Transmission and Drive Involving Parasitic B Chromosomes
Abstract
:1. Introduction
2. Plants
2.1. Rye (Secale Cereal, 2n = 2x = 14 + Bs)
2.2. Maize (Zea Mays, 2n = 2x = 20 + Bs)
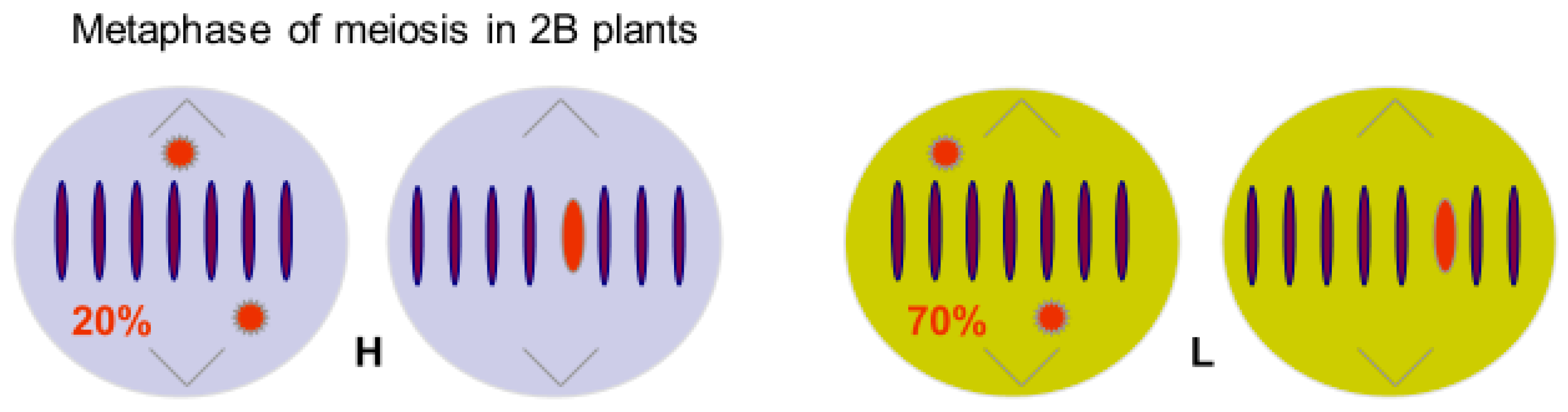
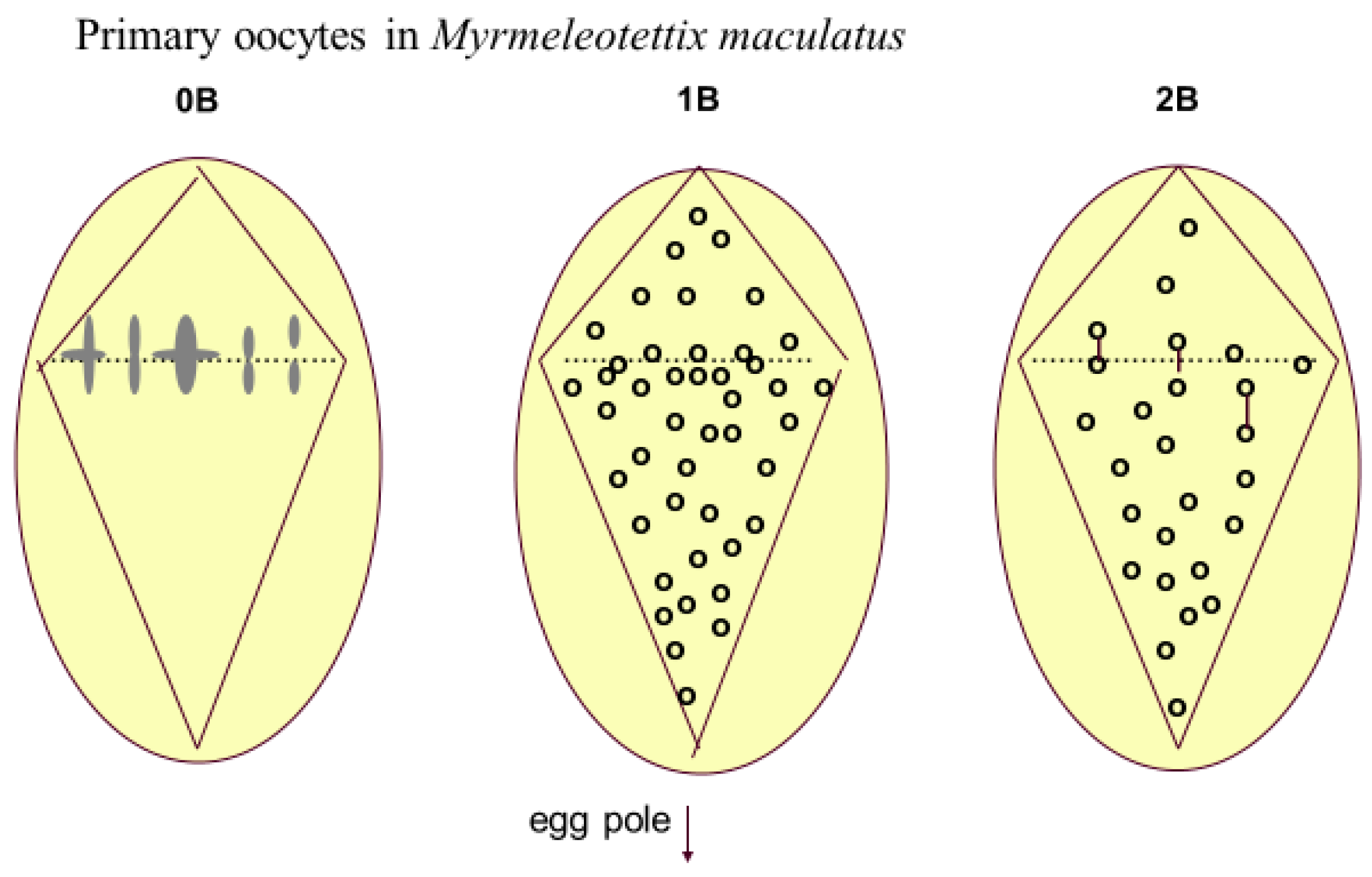
3. Animals
4. Conclusions
Funding
Conflicts of Interest
References
- Jones, R.N. B chromosome drive. Am. Nat. 1991, 137, 430–442. [Google Scholar] [CrossRef]
- Jones, R.N.; Rees, H. B Chromosomes; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Camacho, J.P.M. B chromosomes. In The Evolution of the Genome; Gregory, T.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Houben, A.; Banaei-Moghaddam, A.M.; Klemme, S. Biology and evolution of B chromosomes. In Plant Genome Diversity Volume 2; Greilhuber, J., Dolezel, J., Wendel, J.F., Eds.; Springer: Vienna, Austria, 2013; pp. 149–165. [Google Scholar]
- Houben, A.; Banaei-Moghaddam, A.M.; Klemme, S.; Timmis, J.N. Evolution and biology of supernumerary B chromosomes. Cell. Mol. Life Sci. 2014, 71, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Banaei-Moghaddam, A.M.; Martis, M.M.; Macas, J.; Gundlach, H.; Himmelbach, A.; Altschmied, L.; Mayer, K.F.; Houben, A. Genes on B chromosomes: Old questions revisited with new tools. Biochim. Biophys. Acta 2015, 1849, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Valente, G.T.; Nakajima, R.T.; Fantinatti, B.E.; Marques, D.F.; Almeida, R.O.; Simoes, R.P.; Martins, C. B chromosomes: From cytogenetics to systems biology. Chromosoma 2017, 126, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Houben, A. B chromosomes—A matter of Chromosome Drive. Front. Plant Sci. 2017, 8, 210. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.; Schmutzer, T.; Scholz, U.; Houben, A. How Next-Generation sequencing has aided our understanding of the sequence composition and origin of B chromosomes. Genes 2017, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Coan, R.L.B.; Martins, C. Landscape of transposable elements focusing on the B chromosome of the cichlid fish Astatotilapia latifasciata. Genes 2018, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, N. A cytological study on 8-chromosome rye. Cytologia 1934, 6, 68–77. [Google Scholar] [CrossRef]
- Lindström, J. Transfer to wheat of accessory chromosomes from rye. Hereditas 1965, 54, 149–155. [Google Scholar] [CrossRef]
