CCN Properties of Organic Aerosol Collected Below and within Marine Stratocumulus Clouds near Monterey, California
Abstract
:1. Introduction
2. Experimental Methods
2.1. Aerosol Sampling and Chemical Composition
| Property | Cloud Residuals (CR) | Sub-Cloud (SC) |
|---|---|---|
| WSOC | 220 ± 14 | 202 ± 7 |
| Ca2+ | 3.10 | 2.75 |
| Mg2+ | 0.07 | 0.58 |
| Na+ | 21.07 | 20.90 |
| Cl− | 25.30 | 22.61 |
| NH4+ | 21.00 | 36.38 |
| NO3− | 16.12 | 17.30 |
| SO42− | 35.30 | 87.85 |
| Oxalate | 3.61 | 4.22 |
| α | 8.99 × 10−4 | 2.91 × 10−3 |
| β | 3.84 × 10−2 | 1.63 × 10−2 |

2.2. CCN Activity of Soluble Material Collected

2.3. Surface Tension Measurements
2.4. Droplet Size Measurements of Activated CCN
3. Analytical Theory
3.1. Köhler Theory
3.2. Inferring Surface Tension
4. Results and Discussion
4.1. Surface Tension

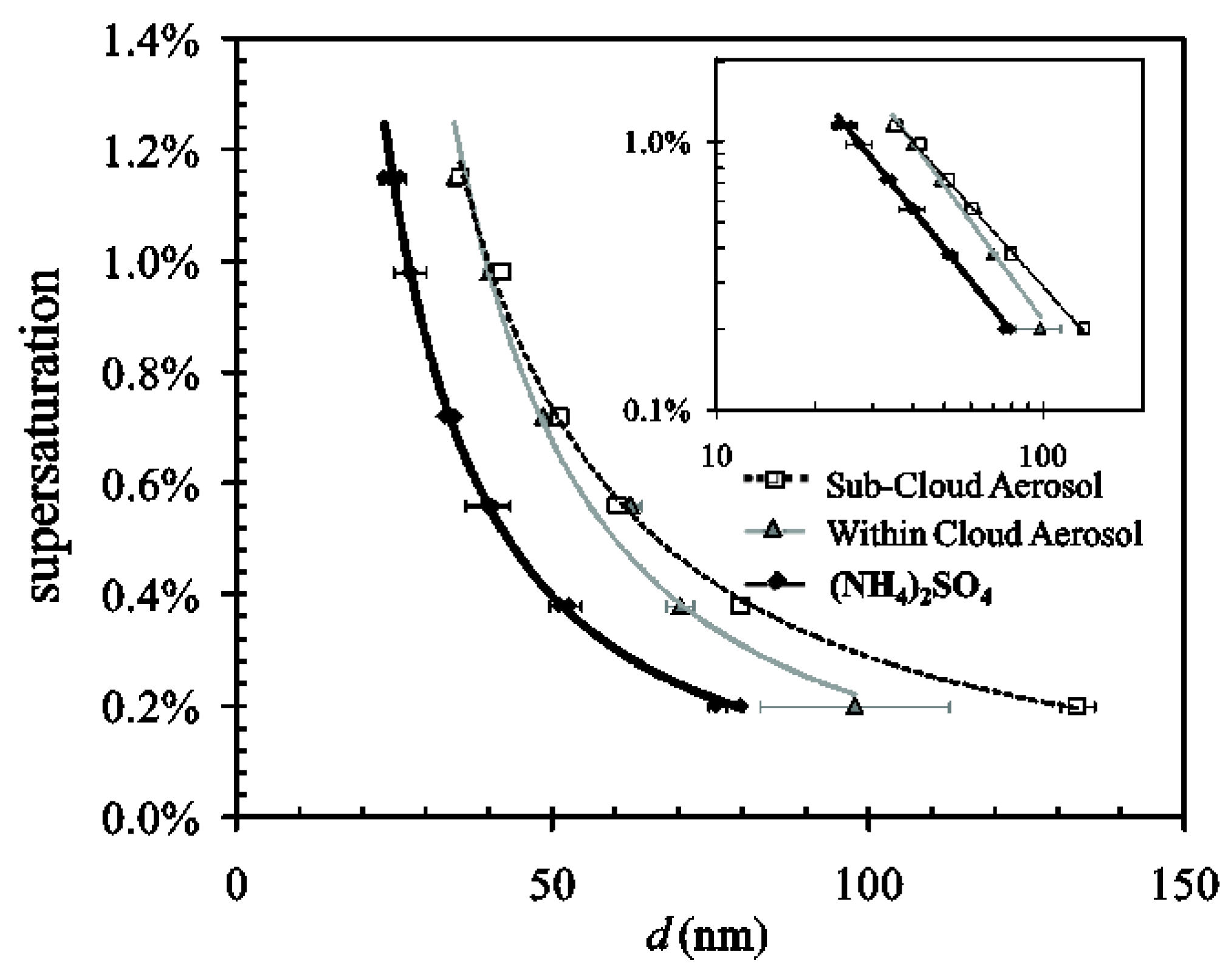
4.2. CCN Activity

4.3. Inferred Molar Volumes and Uncertainties
| Cloud Residuals (CR) | Sub-Cloud (SC) | |
|---|---|---|
| Organic | 88.3 | 82 |
| NH4 | 3.4 | 3.6 |
| NaCl | 0.6 | 0 |
| (NH4)2SO4 | 0.0 | 5.1 |
| NaNO3 | 2.3 | 0.0 |
| NH4NO3 | 0.0 | 2.4 |
| Na2SO4 | 4.1 | 7.0 |
| CaSO4 | 1.1 | 0.0 |
| Property x (units) | Average Value of x | Uncertainty Δx | Sensitivity, Φx (m3·mol−1·x−1) | Contribution (%) |
|---|---|---|---|---|
| σ (N·m−1) | 6.89 × 10−2 | 1.38 × 10−3 | 3.83 × 10−3 | 5.72 |
| ω (m1.5) | 6.80 × 10−2 | 5.53 × 10−15 | 2.59 × 109 | 12.42 |
| 2 | 0.5 | 6.43 × 10−6 | 3.49 | |
| 2 | 0.5 | 1.54 × 10−6 | 0.83 | |
| 2 | 0.5 | 4.18 × 10−6 | 2.27 | |
| 3 | - | - | - | |
| 1 | 0.20 | 4.39 × 10−5 | 9.52 | |
| 0.05 | - | - | - | |
| 0.07 | - | - | - | |
| 0.10 | 1.95 × 10−3 | 8.50 × 10−4 | 0.80 | |
| 0.88 | 6.36 × 10−3 | 4.10 × 10−3 | 2.82 | |
| 1.53 | - | - | - | |
| 2.16 | - | - | - | |
| 2.3 | - | - | - | |
| 2.68 | - | - | - | |
| 1.4 | - | - | - | |
| 1.59 | - | - | - | |
| (cm3·mol−1) | 1.60 × 10−4 | 17.5% | - | - |
| (g·mol−1) | 143 | 25 | - | - |
| Property x (units) | Average Value of x | Uncertainty Δx | Sensitivity, Φx (m3·mol−1·x−1) | Contribution (%) |
|---|---|---|---|---|
| σ (N·m−1) | 6.58 × 10−2 | 1.32 × 10−3 | 1.97 × 10−3 | 8.37 |
| ω (m1.5) | 8.75 × 10−14 | 4.43 × 10−15 | 9.89 × 109 | 14.13 |
| 2 | 0.5 | 6.35 × 10−5 | 10.24 | |
| 2.5 | 0.5 | 3.74 × 10−6 | 0.60 | |
| 2 | 0.5 | 2.82 × 10−6 | 0.45 | |
