The Moss Physcomitrella patens Is Hyperresistant to DNA Double-Strand Breaks Induced by γ-Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Suspension Culture
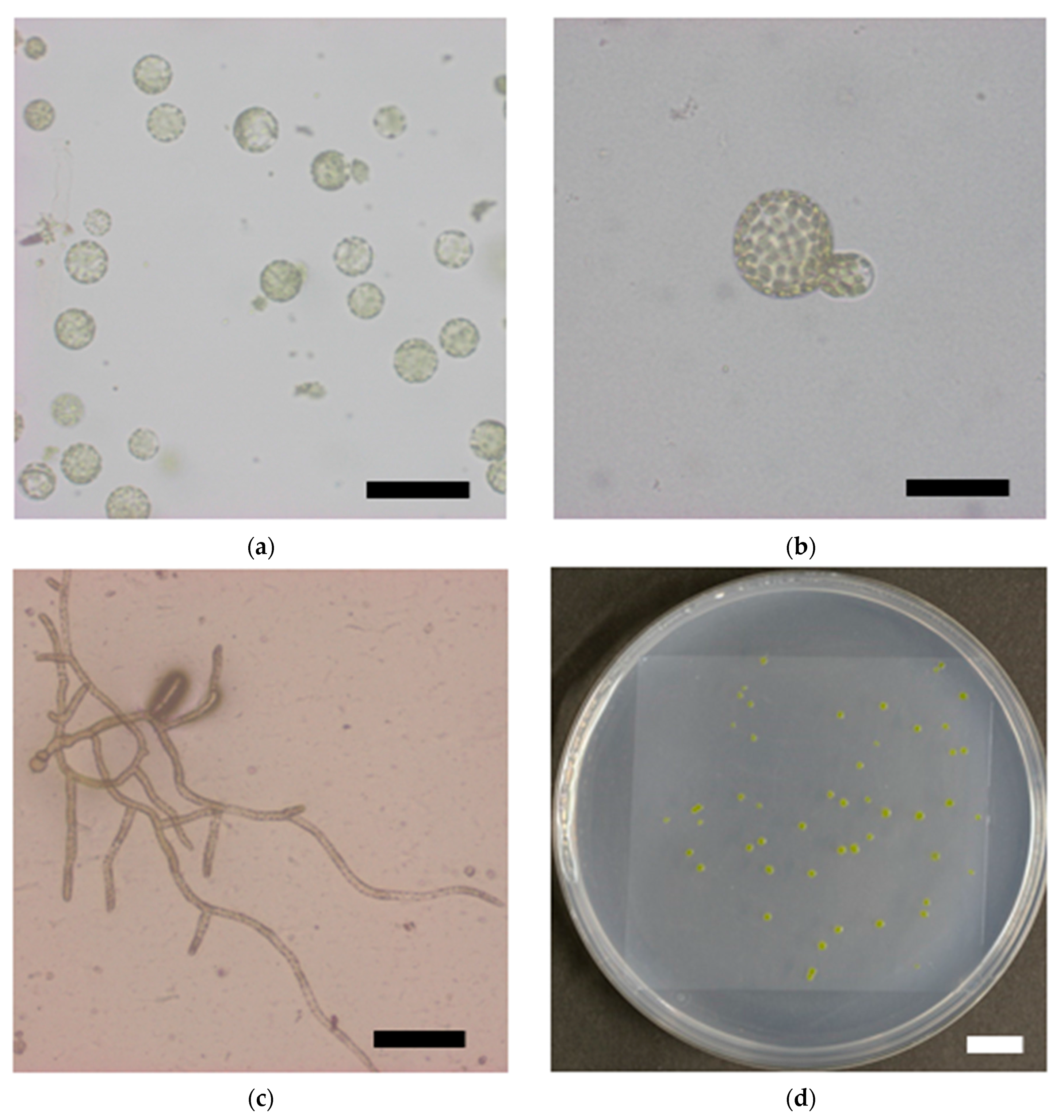
2.2. Protoplast Isolation
2.3. Analysis of Cell Cycle Phase Distribution
2.4. γ-Irradiation
2.5. Growth Assay
2.6. Colony Formation Assay
2.7. Pulsed-Field Gel Electrophoresis Assay
2.8. Quantification of Double-Strand Breaks
3. Results
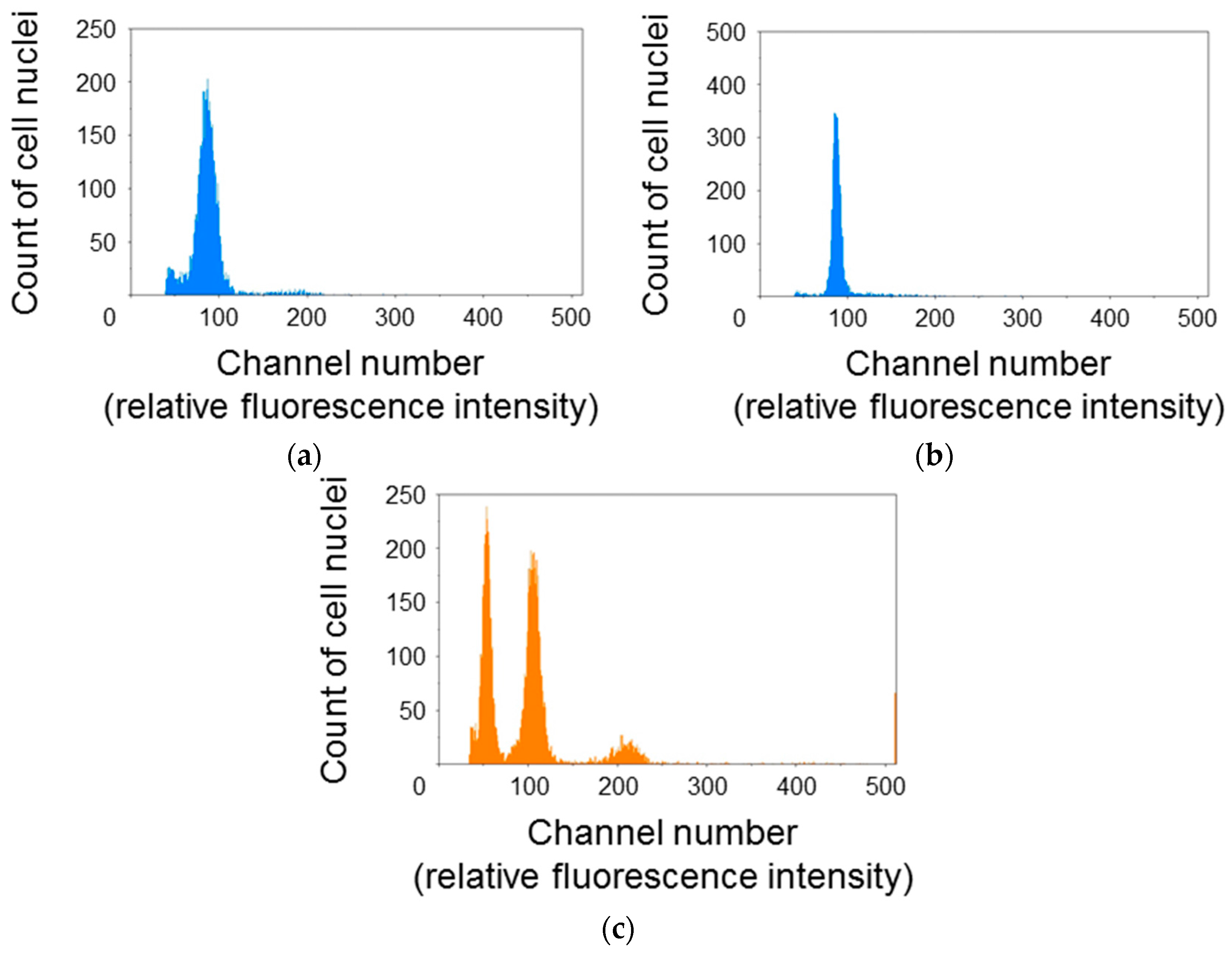
3.1. Major Fractions of Protonema Protoplasts Are Haploid and in G0/G1 Phase
3.2. Growth Potential of Protonemata Decreased with Increasing Dose of γ-rays
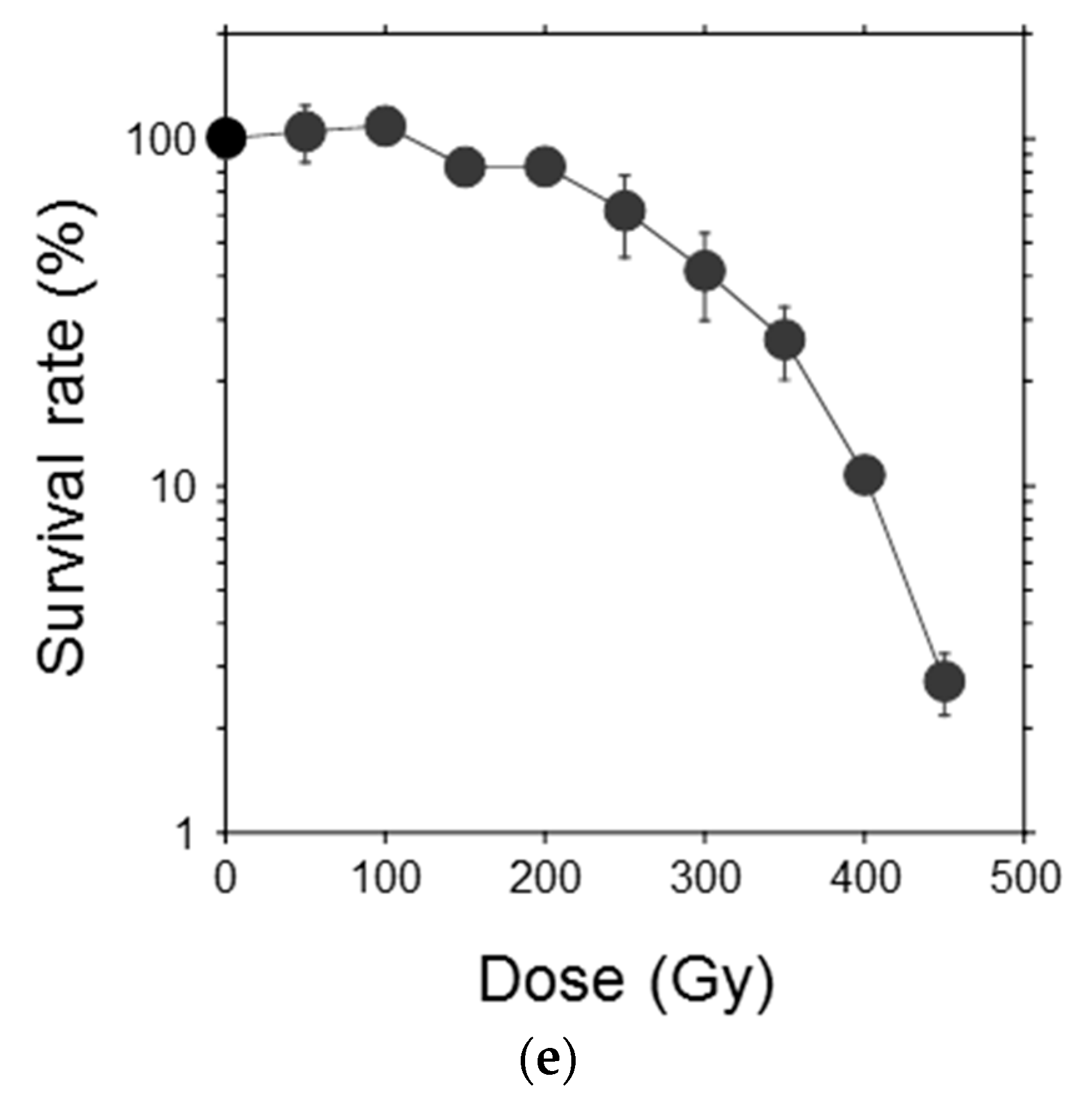
3.3. Survival Rate of Protoplasts Decreased with Increasing γ-ray Dose
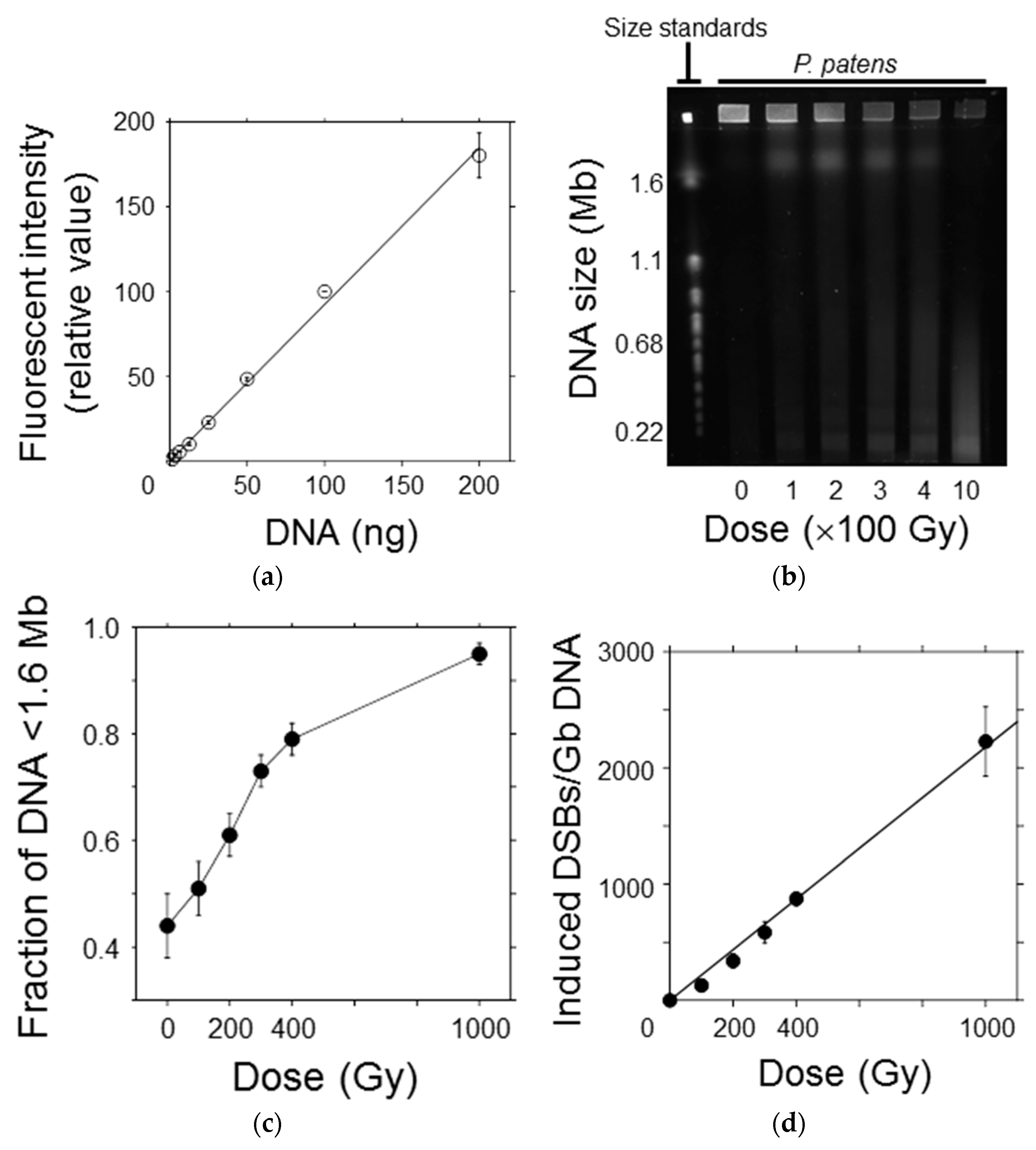
3.4. Double-Strand Break Yields Linearly Increased with Increasing Dose
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation; United Nations: New York, NY, USA, 1996; p. 76. ISBN 92-1-142219-1. [Google Scholar]
- Underbrink, A.G.; Sparrow, A.H.; Pond, V. Chromosomes and cellular radiosensitivity-II. Use of interrelationships among chromosome volume, nucleotide content and D0 of 120 diverse organisms in predicting radiosensitivity. Radiat. Bot. 1968, 8, 205–237. [Google Scholar] [CrossRef]
- Tanaka, A.; Shikazono, N.; Yokota, Y.; Watanabe, H.; Tano, S. Effects of heavy ions on the germination and survival of Arabidopsis thaliana. Int. J. Radiat. Biol. 1997, 72, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Hase, Y.; Yamaguchi, M.; Inoue, M.; Tanaka, A. Reduction of survival and induction of chromosome aberrations in tobacco irradiated by carbon ions with different linear energy