- Müntzing, A. Chromosomal variation in the Lindström strain of wheat carrying accessory chromosomes of rye. Hereditas 1970, 66, 279–286. [Google Scholar] [CrossRef]
- Matthews, R.B.; Jones, R.N. Dynamics of the B chromosome polymorphism in rye. I. Simulated populations. Heredity 1982, 48, 345–369. [Google Scholar] [CrossRef]
- Matthews, R.B.; Jones, R.N. Dynamics of the B chromosome polymorphism in rye. II. Estimates of parameters. Heredity 1983, 50, 119–137. [Google Scholar] [CrossRef]
- Kishikawa, H. Cytogenetic studies of B chromosomes in rye, Secale cereale L. in Japan. Agric. Bull. Saga Univ. 1965, 21, 1–81. [Google Scholar]
- Müntzing, A. Cytological studies of extra fragment chromosomes in rye II. Transmission and multiplication of standard fragments and iso-fragments. Hereditas 1945, 31, 457–477. [Google Scholar] [CrossRef] [PubMed]
- Lima-De-Faria, A. B chromosomes of rye at pachytene. Port. Acta Biol. 1948, 2, 167–174. [Google Scholar]
- Lima-De-Faria, A. The evolution of the structural pattern in a rye B chromosome. Evolution 1963, 17, 289–295. [Google Scholar] [CrossRef]
- Jiménez, M.M.; Romero, F.; Puertas, M.J. B Chromosomes in inbred lines of rye (Secale cereal L.) I. Vigour and fertility. Genetica 1994, 92, 149–154. [Google Scholar] [CrossRef]
- Puertas, M.J.; Jiménez, G.; Manzanero, S.; Chiavarino, A.M.; Rosato, M.; Naranjo, C.A.; Poggio, L. Genetic control of B chromosome transmission in maize and rye. Chromosom. Today 2000, 13, 79–82. [Google Scholar]
- Gonzáez-Sánchez, M.; Chiavarino, M.; Jiménez, G.; Manzanero, S.; Rosato, M.; Puertas, M.J. The parasitic effects of rye B chromosomes might be beneficial in the long term. Cytogenet. Genome Res. 2004, 106, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Östergren, G. Parasitic nature of extra fragment chromosomes. Bot. Not. 1945, 2, 157–163. [Google Scholar]
- Roman, H. Mitotic nondisjunction in the case of interchanges involving the B-type chromosome in maize. Genetics 1947, 32, 391–409. [Google Scholar] [PubMed]
- Jones, R.N.; Ruban, A. Are B chromosomes useful? In Plants, People, Planet; 2018; submitted for publication. [Google Scholar]
- Carlson, W.R. The B chromosome of maize. Crit. Rev. Plant Sci. 1986, 3, 201–226. [Google Scholar] [CrossRef]
- Rosato, M.; Chiavarino, A.M.; Naranjo, C.; Puertas, M.; Poggio, L. Genetic control of B chromosome transmission rate in Zea mays ssp. mays (Poaceae). Am. J. Bot. 1996, 83, 1107–1112. [Google Scholar] [CrossRef]
