Rosinger, S.; Smyth, D.J.; Boehm, B.O.; ADBW-END Study Group; Todd, J.A. Genetic analysis of adult-onset autoimmune diabetes. Diabetes 2011, 60, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Pociot, F.; Lernmark, Å. Genetic risk factors for type 1 diabetes. Lancet 2016, 387, 2331–2339. [Google Scholar] [CrossRef]
- Black, M.H.; Lawrence, J.M.; Pihoker, C.; Dolan, L.M.; Anderson, A.; Rodriguez, B.; Marcovina, S.M.; Mayer-Davis, E.J.; Imperatore, G.; Dabelea, D. SEARCH for Diabetes in Youth Study Group. HLA-associated phenotypes in youth with autoimmune diabetes. Pediatr. Diabet. 2013, 14, 121–128. [Google Scholar] [CrossRef] [PubMed]
- García Cabezas, M.A.; Giralt Muiña, P.; Fernández Valle, B.; Benito López, P. Outcome differences in pediatric patients with type 1 diabetes mellitus depending on their HLA-DQ genotypes. Med. Clin. 2010, 134, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Stahl, A.; Straßburger, K.; Lange, K.; Bächle, C.; Holl, R.W.; Giani, G.; Rosenbauer, J. Health-related quality of life among German youths with early-onset and long-duration type 1 diabetes. Diabet. Care 2012, 35, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Karges, B.; Rosenbauer, J.; Kapellen, T.; Wagner, V.M.; Schober, E.; Karges, W.; Holl, R.W. Hemoglobin A1c Levels and risk of severe hypoglycemia in children and young adults with type 1 diabetes from Germany and Austria: A trend analysis in a cohort of 37,539 patients between 1995 and 2012. PLoS Med. 2014, 11, e1001742. [Google Scholar] [CrossRef] [PubMed]
- Rosario, A.S.; Kurth, B.M.; Stolzenberg, H.; Ellert, U.; Neuhauser, H. Body mass index percentiles for children and adolescents in Germany based on a nationally representative sample (KiGGS 2003–2006). Eur. J. Clin. Nutr. 2010, 64, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Green, P.J. Smoothing reference centile curves: The LMS method and penalized likelihood. Stat. Med. 1992, 11, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Knipper, A.J.; Hakenberg, P.; Enczmann, J.; Kuhröber, A.; Kiesel, U.; Kögler, G.; Wernet, P. HLA-DRB1,3,4,5 and -DQB1 allele frequencies and HLA-DR/DQ linkage disequilibrium of 231 German caucasoid patients and their corresponding 821 potential unrelated stem cell transplants. Hum. Immunol. 2000, 61, 605–614. [Google Scholar] [CrossRef]
- Dalva, K.; Beksac, M. HLA typing with sequence-specific oligonucleotide primed PCR (PCR-SSO) and use of the Luminex technology. Methods Mol. Med. 2007, 134, 61–69. [Google Scholar] [PubMed]
- Mack, S.J.; Cano, P.; Hollenbach, J.A.; He, J.; Hurley, C.K.; Middleton, D.; Moraes, M.E.; Pereira, S.E.; Kempenich, J.H.; Reed, E.F.; et al. Common and well-documented HLA alleles: 2012 update to the CWD catalogue. Tissue Antigens 2013, 81, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.; Halliwell, J.A.; Hayhurst, J.D.; Flicek, P.; Parham, P.; Marsh, S.G. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res. 2015, 43, D423–D431. [Google Scholar] [CrossRef] [PubMed]