transfers. Int. J. Radiat. Biol. 2002, 78, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Yokota, Y.; Hase, Y.; Shikazono, N.; Tanaka, A.; Inoue, M. LET dependence of lethality of carbon ion irradiation to single tobacco cells. Int. J. Radiat. Biol. 2003, 79, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.F. DNA damage produced by ionizing-radiation in mammalian-cells—Identities, mechanisms of formation, and reparability. Prog. Nucl. Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar] [CrossRef]
- Prise, K.M.; Ahnström, G.; Belli, M.; Carlsson, J.; Frankenberg, D.; Kiefer, J.; Löbrich, M.; Michael, B.D.; Nygren, J.; Simone, G.; et al. A review of dsb induction data for varying quality radiations. Int. J. Radiat. Biol. 1998, 74, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Yokota, Y.; Shikazono, N.; Tanaka, A.; Hase, Y.; Funayama, T.; Wada, S.; Inoue, M. Comparative radiation tolerance based on the induction of DNA double-strand breaks in tobacco BY-2 cells and CHO-K1 cells irradiated with gamma rays. Radiat. Res. 2005, 163, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Friesner, J.D.; Liu, B.; Culligan, K.; Britt, A.B. Ionizing radiation-dependent γ-H2AX focus formation requires ataxia telangiectasia mutated and ataxia telangiectasia mutated and Rad3-related. Mol. Biol. Cell 2005, 16, 2566–2576. [Google Scholar] [CrossRef] [PubMed]
- Rensing, S.A.; Lang, D.; Zimmer, A.D.; Terry, A.; Salamov, A.; Shapiro, H.; Nishiyama, T.; Perroud, P.F.; Lindquist, E.A.; Kamisugi, Y.; et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 2008, 319, 64–69. [Google Scholar] [CrossRef] [PubMed]
- PHYSCObase. Available online: http://moss.nibb.ac.jp/index.html (accessed on 3 October 2017).
