Optimization of Vibrating Mesh Nebulizer Air Inlet Structure for Pulmonary Drug Delivery
Abstract
:1. Introduction
2. Methods
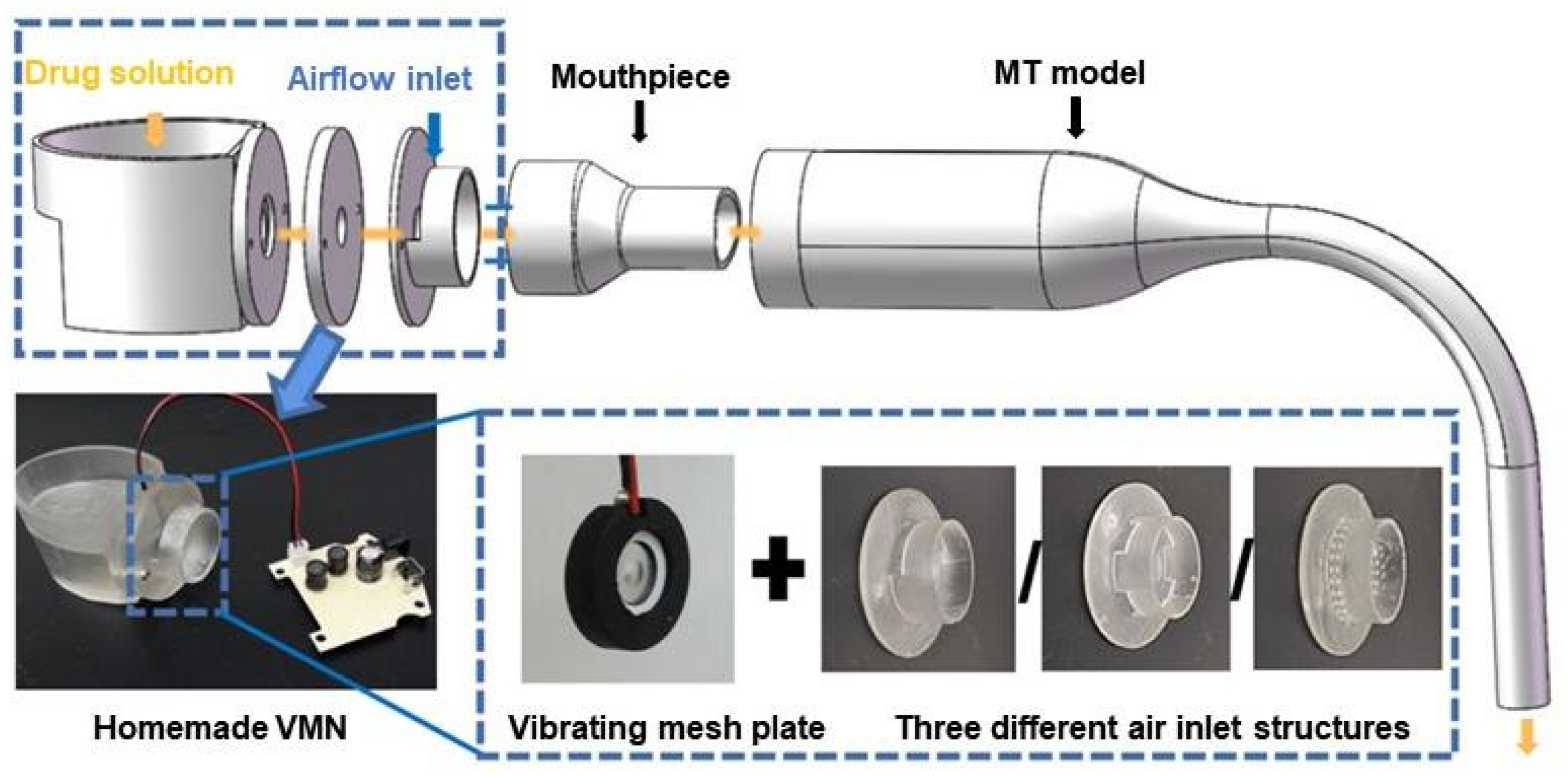
2.1. Experimental Setup
2.2. In Vitro Droplet Deposition Test
2.3. Numerical Methods
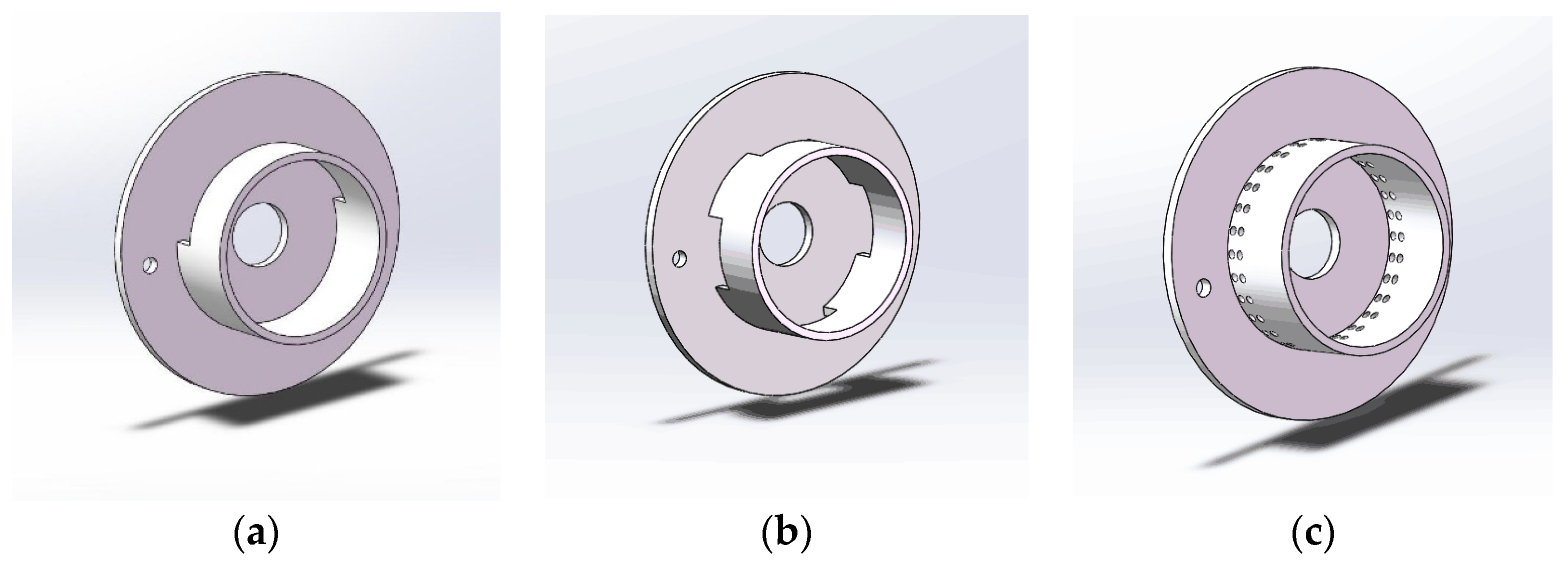
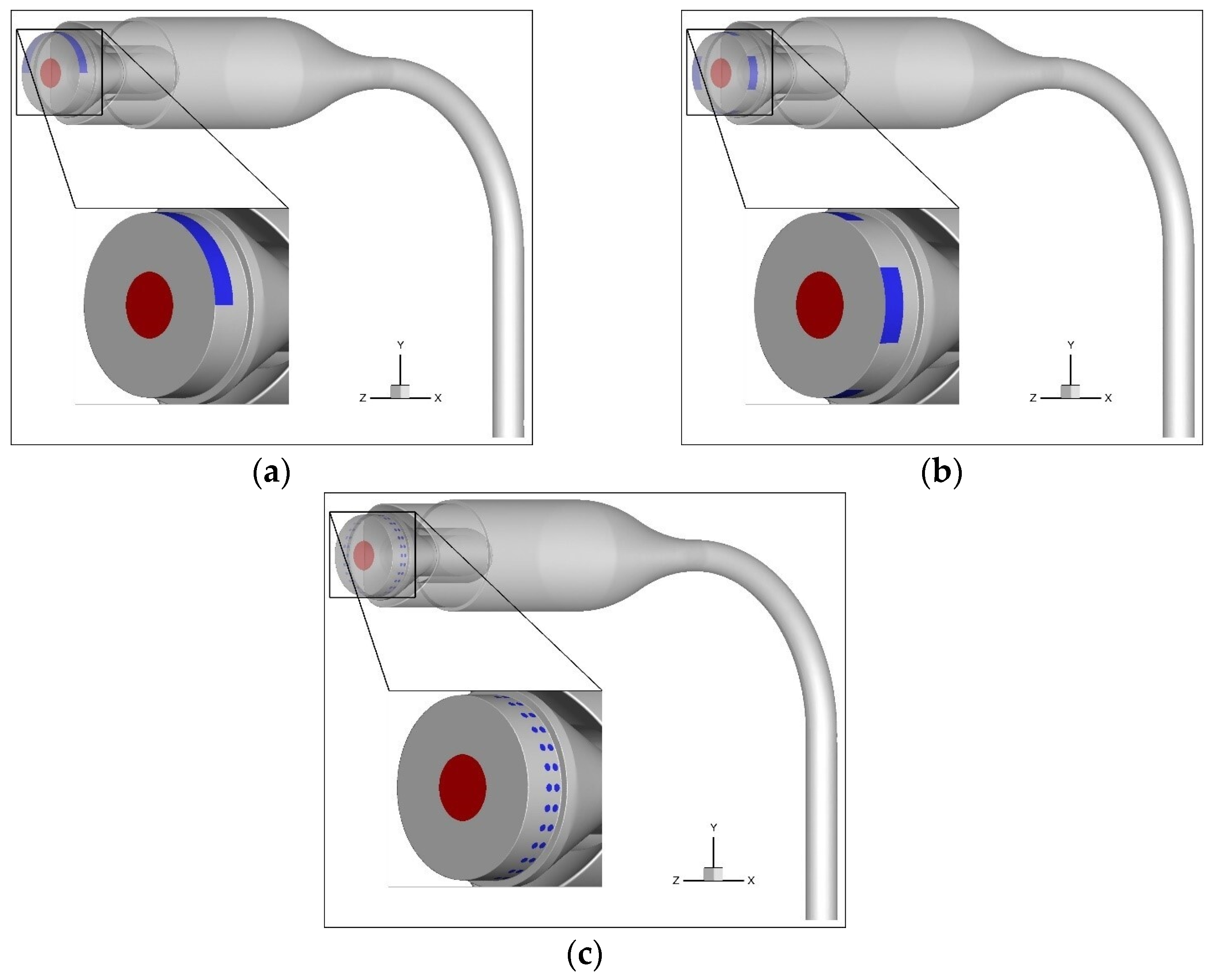
2.4. Geometries for Simulations
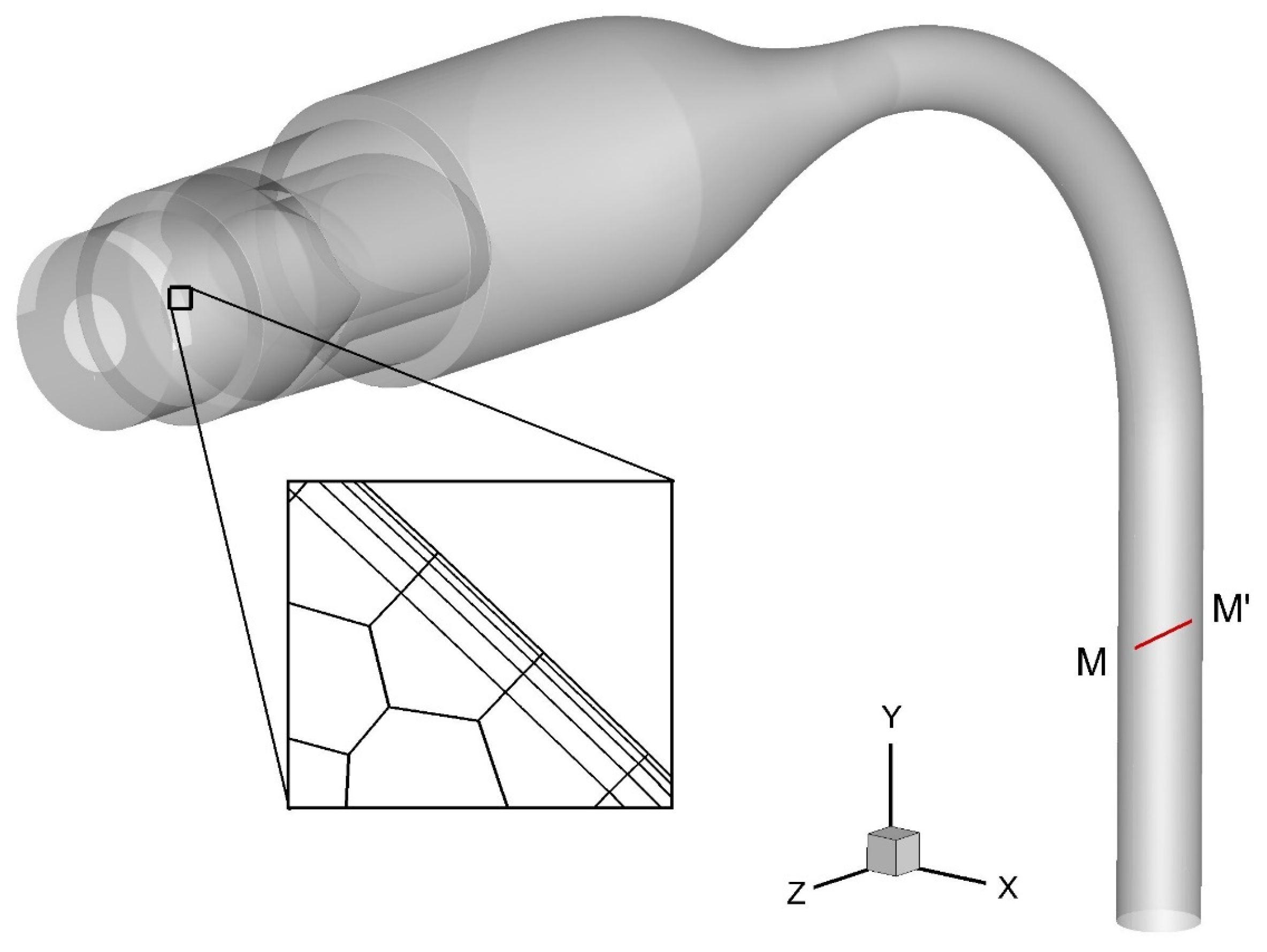
2.5. Simulation Setup
2.6. DF
3. Results and Discussion
3.1. Numerical Simulation of Flow Field Distribution
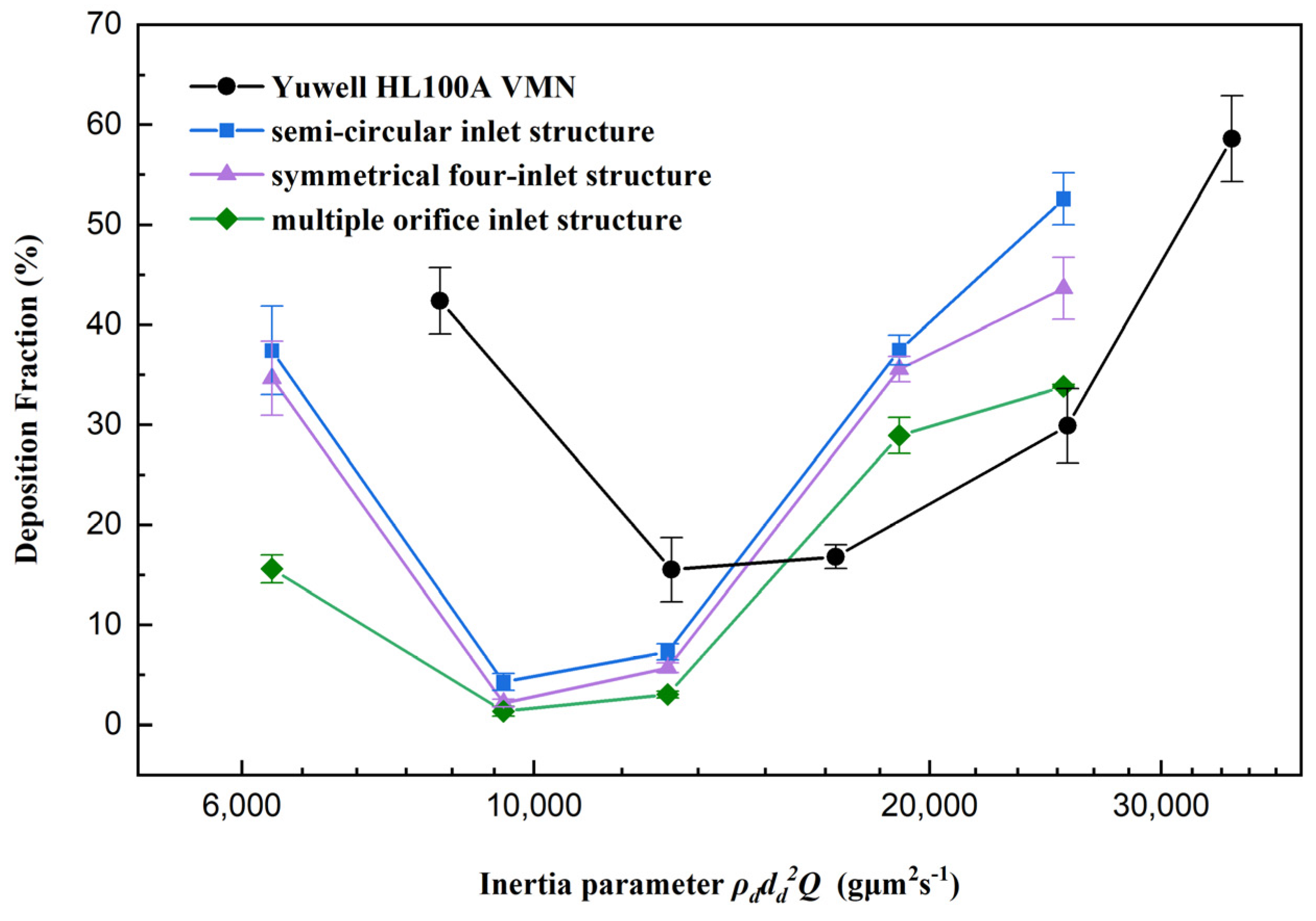
3.2. Experimental Study of DFs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Le Brun, P.P.H.; de Boer, A.H.; Frijlink, H.W.; Heijerman, H.G.M. A review of the technical aspects of drug nebulization. Pharm. World Sci. 2000, 22, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Dolovich, M.B.; Dhand, R. Aerosol drug delivery: Developments in device design and clinical use. Lancet 2011, 377, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Watts, A.B.; McConville, J.T.; Williams, R.O. Current Therapies and Technological Advances in Aqueous Aerosol Drug Delivery. Drug Dev. Ind. Pharm. 2008, 34, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Skaria, S.; Smaldone, G.C. Omron NE U22: Comparison Between Vibrating Mesh and Jet Nebulizer. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Elhissi, A.M.A.; Karnam, K.K.; Danesh-Azari, M.