- Carlson, W.R.; Roseman, R. A new property of the maize B chromosome. Genetics 1992, 131, 211–223. [Google Scholar] [PubMed]
- Komatsu, T.; Nakajima, K. B chromosomes in diploid Guineagrass (Panicum maximum JACQ). Jpn. J. Breed. 1988, 38, 151–157. [Google Scholar] [CrossRef]
- Ohta, S. Mechanisms of B-chromosome ac in Aegilops mutica Boiss. Genes Genet. Syst. 1996, 71, 23–29. [Google Scholar] [CrossRef]
- Darlington, C.D.; Thomas, P.T. Morbid mitosis and the activity of inert chromosomes in Sorghum. Proc. R. Soc. B 1941, 130, 127–150. [Google Scholar] [CrossRef]
- Parker, J.S.; Jones, G.H.; Edgar, L.; Whitehouse, C. The population cytogenetics of Crepis capillaris. II. The stability and inheritance of B-chromosomes. Heredity 1989, 63, 19–27. [Google Scholar] [CrossRef]
- Kimura, M.; Kayano, H. The maintenance of supernumerary chromosomes in wild populations of Lilium callosum by preferential segregation. Genetics 1961, 46, 1699–1712. [Google Scholar] [PubMed]
- Fröst, S. The inheritance of accessory chromosomes in plants, especially in Ranunculus acris and Phleum nodosum. Hereditas 1969, 61, 317–326. [Google Scholar] [CrossRef]
- Fröst, S. The cytological be and mode of transmission of accessory chromosomes in Plantago serraria. Hereditas 1959, 45, 191–210. [Google Scholar] [CrossRef]
- Rutishauser, A. Genetics of fragment chromosomes in Trillium grandiflorum. Heredity 1956, 10, 195–204. [Google Scholar] [CrossRef]
- Kean, V.M.; Fox, D.P.; Faulkner, R. The Ac mechanism of the supernumerary (B-) chromosome in Picea sitchensis (Bong.) Carr. and the effect of this on male and female flowering. Silvae Genet. 1982, 31, 126–131. [Google Scholar]
- Parker, J.S.; Taylor, S.; Ainsworth, C.C. The B-Chromosome system of Hypochoeris maculata III. Variation in B-Chromosome transmission rates. Chromosoma 1982, 85, 299–310. [Google Scholar] [CrossRef]
- Murray, B.G. The structure, meiotic behaviour and effects of B chromosomes in Briza humilis Bieb. (Gramineae). Genetica 1984, 63, 213–219. [Google Scholar] [CrossRef]
- Rutishauser, A.; Roethlisberger, E. Boosting of B chromosomes in Crepis capillaris. Chromosom. Today 1966, 1, 28–30. [Google Scholar]