- Klitz, W.; Maiers, M.; Spellman, S.; Baxter-Lowe, L.A.; Schmeckpeper, B.; Williams, T.M.; Fernandez-Viña, M. New HLA haplotype frequency reference standards: High-resolution and large sample typing of HLA DR-DQ haplotypes in a sample of European Americans. Tissue Antigens 2003, 62, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Cabezas, O.; Flanagan, S.E.; Damhuis, A.; Hattersley, A.T.; Ellard, S. K(ATP) channel mutations in infants with permanent diabetes diagnosed after 6 months of life. Pediatr. Diabet. 2012, 13, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lovejoy, N.F.; Faustman, D.L. Persistence of prolonged C-peptide production in type 1 diabetes as measured with an ultrasensitive C-peptide assay. Diabet. Care 2012, 35, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Awa, W.L.; Boehm, B.O.; Kapellen, T.; Rami, B.; Rupprath, P.; Marg, W.; Becker, M.; Holl, R.W.; DPV-Wiss Study Group and the German Competence Network Diabetes Mellitus. HLA-DR genotypes influence age at disease onset in children and juveniles with type 1 diabetes mellitus. Eur. J. Endocrinol. 2010, 163, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Awa, W.L.; Boehm, B.O.; Rosinger, S.; Achenbach, P.; Ziegler, A.G.; Krause, S.; Meissner, T.; Wiegand, S.; Reinehr, T.; DPV Initiative and the German BMBF Competence Networks Diabetes Mellitus and Obesity; et al. HLA-typing, clinical, and immunological characterization of youth with type 2 diabetes mellitus phenotype from the German/Austrian DPV database. Pediatr. Diabet. 2013, 4, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Komulainen, J.; Kulmala, P.; Savola, K.; Lounamaa, R.; Ilonen, J.; Reijonen, H.; Knip, M.; Akerblom, H.K. Clinical, autoimmune, and genetic characteristics of very young children with type 1 diabetes. Childhood Diabetes in Finland (DiMe) Study Group. Diabet. Care 1999, 22, 1950–1955. [Google Scholar] [CrossRef]
- Rewers, M.; Bugawan, T.L.; Norris, J.M.; Blair, A.; Beaty, B.; Hoffman, M.; McDuffie, R.S., Jr.; Hamman, R.F.; Klingensmith, G.; Eisenbarth, G.S.; et al. Newborn screening for HLA markers associated with IDDM: Diabetes autoimmunity study in the young (DAISY). Diabetologia 1996, 39, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Roark, C.L.; Anderson, K.M.; Simon, L.J.; Schuyler, R.P.; Aubrey, M.T.; Freed, B.M. Multiple HLA epitopes contribute to type 1 diabetes susceptibility. Diabetes 2014, 63, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Thomson, G.; Valdes, A.M.; Noble, J.A.; Kockum, I.; Grote, M.N.; Najman, J.; Erlich, H.A.; Cucca, F.; Pugliese, A.; Steenkiste, A.; et