| 3 | - | - | - | |
| 1 | 0.20 | 7.21 × 10−5 | 4.65 | |
| 0.06 | - | - | - | |
| 0.10 | - | - | - | |
| 0.09 | 1.95 × 10−3 | 4.21 × 10−3 | 2.64 | |
| 0.12 | 2.03 × 10−3 | 4.10 × 10−3 | 2.69 | |
| 0.82 | 6.36 × 10−3 | 4.63 × 10−3 | 9.50 | |
| 1.53 | - | - | - | |
| 1.77 | - | - | - | |
| 1.73 | - | - | - | |
| 2.68 | - | - | - | |
| (cm3·mol−1) | 1.72 × 10−3 | 22.2% | - | - |
| (g·mol−1) | 2413 | 536 | - | - |
4.4. The Effect of Organics on Droplet Growth Kinetics

5. Summary and Implications
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saxena, P.; Hildemann, L.M.; McMurry, P.H.; Seinfeld, J.H. Organics alter hygroscopic behavior of atmospheric particles. J. Geophys. Res. 1995, 100, 18755–18770. [Google Scholar] [CrossRef]
- Facchini, M.C.; Fuzzi, S.; Zappoli, S.; Andracchio, A.; Gelencsér, A.; Kiss, G.; Krivácsy, Z.; Mészáros, E.; Hansson, H.-C.; Alsberg, T.; et al. Partitioning of the organic aerosol component between fog droplets and interstitial air. J. Geophys. Res. 1999, 104, 26821–26832. [Google Scholar] [CrossRef]
- Nenes, A.; Charlson, R.J.; Facchini, M.C.; Kulmala, M.; Laaksonen, A.; Seinfeld, J.H. Can chemical effects on cloud droplet number rival the first indirect effect? Geophys. Res. Lett. 2002, 29, 29–21. [Google Scholar]
- Decesari, S.; Facchini, M.C.; Mircea, M.; Cavalli, F.; Fuzzi, S. Solubility properties of surfactants in atmospheric aerosol and cloud/fog water samples. J. Geophys. Res. 2003, 108, 1984–2012. [Google Scholar]
- Ervens, B.; Feingold, G.; Clegg, S.L.; Kreidenweis, S.M. A modeling study of aqueous production of dicarboxylic acids: 2. Implications for cloud microphysics. J. Geophys. Res. 2004, 109, D15206. [Google Scholar] [CrossRef]
- Saxena, P.; Hildemann, L.M. Water-soluble organics in atmospheric particles: A critical review of the literature and application of thermodynamics to identify candidate compounds. J. Atmos. Chem. 1996, 24, 57–109. [Google Scholar] [CrossRef]
- Mircea, M.; Facchini, M.C.; Decesari, S.; Fuzzi, S.; Charlson, R.J. The influence of the organic aerosol component on CCN supersaturation spectra for different aerosol types. Tellus. B Chem. Phys. Meteorol. 2002, 54, 74–81. [Google Scholar] [CrossRef]