- Bougourd, S.M.; Plowman, A.B.; Ponsford, N.R.; Elias, M.L.; Holmes, D.S.; Taylor, S. The case for unselfish B-chromosomes: Evidence from Allium schoenoprasum. In Proceedings of the Kew Chromosome Conference IV, Kew, UK, 30 August–2 September 1994; Royal Botanic Gardens: Kew, UK, 1995; pp. 21–34. [Google Scholar]
- Berger, C.A.; Feely, E.J.; Witkus, E.R. The cytology of Xanthisma texanum D.C. IV. Megasporogenesis and embryo formation, pollen mitosis and embryo formation. Bull. Torrey Bot. Club 1956, 83, 428–434. [Google Scholar] [CrossRef]
- Lopez-Leon, M.D.; Cabrero, J.; Camacho, J.P.M.; Cano, M.I.; Santos, J.L. A widespread B chromosome polymorphism maintained without apparent drive. Evolution 1992, 46, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.; Saboya, S.M.R.; Foresti, F.; Senhorini, J.O.; Bernardino, G. Increased B chromosome frequency and absence of drive in the fish Prochiodus lineatus. Heredity 1997, 79, 473–476. [Google Scholar] [CrossRef]
- Gorlov, I.P.; Tsurusaki, N. Morphology and meiotic/mitotic behavior of B Chromosomes in a Japanese harvestman, Metagagrella tenuipes (Arachnida: Opiliones): No evidence for B accumulation mechanisms. Zool. Sci. 2000, 17, 349–355. [Google Scholar] [PubMed]
- Pearse, F.K.; Ehrlich, P.R. B Chromosome variation in Euphydryas colon (Lepidoptera: Nymphalidae). Chromosoma 1979, 73, 263–274. [Google Scholar] [CrossRef]
- Nur, U.; Brett, B.L.H. Genotypes suppressing meiotic drive in a B chromosome in the mealy bug, Pseudococcus obscurus. Genetics 1985, 110, 73–92. [Google Scholar] [PubMed]
- Hewitt, G.M. Meiotic drive for a B-chromosomes in the primary oocytes of Myrmeleotettix maculatus (Orthoptera: Acrididae). Chromosoma 1976, 56, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Nur, U. Maintenance of a “parasitic” B chromosome in the grasshopper Melanoplus femur-rubrum. Genetics 1977, 87, 499–512. [Google Scholar] [PubMed]
- Cano, M.I.; Santos, J.L. Cytological basis of the B chromosome accumulation mechanism in the grasshopper Heteracris littoralis (Ramb). Heredity 1989, 62, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.; Del Cerro, A.L.; Fernandez, A.; Diez, M. Meiotic behaviour of B chromosomes in the grasshopper Omocestus burri: A case of drive in females. Hereditas 1993, 118, 139–143. [Google Scholar] [CrossRef]
- Thompson, R.L.; Westerman, M.; Murray, D. B chromosomes in Rattus fuscipes I. Mitotic and meiotic chromosomes and the effects of B chromosomes on chiasma frequency. Heredity 1984, 52, 355–362. [Google Scholar] [CrossRef]