al. Relative predispositional effects of HLA class II DRB1-DQB1 haplotypes and genotypes on type 1 diabetes: A meta-analysis. Tissue Antigens 2007, 70, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.A.; Valdes, A.M. Genetics of the HLA region in the prediction of type 1 diabetes. Curr. Diabet. Rep. 2011, 11, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Koeleman, B.P.; Lie, B.A.; Undlien, D.E.; Dudbridge, F.; Thorsby, E.; de Vries, R.R.; Cucca, F.; Roep, B.O.; Giphart, M.J.; Todd, J.A. Genotype effects and epistasis in type 1 diabetes and HLA-DQ trans dimer associations with disease. Genes Immun. 2004, 5, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Van Lummel, M.; Duinkerken, G.; van Veelen, P.A.; de Ru, A.; Cordfunke, R.; Zaldumbide, A.; Gomez-Touriño, I.; Arif, S.; Peakman, M.; Drijfhout, J.W.; et al. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes 2014, 63, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Thomson, G.; Robinson, W.P.; Kuhner, M.K.; Joe, S.; MacDonald, M.J.; Gottschall, J.L.; Barbosa, J.; Rich, S.S.; Bertrams, J.; Baur, M.P.; et al. Genetic heterogeneity, modes of inheritance, and risk estimates for a joint study of Caucasians with insulin-dependent diabetes mellitus. Am. J. Hum. Genet. 1988, 43, 799–816. [Google Scholar] [PubMed]
- Schmidt, D.; Amrani, A.; Verdaguer, J.; Bou, S.; Santamaria, P. Autoantigen-independent deletion of diabetogenic CD4+ thymocytes by protective MHC class II molecules. J. Immunol. 1999, 162, 4627–4636. [Google Scholar] [PubMed]
- Miyadera, H.; Ohashi, J.; Lernmark, Å.; Kitamura, T.; Tokunaga, K. Cell-surface MHC density profiling reveals instability of autoimmunity-associated HLA. J. Clin. Investig. 2015, 125, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A.; Boulware, D.; Yu, L.; Babu, S.; Steck, A.K.; Becker, D.; Rodriguez, H.; DiMeglio, L.; Evans-Molina, C.; Harrison, L.C.; et al. Type Diabetes TrialNet Study Group. HLA-DRB1*15:01-DQA1*01:02-DQB1*06:02 Haplotype Protects Autoantibody-Positive Relatives from Type 1 Diabetes Throughout the Stages of Disease Progression. Diabetes 2016, 65, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Deutsch, A.J.; Lenz, T.L.; Onengut-Gumuscu, S.; Han, B.; Chen, W.M.; Howson, J.M.; Todd, J.A.; de Bakker, P.I.; Rich, S.S.; et al. Additive and interaction effects at three amino acid positions in HLA-DQ and HLA-DR molecules drive type 1 diabetes risk. Nat. Genet. 2015, 47, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Boehm, B.O.; Manfras, B.; Rosak, C.; Schöffling, K.; Trucco, M. Aspartic acid at position 57 of the HLA-DQ beta chain is protective against future development of insulin-dependent (type 1) diabetes mellitus. Klin. Wochenschr. 