- Alfonso, L.; Raga, G.B. The influence of organic compounds on the development of precipitation acidity in maritime clouds. Atmos. Chem. Phys. 2004, 4, 1097–1111. [Google Scholar] [CrossRef]
- O’Dowd, C.D.; Facchini, M.C.; Cavalli, F.; Ceburnis, D.; Mircea, M.; Decesari, S.; Fuzzi, S.; Yoon, Y.J.; Putaud, J.-P. Biogenically driven organic contribution to marine aerosol. Nature 2004, 431, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Ishizaka, Y.; Peng, D. Numerical Study on Impacts of Multi-Component Aerosols on Marine Cloud Microphysical Properties. J. Meteorol. Soc. Jpn. Ser. II 2005, 83, 977–986. [Google Scholar] [CrossRef]
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J.; et al. Organic aerosol and global climate modelling: A review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Ceburnis, D.; Cavalli, F.; Jourdan, O.; Putaud, J.P.; Facchini, M.C.; Decesari, S.; Fuzzi, S.; Sellegri, K.; Jennings, S.G.; et al. Seasonal characteristics of the physicochemical properties of North Atlantic marine atmospheric aerosols. J. Geophys. Res. 2007, 112, D04206. [Google Scholar] [CrossRef]
- Ovadnevaite, J.; Ceburnis, D.; Martucci, G.; Bialek, J.; Monahan, C.; Rinaldi, M.; Facchini, M.C.; Berresheim, H.; Worsnop, D.R.; O’Dowd, C. Primary marine organic aerosol: A dichotomy of low hygroscopicity and high CCN activity. Geophys. Res. Lett. 2011, 38, L21806. [Google Scholar] [CrossRef]
- Moore, R.H.; Raatikainen, T.; Langridge, J.M.; Bahreini, R.; Brock, C.A.; Holloway, J.S.; Lack, D.A.; Middlebrook, A.M.; Perring, A.E.; Schwarz, J.P.; et al. CCN spectra, hygroscopicity, and droplet activation kinetics of secondary organic aerosol resulting from the 2010 Deepwater Horizon oil spill. Environ. Sci. Technol. 2012, 46, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Sorooshian, A.; Lu, M.-L.; Brechtel, F.J.; Jonsson, H.; Feingold, G.; Flagan, R.C.; Seinfeld, J.H. On the source of organic acid aerosol layers above clouds. Environ. Sci. Technol. 2007, 41, 4647–4654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceburnis, D.; O’Dowd, C.D.; Jennings, G.S.; Facchini, M.C.; Emblico, L.; Decesari, S.; Fuzzi, S.; Sakalys, J. Marine aerosol chemistry gradients: Elucidating primary and secondary processes and fluxes. Geophys. Res. Lett. 2008, 35, L07804. [Google Scholar] [CrossRef]