- Gregg, P.C.; Webb, G.C.; Adena, M.A. The dynamics of B chromosomes in populations of the Australian plague locust, Chortoicetes terminifera (Walker). Can. J. Genet. Cytol. 1984, 26, 194–208. [Google Scholar] [CrossRef]
- Nur, U.; Werren, J.H.; Eickbush, D.G.; Burke, W.D.; Eickbush, T.H. A “selfish” B chromosome that enhances its transmission by eliminating the paternal genome. Science 1988, 240, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Shaw, M.W.; Hewitt, G.M. The effect of temperature on meiotic transmission rates of the B chromosome of Myrmeleotettix maculatus (Orthoptea: Acrididiae). Heredity 1984, 53, 259–268. [Google Scholar] [CrossRef]
- Pardo, M.C.; Lopez-Leon, M.D.; Cabrerro, J.; Camacho, J.P.M. Transmission analysis of mitotically unstable B chromosomes in Locusta migratoria. Genome 1994, 37, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Cabrero, J.; Martin-Pecina, M.; Ruiz-Ruano, F.J.; Gomez, R.; Camacho, J.P.M. Post-meiotic B chromosome expulsion, during spermiogenesis, in two grasshopper species. Chromosoma 2017, 26, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Camacho, J.P.; Cabrero, J.; Lopez-Leon, M.D.; Shaw, M.W. Evolution of a near-neutral B chromosome. Chromosom. Today 1997, 12, 301–318. [Google Scholar]

| Author | Title | Reference |
|---|---|---|
| Jones, R.N. | B Chromosome Drive | [1] |
| Jones, R.N. et al. | B Chromosomes | [2] |
| Camacho, J.P.M. | B Chromosomes | [3] |
| Houben, A.; et al. | Biology and Evolution of B Chromosomes | [4] |
| Houben, A.; et al. | Evolution and Biology of Supernumerary B Chromosomes | [5] |
| Banaei-Moghaddam, A.M. et al. | Genes on B Chromosomes: Old Questions Revisited with New Tools | [6] |
| Valente, G.T. et al. | B Chromosomes: from Cytogenetics to Systems Biology | [7] |
| Houben, A. | B Chromosomes—A Matter of Chromosome Drive | [8] |
| Ruban, A. et al. | How Next-Generation Sequencing Has Aided Our Understanding of the Sequence Composition and Origin of B Chromosomes | [9] |
| Coan, R.L.B. et al. | Landscape of Transposable Elements Focusing on the B Chromosome of the Cichlid Fish Astatotilapia latifasciata | [10] |
| Nondisjunction at First Pollen Grain Mitosis |