1991, 69, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Harfouch-Hammoud, E.; Walk, T.; Otto, H.; Jung, G.; Bach, J.F.; van Endert, P.M.; Caillat-Zucman, S. Identification of peptides from autoantigens GAD65 and IA-2 that bind to HLA class II molecules predisposing to or protecting from type 1 diabetes. Diabetes 1999, 48, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Durinovic-Belló, I.; Schlosser, M.; Riedl, M.; Maisel, N.; Rosinger, S.; Kalbacher, H.; Deeg, M.; Ziegler, M.; Elliott, J.; Roep, B.O.; et al. Pro- and anti-inflammatory cytokine production by autoimmune T cells against preproinsulin in HLA-DRB1*04, DQ8 Type 1 diabetes. Diabetologia 2004, 47, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.R.; Dolton, G.; Kronenberg-Versteeg, D.; Eichmann, M.; Zhao, M.; Huang, G.C.; Beck, K.; Cole, D.K.; Sewell, A.K.; Skowera, A.; et al. A distinct immunogenic region of glutamic acid decarboxylase 65 is naturally processed and presented by human islet cells to cytotoxic CD8 T cells. Clin. Exp. Immunol. 2015, 179, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Kracht, M.J.; Zaldumbide, A.; Roep, B.O. Neoantigens and microenvironment in type 1 diabetes: Lessons from antitumor immunity. Trends Endocrinol. Metab. 2016, 7, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Sollid, L.M.; Qiao, S.W.; Anderson, R.P.; Gianfrani, C.; Koning, F. Nomenclature and listing of celiac disease relevant gluten T-cell epitopes restricted by HLA-DQ molecules. Immunogenetics 2012, 64, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Warncke, K.; Fröhlich-Reiterer, E.E.; Thon, A.; Hofer, S.E.; Wiemann, D.; Holl, R.W.; DPV Initiative of the German Working Group for Pediatric Diabetology; German BMBF Competence Network for Diabetes Mellitus. Polyendocrinopathy in children, adolescents, and young adults with type 1 diabetes: A multicenter analysis of 28,671 patients from the German/Austrian DPV-Wiss database. Diabet. Care 2010, 33, 2010–2012. [Google Scholar]
- Zeitlin, A.A.; Heward, J.M.; Newby, P.R.; Carr-Smith, J.D.; Franklyn, J.A.; Gough, S.C.; Simmonds, M.J. Analysis of HLA class II genes in Hashimoto’s thyroiditis reveals differences compared to Graves’ disease. Genes Immun. 2008, 9, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; Penny, M.A.; Fletcher, J.A.; Jacobs, K.H.; Mijovic, C.H.; Franklyn, J.A.; Sheppard, M.C. HLA class II gene polymorphism contributes little to Hashimoto’s thyroiditis. Clin. Endocrinol. 1992, 37, 141–145. [Google Scholar] [CrossRef]