- Rinaldi, M.; Decesari, S.; Finessi, E.; Giulianelli, L.; Carbone, C.; Fuzzi, S.; O’Dowd, C.D.; Ceburnis, D.; Facchini, M.C. Primary and secondary organic marine aerosol and oceanic biological activity: Recent results and new perspectives for future studies. Adv. Meteorol. 2010, 2010, 1–10. [Google Scholar] [CrossRef]
- Coggon, M.M.; Sorooshian, A.; Wang, Z.; Metcalf, A.R.; Frossard, A.A.; Lin, J.J.; Craven, J.S.; Nenes, A.; Jonsson, H.H.; Russell, L.M.; et al. Ship impacts on the marine atmosphere: Insights into the contribution of shipping emissions to the properties of marine aerosol and clouds. Atmos. Chem. Phys. 2012, 12, 8439–8458. [Google Scholar] [CrossRef]
- Sorooshian, A.; Wang, Z.; Coggon, M.M.; Jonsson, H.H.; Ervens, B. Observations of sharp oxalate reductions in stratocumulus clouds at variable altitudes: Organic acid and metal measurements during the 2011 E-PEACE campaign. Environ. Sci. Technol. 2013, 47, 7747–7756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorooshian, A.; Varutbangkul, V.; Brechtel, F.J.; Ervens, B.; Feingold, G.; Bahreini, R.; Murphy, S.M.; Holloway, J.S.; Atlas, E.L.; Buzorius, G.; et al. Oxalic acid in clear and cloudy atmospheres: Analysis of data from International Consortium for Atmospheric Research on Transport and Transformation 2004. J. Geophys. Res. D: Atmos. 2006, 111, D10S27. [Google Scholar] [CrossRef]
- Ervens, B.; Feingold, G.; Frost, G.J.; Kreidenweis, S.M. A modeling study of aqueous production of dicarboxylic acids: 1. Chemical pathways and speciated organic mass production. J. Geophys. Res. 2004, 109, D15205. [Google Scholar] [CrossRef]
- Lim, H.; Carlton, A.G.; Turpin, B.J. Isoprene forms secondary organic aerosol in Atlanta: Results from time-resolved measurements during the Atlanta supersite experiment. Environ. Sci. Technol. 2005, 39, 4441–4446. [Google Scholar] [CrossRef] [PubMed]
- Carlton, A.G.; Turpin, B.J.; Lim, H.-J.; Altieri, K.E.; Seitzinger, S. Link between isoprene and secondary organic aerosol (SOA): Pyruvic acid oxidation yields low volatility organic acids in clouds. Geophys. Res. Lett. 2006, 33, L06822. [Google Scholar] [CrossRef]
- Coggon, M.M.; Sorooshian, A.; Wang, Z.; Craven, J.S.; Metcalf, A.R.; Lin, J.J.; Nenes, A.; Jonsson, H.H.; Flagan, R.C.; Seinfeld, J.H. Observations of continental biogenic impacts on marine aerosol and clouds off the coast of California. J. Geophys. Res. D: Atmos. 2014, 119, 6724–6748. [Google Scholar] [CrossRef]