-R.; Gill, H.S.; Taylor, K.M.G. Formulations generated from ethanol-based proliposomes for delivery via medical nebulizers. J. Pharm. Pharmacol. 2006, 58, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.C.; McConville, J.T. The function and performance of aqueous aerosol devices for inhalation therapy. J. Pharm. Pharmacol. 2016, 68, 556–578. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahim, M.E.; Plant, P.K.; Chrystyn, H. The relative lung and systemic bioavailability of terbutaline following nebulisation in non-invasively ventilated patients. Int. J. Pharm. 2011, 420, 313–318. [Google Scholar] [CrossRef]
- ElHansy, M.H.E.; Boules, M.E.; Farid, H.; Chrystyn, H.; El-Maraghi, S.K.; Al-Kholy, M.B.; El-Essawy, A.F.M.; Abdelrahman, M.M.; Said, A.S.A.; Hussein, R.R.S.; et al. In vitro aerodynamic characteristics of aerosol delivered from different inhalation methods in mechanical ventilation. Pharm. Dev. Technol. 2017, 22, 844–849. [Google Scholar] [CrossRef]
- Pitance, L.; Vecellio, L.; Leal, T.; Reychler, G.; Reychler, H.; Liistro, G. Delivery efficacy of a vibrating mesh nebulizer and a jet nebulizer under different configurations. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 389–396. [Google Scholar] [CrossRef]