- Fröhlich-Reiterer, E.E.; Hofer, S.; Kaspers, S.; Herbst, A.; Kordonouri, O.; Schwarz, H.P.; Schober, E.; Grabert, M.; Holl, R.W.; DPV-Wiss Study Group. Screening frequency for celiac disease and autoimmune thyroiditis in children and adolescents with type 1 diabetes mellitus—Data from a German/Austrian multicentre survey. Pediatr. Diabet. 2008, 9, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Bakker, S.F.; Tushuizen, M.E.; von Blomberg, B.M.; Bontkes, H.J.; Mulder, C.J.; Simsek, S. Screening for coeliac disease in adult patients with type 1 diabetes mellitus: Myths, facts and controversy. Diabetol. Metab. Syndr. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Monsuur, A.J.; Wijmenga, C. Understanding the molecular basis of celiac disease: What genetic studies reveal. Ann. Med. 2006, 38, 578–591. [Google Scholar] [CrossRef] [PubMed]
- Elias, J.; Hoorweg-Nijman, J.J.; Balemans, W.A. Clinical relevance and cost-effectiveness of HLA genotyping in children with Type 1 diabetes mellitus in screening for coeliac disease in The Netherlands. Diabet. Med. 2015, 32, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Achury, J.; Romanos, J.; Bakker, S.F.; Kumar, V.; de Haas, E.C.; Trynka, G.; Ricaño-Ponce, I.; Steck, A.; Type 1 Diabetes Genetics Consortium; Chen, W.M.; et al. Contrasting the Genetic Background of Type 1 Diabetes and Celiac Disease Autoimmunity. Diabet. Care 2015, 38 (Suppl. S2), 37–44. [Google Scholar] [CrossRef] [PubMed]
- Törn, C.; Landin-Olsson, M.; Lernmark, A.; Palmer, J.P.; Arnqvist, H.J.; Blohmé, G.; Lithner, F.; Littorin, B.; Nyström, L.; Scherstén, B.; et al. Prognostic factors for the course of beta cell function in autoimmune diabetes. J. Clin. Endocrinol. Metab. 2000, 85, 4619–4623. [Google Scholar] [PubMed]
- Greenbaum, C.J.; Anderson, A.M.; Dolan, L.M.; Mayer-Davis, E.J.; Dabelea, D.; Imperatore, G.; Marcovina, S.; Pihoker, C.; SEARCH Study Group. Preservation of beta-cell function in autoantibody-positive youth with diabetes. Diabet. Care 2009, 32, 1839–1844. [Google Scholar] [CrossRef] [PubMed]
- Oram, R.A.; Jones, A.G.; Besser, R.E.; Knight, B.A.; Shields, B.M.; Brown, R.J.; Hattersley, A.T.; McDonald, T.J. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia 2014, 57, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, A.G.; Bonifacio, E.; Powers, A.C.; Todd, J.A.; Harrison, L.C.; Atkinson, M.A. Type 1 Diabetes Prevention: A Goal Dependent on Accepting a Diagnosis of an Asymptomatic Disease. Diabetes 2016, 65, 3233–3239. [Google Scholar] [CrossRef] [PubMed]

| DRB1* | DQA1* | DQB1* | Controls | T1D Patients | Odds Ratio (CI) | p |
|---|---|---|---|---|---|---|
| n (%) | n (%) | |||||
| 01:01 | 01:01 | 05:01 | 3582 (9.2) | 32 (6.9) | 0.73 (0.51; 1.05) | 0.087 |
| 01:02 | 01:01 | 05:01 | 471 (1.2) | 5 (1.1) | 0.89 (0.39; 2.08) | 0.795 |
| 03:01 | 05:01 | 02:01 | 4089 (10.5) | 155 (33.3) | 4.27 (3.51; 5.17) | <0.001 |
| 04:01 | 03:01 | 03:02 | 1801 (4.6) | 149 (32.0) | 9.73 (7.96; 11.86) | <0.001 |