- Schaefer, D.G.; Zryd, J.P. Efficient gene targeting in the moss Physcomitrella patens. Plant J. 1997, 11, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Yokota, Y.; Funayama, T.; Mutou-Yoshihara, Y.; Ikeda, H.; Kobayashi, Y. The bystander cell-killing effect mediated by nitric oxide in normal human fibroblasts varies with irradiation dose but not with radiation quality. Int. J. Radiat. Biol. 2015, 91, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Kiltie, A.E.; Ryan, A.J. SYBR Green I staining of pulsed field agarose gels is a sensitive and inexpensive way of quantitating DNA double-strand breaks in mammalian cells. Nucleic Acids Res. 1997, 25, 2945–2946. [Google Scholar] [CrossRef] [PubMed]
- Blöcher, D. In CHEF electrophoresis a linear induction of dsb corresponds to a nonlinear fraction of extracted DNA with dose. Int. J. Radiat. Biol. 1990, 57, 7–12. [Google Scholar] [CrossRef] [PubMed]
- The Arabidopsis Information Resource (TAIR). Available online: https://www.arabidopsis.org/index.jsp (accessed on 29 January 2018).
- Schween, G.; Gorr, G.; Hohe, A.; Reski, R. Unique tissue-specific cell cycle in Physcomitrella. Plant Biol. 2003, 5, 50–58. [Google Scholar] [CrossRef]
- Cedervall, B.; Wong, R.; Albright, N.; Dynlacht, J.; Lambin, P.; Dewey, W.C. Methods for the quantification of DNA double-strand breaks determined from the distribution of DNA fragment sizes measured by pulsed-field gel electrophoresis. Radiat. Res. 1995, 143, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Kurahayashi, H.; Kobayashi, Y.; Funayama, T.; Yamamoto, K.; Natsuhori, M.; Ito, N. The relationship between cellular radiosensitivity and radiation-induced DNA damage measured by the comet assay. J. Vet. Med. Sci. 2003, 65, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Resnick, M.A. Genetic control of radiation sensitivity in Saccharomyces cerevisiae. Genetics 1969, 62, 519–531. [Google Scholar] [PubMed]