- Moody, G.B.; Luckett, P.M.; Shockley, C.M.; Huang, R.; Ari, A. Clinical Efficacy of Vibrating Mesh and Jet Nebulizers With Different Interfaces in Pediatric Subjects With Asthma. Respir. Care 2020, 65, 1451–1463. [Google Scholar] [CrossRef]
- Cheng, K.-H.; Cheng, Y.-S.; Yeh, H.-C.; Swift, D.L. An Experimental Method for Measuring Aerosol Deposition Efficiency in the Human Oral Airway. Am. Ind. Hyg. Assoc. J. 1997, 58, 207–213. [Google Scholar] [CrossRef]
- Lin, J.; Fan, J.R.; Zheng, Y.Q.; Hu, G.L.; Pan, D. Numerical simulation of inhaled aerosol particle deposition within 3D realistic human upper respiratory tract. AIP Conf. Proc. 2010, 1207, 992–997. [Google Scholar]
- Chen, X.; Feng, Y.; Zhong, W.; Sun, B.; Tao, F. Numerical investigation of particle deposition in a triple bifurcation airway due to gravitational sedimentation and inertial impaction. Powder Technol. 2018, 323, 284–293. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Zhang, Z. Airflow and Particle Transport in the Human Respiratory System. Annu. Rev. Fluid Mech. 2010, 42, 301–334. [Google Scholar] [CrossRef]
- Feng, Y.; Kleinstreuer, C.; Castro, N.; Rostami, A. Computational transport, phase change and deposition analysis of inhaled multicomponent droplet–vapor mixtures in an idealized human upper lung model. J. Aerosol Sci. 2016, 96, 96–123. [Google Scholar] [CrossRef]