| 04:01 | 03:01 | 03:01 | 989 (2.5) | 3 (0.6) | 0.25 (0.08; 0.71) | 0.010 |
| 04:02 | 03:01 | 03:02 | 391 (1.0) | 12 (2.6) | 2.62 (1.46; 4.63) | <0.001 |
| 04:03 | 03:01 | 03:02 | 271 (0.7) | 1 (0.2) | 0.31 (0.03; 1.69) | 0.214 |
| 04:04 | 03:01 | 03:02 | 809 (2.1) | 19 (4.1) | 2.01 (1.28; 3.17) | 0.003 |
| 04:05 | 03:01 | 02:02 | 36 (0.1) | 3 (0.6) | 7.03 (2.26; 21.65) | <0.001 |
| 04:05 | 03:01 | 03:02 | 111 (0.3) | 8 (1.7) | 6.13 (2.94; 12.36) | <0.001 |
| 07:01 | 02:01 | 02:02 | 3408 (8.7) | 5 (1.1) | 0.11 (0.05; 0.26) | <0.001 |
| 07:01 | 02:01 | 03:03 | 1411 (3.6) | 1 (0.2) | 0.06 (0.01; 0.31) | <0.001 |
| 08:01 | 04:01/02* | 04:02 | 971 (2.5) | 22 (4.7) | 1.95 (1.25; 2.97) | 0.002 |
| 11:01 | 05:01 | 03:01 | 2987 (7.6) | 3 (0.6) | 0.08 (0.03; 0.22) | <0.001 |
| 11:04 | 05:01 | 03:01 | 1363 (3.5) | 1 (0.2) | 0.06 (0.01; 0.32) | <0.001 |
| 12:01 | 05:01 | 03:01 | 757 (1.9) | 4 (0.9) | 0.44 (0.17; 1.11) | 0.092 |
| 13:01 | 01:03 | 06:03 | 2737 (7.0) | 8 (1.7) | 0.23 (0.11; 0.45) | <0.001 |
| 13:02 | 01:02 | 06:04 | 1441 (3.7) | 12 (2.6) | 0.69 (0.39; 1.20) | 0.205 |
| 13:03 | 05:01 | 03:01 | 542 (1.4) | 1 (0.2) | 0.15 (0.02; 0.83) | 0.031 |
| 14:01/54* | 01:01 | 05:03 | 726 (1.9) | 0 (0.0) | 0.00 (0; 0.39) | 0.003 |
| 15:01 | 01:02 | 06:02 | 4634 (11.9) | 0 (0.0) | 0.00 (0; 0.05) | <0.001 |
| 16:01 | 01:02 | 05:02 | 1014 (2.6) | 13 (2.8) | 1.08 (0.61; 1.86) | 0.792 |
| others | 4547 (11.6) | 9 (1.9) |
| Patients with DRB1*03/x | ||||||
| DRB1* | DQA1* | DQB1* | Controls | T1D Patients | Odds Ratio (CI) | p |
| n (%) | n (%) | |||||
| 01:01 | 01:01 | 05:01 | 359 (10.5) | 11 (29.7) | 1.29 (0.62; 2.65) | 0.447 |
| 07:01 | 02:01 | 02:02 | 332 (9.7) | 2 (5.4) | 0.19 (0.05; 0.72) | 0.009 |
| 08:01 | 04:01/02 | 04:02 | 105 (3.1) | 5 (13.5) | 2.01 (0.83; 4.98) | 0.188 |
| 09:01 | 03:02 | 03:03 | 23 (0.7) | 4 (10.8) | 7.55 (2.68; 22.77) | 0.007 |
| 12:01 | 05:05 | 03:01 | 94 (2.7) | 2 (5.4) | 0.83 (0.19; 3.18) | 0.999 |
| 13:01 | 01:03 | 06:03 | 281 (8.2) | 2 (5.4) | 0.24 (0.06; 0.89) | 0.032 |
| 13:02 | 01:02 | 06:04 | 142 (4.1) | 3 (8.1) | 0.81 (0.26; 2.41) | 0.999 |
| 16:01 | 01:02 | 05:02 | 120 (3.5) | 8 (21.6) | 3.07 (1.44; 6.69) | 0.011 |
| others | 1456 (42.5) | |||||
| Patients with DRB1*04/x | ||||||
| DRB1* | DQA1* | DQB1* | Controls | T1D Patients | Odds Ratio (CI) | p |
| n (%) | n (%) | |||||
| 01:01 | 01:01 | 05:01 | 170 (11.1) | 15 (25.0) | 2.68 (1.44; 4.85) | 0.003 |
| 01:02 | 01:01 | 05:01 | 25 (1.6) | 2 (3.3) | 2.08 (0,48; 7.88) | 0.270 |
| 04:01 | 03:02 | 03:01 | 44 (2.9) | 2 (3.3) | 1.17 (0.27; 4.50) | 0.691 |
| 07:01 | 02:01 | 02:02 | 124 (8.1) | 3 (5.0) | 0.59 (0.19; 1.80) | 0.624 |
| 07:01 | 02:01 | 03:03 | 59 (3.8) | 1 (1.7) | 0.42 (0.04; 2.38) | 0.725 |
| 08:01 | 04:01/02 | 04:02 | 67 (4.4) | 14 (23.3) | 6.66 (3.55; 12.56) | <0.001 |