| Aegilops speltoides, Alopecurus pratensis, Anthoxanthum aristatum, Brachycome lineariloba, Briza media. Dactylis glomerata, Deschampsia bottnica, Deschampsia caespitosa, Deschampsia wibeliana, Festuca arundinacea, Festuca pratensis, Haplopappus gracilis, Holcus lanatus, Phleum phleoides, [2] Panicum maximum [29] Aegilops mutica [30] |
| Pollen Grain Mitosis of Extra Divisions |
| Sorghum-purpureo-sericium [31] |
| Somatic Non-Disjunction in the Developing Inflorescences |
| Crepis capillaris [32] |
| Female Meiotic Drive |
| Lilium callosum [33], Phleum nodosum [34] Plantago serraria [35] Trillium grandiflorum [36] |
| Female Meiotic Drive and Male Meiotic Drag |
| Picea sitchensis [37] Hypochoeris maculata [38] |
| Male Drive |
| Haplopappus validus, Clarkia elegans, Iseilema laxum [2] Briza humilis BL [39] |
| Somatic Nondisjunction Coincident with Flower Initiation |
| Crepis capillaris [40] |
| No Apparent Mechanism |
| Allium schoenoprasum [41] Xanthisma texanum [42] Centauria scabiosa, Poa alpina, Ranunculus acris, Ranunculus ficaria [2]. |
| No Drive |
| Eyprepocnemis plorans (grasshopper) [43] Prochilodus lineatus (fish) [44] Metagagrella tenuipes (Arachnida, Japanese harvestman) [45] |
| Mechanism (?) to Boost B-number in Males |
| Euphydryas colon (Lepidoptera). [46] |
| Female Meiotic Drive |
| Pseudococcus obscurus (mealy bug) [47] Myrmeleotettix maculatus (grasshopper) [48] Melanoplus femur-rubrum (grasshopper) [49] Heteracris littoralis (grasshopper) [50] Omocestus burri (grasshopper) [51] |
| Male Drive |
| Rattus fuscipes (Australian bushrat) [52] |
| Male Meiotic Drive and the Opposite of Drive, i.e., Female Drag |
| Chortoicetes terminifera (Australian plague locust) [53] |
| PSR Enhances Transmission by Losing Paternal Chromosomes Except Itself |
| Nasonia vitripennis (parasitic wasp) [54] |
| Female Meiotic Drive and Male Meiotic Drag |
| Myrmeleotettix maculatus (grasshopper) [55] Locusta migratoria [56] |
| B Elimination during Spermiogenesis |
| Eumigus monticola, Eyprepocnemis plorans [57] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, R.N. Transmission and Drive Involving Parasitic B Chromosomes. Genes 2018, 9, 388. https://doi.org/10.3390/genes9080388
Jones RN. Transmission and Drive Involving Parasitic B Chromosomes. Genes. 2018; 9(8):388. https://doi.org/10.3390/genes9080388
Chicago/Turabian StyleJones, R.N. 2018. "Transmission and Drive Involving Parasitic B Chromosomes" Genes 9, no. 8: 388. https://doi.org/10.3390/genes9080388