- Golshahi, L.; Noga, M.L.; Finlay, W.H. Deposition of inhaled micrometer-sized particles in oropharyngeal airway replicas of children at constant flow rates. J. Aerosol Sci. 2012, 49, 21–31. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, Y.; Zhang, L.; Zhu, J.; Jin, F. The development of a novel dry powder inhaler. Int. J. Pharm. 2012, 431, 45–52. [Google Scholar] [CrossRef]
- Kakade, P.P.; Versteeg, H.K.; Hargrave, G.K.; Genova, P.; Williams Iii, R.C.; Deaton, D. Design optimization of a novel pMDI actuator for systemic drug delivery. J. Aerosol Med. 2007, 20, 460–474. [Google Scholar] [CrossRef]
- Gong, J.-S.; Fu, W.-B. The experimental study on the flow characteristics for a swirling gas–liquid spray atomizer. Appl. Therm. Eng. 2007, 27, 2886–2892. [Google Scholar] [CrossRef]
- Selvam, P.; McNair, D.; Truman, R.; Smyth, H.D.C. A novel dry powder inhaler: Effect of device design on dispersion performance. Int. J. Pharm. 2010, 401, 1–6. [Google Scholar] [CrossRef]
- Hu, J.; Chen, X.; Li, S.; Zheng, X.; Zhang, R.; Tan, W. Comparison of the performance of inhalation nebulizer solution and suspension delivered with active and passive vibrating-mesh device. J. Drug Deliv. Sci. Technol. 2020, 55, 101353. [Google Scholar] [CrossRef]