| 08:04 | 04:01 | 04:02 | 3 (0.2) | 1 (1.7) | 8.65 (0.66; 58.49) | 0.142 |
| 11:01 | 05:05 | 03:01 | 151 (9.8) | 4 (6.7) | 0.65 (0.25; 1.77) | 0.512 |
| 11:03 | 05:05 | 03:01 | 15 (1.0) | 1 (1.7) | 17.16 (2.98; 84.83) | 0.014 |
| 12:01 | 05:05 | 03:01 | 26 (1.7) | 1 (1.7) | 1.97 (0.45; 7.38) | 0.291 |
| 13:01 | 01:03 | 06:03 | 130 (8.5) | 5 (8.3) | 0.98 (0.41; 2.38) | 0.999 |
| 13:02 | 01:02 | 06:04 | 64 (4.2) | 6 (10.0) | 2.55 (1.13; 5.92) | 0.044 |
| 13:03 | 05:05 | 03:01 | 18 (1.2) | 1 (1.7) | 1.43 (0.13; 8.31) | 0.520 |
| 16:01 | 01:02 | 05:02 | 59 (2.8) | 4 (6.7) | 1.79 (0.67; 4.78) | 0.295 |
| others | 397 (25.9%) | |||||
| Highest-Risk Haplotype (n = 86) | Moderate Risk Haplotype (n = 103) | Low Risk Haplotype (n = 6) | |||||
|---|---|---|---|---|---|---|---|
| n 2 | % or mean (SE) | n 2 | % or mean (SE) | n 2 | % or mean (SE) | p 1 | |
| Weight (kg) | 1008 | 45.3 (43.5; 47.1) | 1286 | 45.8 (44.2; 47.5) | 74 | 45.5 (38.8; 52.2) | 0.917 |
| Height (cm) | 1008 | 146.4 (144.6; 148.2) | 1286 | 146.8 (145.1; 148.4) | 74 | 148.7 (142.0; 155.4) | 0.802 |
| BMI-SDS (KIGGS) | 1007 | 0.37 (0.23; 0.52) | 1285 | 0.42 (0.28; 0.55) | 74 | 0.15 (−0.39; 0.70) | 0.629 |
| SMBG | 925 | 5.4 (5.2; 5.7) | 1228 | 5.1 (4.9; 5.4) | 72 | 5.3 (4.3; 6.3) | 0.304 |
| Insulin dose (IU/d) | 1014 | 38.9 (36.4; 41.4) | 1288 | 40.0 (37.7; 42.3) | 75 | 38.9 (29.5; 48.4) | 0.809 |
| Insulin dose per kg bodyweight (IU/kg/d) | 1008 | 0.81 (0.78; 0.85) | 1286 | 0.83 (0.80; 0.86) | 74 | 0.83 (0.70; 0.97) | 0.820 |
| HbA1c (%) | 1005 | 7.6 (7.4; 7.8) | 1283 | 7.4 (7.3; 7.6) | 73 | 7.6 (6.9; 8.2) | 0.441 |
| HbA1c (mmol/mol) | 1005 | 60 (58; 62) | 1283 | 58 (56; 60) | 73 | 59 (52; 67) | |
| RR systolic (mmHg) | 979 | 113.0 (111.5; 114.5) | 1233 | 112.7 (111.3; 114.1) | 73 | 113.3 (107.7; 118.8) | 0.947 |
| RR diastolic (mmHg) | 979 | 66.9 (65.8; 68.0) | 1233 | 66.8 (65.8; 67.8) | 73 | 64.2 (60.2; 68.3) | 0.447 |
| Cholesterol (mg/dL) | 748 | 174.8 (169.9; 179.8) | 926 | 178.2 (173.6; 182.9) | 55 | 174.9 (156.3; 193.4) | 0.608 |
| HDL-Cholesterol (mg/dL) | 612 | 62.5 (59.); 65.2) | 736 | 64.2 (61.8; 66.7) | 40 | 61.5 (51.8; 71.2) | 0.612 |
| LDL-Cholesterol (mg/dL) | 606 | 96.9 (91.9; 101.9) | 732 | 96.3 (91.7; 101.0) | 40 | 93.8 (75.6; 111.9) | 0.942 |
| Triglyceride (mg/dL) | 709 | 98.9 (91.0; 106.9) | 874 | 112.1 (104.8; 119.4) | 55 | 98.4 (69.5; 127.4) | 0.051 |
| Creatinine (mg/dL) | 718 | 57.0 (55.3; 58.7) | 847 | 58.5 (57.0; 60.1) | 40 | 55.6 (49.0; 62.3) | 0.348 |
| Urine albuminuria (mg/L) | 315 | 11.8 (0.0; 26.0) * | 383 | 16.0 (3.1; 28.9) * | 15 | 1.8 (0.0; 66.0) * | 0.852* |
| Rate of severe hypoglycaemia (1/100 patient years) | 1012 | 18.5 (12.3; 27.9) | 1287 | 28.7 (19.9; 41.4) | 75 | 6.5 (1.2; 35.6) | 0.100 |