- Suzuki, M.; Kase, Y.; Yamaguchi, H.; Kanai, T.; Ando, K. Relative biological effectiveness for cell-killing effect on various human cell lines irradiated with heavy-ion medical accelerator in CHIBA (HIMAC) carbon-ion beams. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 241–250. [Google Scholar] [CrossRef]
- Kamisugi, Y.; Whitaker, J.W.; Cuming, A.C. The transcriptional response to DNA-double-strand breaks in Physcomitrella patens. PLoS ONE 2016, 11, e0161204. [Google Scholar] [CrossRef] [PubMed]
- Trouiller, B.; Schaefer, D.G.; Charlot, F.; Nogué, F. MSH2 is essential for the preservation of genome integrity and prevents homeologous recombination in the moss Physcomitrella patens. Nucleic Acids Res. 2006, 34, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, D.G.; Delacote, F.; Charlot, F.; Vrielynck, N.; Guyon-Debast, A.; Le Guin, S.; Neuhaus, J.M.; Doutriaux, M.P.; Nogué, F. RAD51 loss of function abolishes gene targeting and de-represses illegitimate integration in the moss Physcomitrella patens. DNA Repair 2010, 9, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Little, J.B. Factors influencing repair of potentially lethal radiation-damage in growth-inhibited human cells. Radiat. Res. 1973, 56, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Bertsche, U. The response of diploid yeast to radiations at different LET. I. Potentially lethal and lethal damage to reproductive capacity. Radiat. Res. 1978, 76, 349–367. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.C.; Cantor, C.R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel-electrophoresis. Cell 1984, 37, 67–75. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, D.W.; Olive, P.L.; O’Neill, K.L. The comet assay—A comprehensive review. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1995, 339, 37–59. [Google Scholar] [CrossRef]
- Rothkamm, K.; Löbrich, M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses. Proc. Natl. Acad. Sci. USA 2003, 100, 5057–5062. [Google Scholar] [CrossRef] [PubMed]
- DeLara, C.M.; Jenner, T.J.; Townsend, K.M.S.; Marsden, S.J.; O’Neill, P. The effect of dimethyl-sulfoxide on the induction of DNA double-strand breaks in V79-4 mammalian-cells by alpha-particles. Radiat. Res. 1995, 144, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sak, A.; Stuschke, M.; Wurm, R.; Budach, V. Protection of DNA from radiation-induced double-strand breaks: Influence of replication and nuclear proteins. Int. J. Radiat. Biol. 2000, 76, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Horikawa, D.D.; Saito, Y.; Kuwahara, H.; Kozuka-Hata, H.; Shin-I, T.; Minakuchi, Y.; Ohishi, K.; Motoyama, A.; Aizu, T.; et al. Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Newman, H.C.; Prise, K.M.; Michael, B.D. The role of higher-order chromatin structure in the yield and distribution of DNA double-strand breaks in cells irradiated with X-rays or alpha-particles. Int. J. Radiat. Biol. 2000, 76, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, R.K. Radiobiological and genetic studies on a polyploid series (haploid to hexaploid) of Saccharomyces cerevisiae. Radiat. Res. 1958, 9, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.L.; Murnane, J.; Weichselbaum, R.R. The