- Howe, C.; Momin, M.A.M.; Farkas, D.R.; Bonasera, S.; Hindle, M.; Longest, P.W. Advancement of the Infant Air-Jet Dry Powder Inhaler (DPI): Evaluation of Different Positive-Pressure Air Sources and Flow Rates. Pharm. Res. 2021, 38, 1615–1632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cui, Y.; Liang, R.; Wang, G.; Yue, X.; Zhao, Z.; Huang, Z.; Huang, Y.; Geng, J.; Pan, X.; et al. Novel approach for real-time monitoring of carrier-based DPIs delivery process via pulmonary route based on modular modified Sympatec HELOS. Acta Pharm. Sin. B 2020, 10, 1331–1346. [Google Scholar] [CrossRef]
- Talaat, M.; Si, X.; Liu, X.; Xi, J. Count- and mass-based dosimetry of MDI spray droplets with polydisperse and monodisperse size distributions. Int. J. Pharm. 2022, 623, 121920. [Google Scholar] [CrossRef]
- Kleinstreuer, C.; Shi, H.; Zhang, Z. Computational analyses of a pressurized metered dose inhaler and a new drug-aerosol targeting methodology. J. Aerosol Med. 2007, 20, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Si, X.A.; Talaat, M.; Xi, J. Effects of guiding vanes and orifice jet flow of a metered-dose inhaler on drug dosimetry in human respiratory tract. Exp. Comput. Multiph. Flow 2023, 5, 247–261. [Google Scholar] [CrossRef]
- Leung, C.M.S.; Tong, Z.; Zhou, Q.; Chan, J.G.Y.; Tang, P.; Sun, S.; Yang, R.; Chan, H.-K. Understanding the Different Effects of Inhaler Design on the Aerosol Performance of Drug-Only and Carrier-Based DPI Formulations. Part 1: Grid Structure. AAPS J. 2016, 18, 1159–1167. [Google Scholar] [CrossRef]