| Rate of hypoglycaemic coma (1/100 patient years) | 1012 | 3.6 (2.1; 6.1) | 1287 | 5.6 (3.6; 8.7) | 75 | 2.3 (0.3; 19.9) | 0.364 |
| Hospitalization rate for hypoglycaemia (1/100 patient years) | 1012 | 1.2 (0.6; 2.4) | 1287 | 1.5 (0.8; 2.7) | 75 | 1.4 (0.1; 15.6) | 0.916 |
| Hospitalization rate for ketoacidosis (1/100 patient years) | 1012 | 1.7 (0.9; 3.2) | 1287 | 2.7 (1.6; 4.5) | 75 | 1.4 (0.1; 17.3) | 0.494 |
| n 3 | % or mean (SE) | n 3 | % or mean (SE) | n 3 | % or mean (SE) | p 1 | |
| Microalbuminuria (%) 4 | 80 | 36.9 (27.0; 48.1) | 95 | 43.0 (33.3; 53.2) | 6 | 17.0 (3.8; 66.2) | 0.497 |
| Macroalbuminuria (%) 4 | 80 | 3.5 (1.1; 10.9) | 95 | 4.9 (1.9; 11.9) | 6 | 7.9 (0.4; 63.7) | 0.838 |
| Retinopathy (%) 4 | 82 | 2.2 (0.5; 9.2) | 97 | 3.8 (1.3; 10.4) | 6 | 8.0 (0.4; 63.4) | 0.689 |
| Transglutaminase antibodies (%) 4 | 66 | 26.3 (16.8; 38.6) | 73 | 20.4 (12.4; 31.7) | 4 | 11.0 (0.5; 76.5) | 0.633 |
| Clinical celiac disease (%) 4 | 86 | 10.6 (5.6; 19.3) | 103 | 4.6 (1.9; 10.7) | 6 | 7.1 (0.4; 62.0) | 0.231 |
| TPO antibodies (%) 4 | 72 | 16.3 (9.3; 27.1) | 83 | 25.0 (16.4; 36.2) | 6 | 5.5 (0.2; 59.0) | 0.283 |
| TG antibodies (%) 4 | 56 | 22.8 (12.8; 37.2) | 71 | 16.9 (9.3; 26.6) | 4 | 4.9 (0.2; 60.2) | 0.489 |
| Clinical thyroiditis (%) 4 | 86 | 8.8 (4.4; 17.0) | 103 | 18.3 (11.7; 27.5) | 6 | 5.8 (0.3; 59.7) | 0.148 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinauer, C.; Rosenbauer, J.; Bächle, C.; Herder, C.; Roden, M.; Ellard, S.; De Franco, E.; Karges, B.; Holl, R.W.; Enczmann, J.; et al. The Clinical Course of Patients with Preschool Manifestation of Type 1 Diabetes Is Independent of the HLA DR-DQ Genotype. Genes 2017, 8, 146. https://doi.org/10.3390/genes8050146
Reinauer C, Rosenbauer J, Bächle C, Herder C, Roden M, Ellard S, De Franco E, Karges B, Holl RW, Enczmann J, et al. The Clinical Course of Patients with Preschool Manifestation of Type 1 Diabetes Is Independent of the HLA DR-DQ Genotype. Genes. 2017; 8(5):146. https://doi.org/10.3390/genes8050146
Chicago/Turabian StyleReinauer, Christina, Joachim Rosenbauer, Christina Bächle, Christian Herder, Michael Roden, Sian Ellard, Elisa De Franco, Beate Karges, Reinhard W. Holl, Jürgen Enczmann, and et al. 2017. "The Clinical Course of Patients with Preschool Manifestation of Type 1 Diabetes Is Independent of the HLA DR-DQ Genotype" Genes 8, no. 5: 146. https://doi.org/10.3390/genes8050146
APA StyleReinauer, C., Rosenbauer, J., Bächle, C., Herder, C., Roden, M., Ellard, S., De Franco, E., Karges, B., Holl, R. W., Enczmann, J., & Meissner, T. (2017). The Clinical Course of Patients with Preschool Manifestation of Type 1 Diabetes Is Independent of the HLA DR-DQ Genotype. Genes, 8(5), 146. https://doi.org/10.3390/genes8050146