contribution of DNA ploidy to radiation sensitivity in human tumour cell lines. Br. J. Cancer 1999, 79, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Hase, Y.; Trung, K.H.; Matsunaga, T.; Tanaka, A. A mutation in the uvi4 gene promotes progression of endo-reduplication and confers increased tolerance towards ultraviolet B light. Plant J. 2006, 46, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Yokota, Y.; Wada, S.; Hase, Y.; Funayama, T.; Kobayashi, Y.; Narumi, I.; Tanaka, A. Kinetic analysis of double-strand break rejoining reveals the DNA reparability of gamma-irradiated tobacco cultured cells. J. Radiat. Res. 2009, 50, 171–175. [Google Scholar] [CrossRef] [PubMed]

| Organism | DSB Yield (Gb DNA−1 Gy−1) | DNA Content (Gb DNA Cell−1) | LD50 (Gy) | DSB Yield (Cell−1 LD50−1) |
|---|---|---|---|---|
| Moss | 2.2 | 0.511 [18] | 277 | 311 |
| Tobacco | 2.0 [8] | 12.3 [8] | 27 [5] | 664 |
| Chinese hamster | 6.6 [8] | 6.0 [16] | 2.5 [20] | 99 |
| Yeast | 5.4 [7] | 0.024 | 400 [21] | 52 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokota, Y.; Sakamoto, A.N. The Moss Physcomitrella patens Is Hyperresistant to DNA Double-Strand Breaks Induced by γ-Irradiation. Genes 2018, 9, 76. https://doi.org/10.3390/genes9020076
Yokota Y, Sakamoto AN. The Moss Physcomitrella patens Is Hyperresistant to DNA Double-Strand Breaks Induced by γ-Irradiation. Genes. 2018; 9(2):76. https://doi.org/10.3390/genes9020076
Chicago/Turabian StyleYokota, Yuichiro, and Ayako N. Sakamoto. 2018. "The Moss Physcomitrella patens Is Hyperresistant to DNA Double-Strand Breaks Induced by γ-Irradiation" Genes 9, no. 2: 76. https://doi.org/10.3390/genes9020076