- Fossat, P.; Ichchou, M.; Bareille, O. Analytical model of the dynamic behavior of a vibrating mesh nebulizer for optimal atomization efficiency. Sens. Actuators A Phys. 2022, 343, 113646. [Google Scholar] [CrossRef]
- Xi, J.; Yang, T.; Talaat, K.; Wen, T.; Zhang, Y.; Klozik, S.; Peters, S. Visualization of local deposition of nebulized aerosols in a human upper respiratory tract model. J. Vis. 2018, 21, 225–237. [Google Scholar] [CrossRef]
- Zhang, Y.; Finlay, W.H.; Matida, E.A. Particle deposition measurements and numerical simulation in a highly idealized mouth-throat. J. Aerosol Sci. 2004, 35, 789–803. [Google Scholar] [CrossRef]
- Xia, X.Y.; Ding, T.; Chen, X.L.; Tao, F.; Sun, B.B.; Lu, T.; Wang, J.W.; Huang, Y.; Xu, Y. Evaporation Affects the In Vitro Deposition of Nebulized Droplet in an Idealized Mouth-Throat Model. Atmosphere 2023, 14, 93. [Google Scholar] [CrossRef]
- Yang, H.Z.; Wang, Y.; Chen, X.L.; Sun, B.B.; Tao, F.; Xie, X.J.; Zhang, Y. The effects of temperature and humidity on the deposition of nebulized droplet in an idealized mouth-throat model. Flow Meas. Instrum. 2023, 91, 102359. [Google Scholar] [CrossRef]
- Menter, F.R.; Langtry, R.; Volker, S. Transition modelling for general purpose CFD codes. Flow Turbul. Combust. 2006, 77, 277–303. [Google Scholar] [CrossRef]
- Zhang, Z.; Kleinstreuer, C. Laminar-to-turbulent fluid-nanoparticle dynamics simulations: Model comparisons and nanoparticle-deposition applications. Int. J. Numer. Methods Biomed. Eng. 2011, 27, 1930–1950. [Google Scholar] [CrossRef]
- Menter, F.R.; Langtry, R.B.; Likki, S.R.; Suzen, Y.B.; Huang, P.G.; Volker, S. A correlation-based transition model using local variables—Part I: Model formulation. J. Turbomach.—Trans. Asme 2006, 128, 413–422. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, X.; Feng, Y.; Jia, Z.; Zhang, J.; Xie, X.; Zhang, Y. A novel experimental approach to measure nebulized droplet deposition pattern and deposition fraction in an idealized mouth-to-throat model. Phys. Fluids 2023, 35, 083322. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, X.; Li, Z.; Yang, H.; Wang, J. Optimization of Vibrating Mesh Nebulizer Air Inlet Structure for Pulmonary Drug Delivery. Atmosphere 2023, 14, 1509. https://doi.org/10.3390/atmos14101509
Liu Y, Chen X, Li Z, Yang H, Wang J. Optimization of Vibrating Mesh Nebulizer Air Inlet Structure for Pulmonary Drug Delivery. Atmosphere. 2023; 14(10):1509. https://doi.org/10.3390/atmos14101509
Chicago/Turabian StyleLiu, Yu, Xiaole Chen, Zhengqi Li, Huizhen Yang, and Jianwei Wang. 2023. "Optimization of Vibrating Mesh Nebulizer Air Inlet Structure for Pulmonary Drug Delivery" Atmosphere 14, no. 10: 1509. https://doi.org/10.3390/atmos14101509