- Eyring, V.; Köhler, H.W.; van Aardenne, J.; Lauer, A. Emissions from international shipping: 1. The last 50 years. J. Geophys. Res. 2005, 110, D17305. [Google Scholar] [CrossRef]
- Zheng, Z.; Tang, X.; Asa-Awuku, A.; Jung, H.S. Characterization of a method for aerosol generation from heavy fuel oil (HFO) as an alternative to emissions from ship diesel engines. J. Aerosol Sci. 2010, 41, 1143–1151. [Google Scholar] [CrossRef]
- Russell, L.M.; Noone, K.J.; Ferek, R.J.; Pockalny, R.A.; Flagan, R.C.; Seinfeld, J.H. Combustion Organic Aerosol as Cloud Condensation Nuclei in Ship Tracks. J. Atmos. Sci. 2000, 57, 2591–2606. [Google Scholar] [CrossRef]
- Twomey, S. Pollution and the planetary albedo. Atmos. Environ. 1974, 8, 1251–1256. [Google Scholar] [CrossRef]
- Coakley, J.A.; Bernstein, R.L.; Durkee, P.A. Effect of ship-stack effluents on cloud reflectivity. Science 1987, 237, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, B.A. Aerosols, cloud microphysics, and fractional cloudiness. Science 1989, 245, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, A.S.; Toon, O.B.; Taylor, J.P.; Johnson, D.W.; Hobbs, P.V.; Ferek, R.J. Effects of aerosols on cloud albedo: Evaluation of Twomey’s parameterization of cloud susceptibility using measurements of ship tracks. J. Atmos. Sci. 2000, 57, 2684–2695. [Google Scholar] [CrossRef]
- Moore, R.H.; Cerully, K.; Bahreini, R.; Brock, C.A.; Middlebrook, A.M.; Nenes, A. Hygroscopicity and composition of California CCN during summer 2010. J. Geophys. Res. 2012, 117, D00V12. [Google Scholar] [CrossRef]
- Padró, L.T.; Asa-Awuku, A.; Morrison, R.; Nenes, A. Inferring thermodynamic properties from CCN activation experiments: Single-component and binary aerosols. Atmos. Chem. Phys. 2007, 7, 5263–5274. [Google Scholar] [CrossRef]
- Moore, R.H.; Ingall, E.D.; Sorooshian, A.; Nenes, A. Molar mass, surface tension, and droplet growth kinetics of marine organics from measurements of CCN activity. Geophys. Res. Lett. 2008, 35, L07801. [Google Scholar] [CrossRef]
- Asa-Awuku, A.; Nenes, A.; Gao, S.; Flagan, R.C.; Seinfeld, J.H. Water-soluble SOA from Alkene ozonolysis: Composition and droplet activation kinetics inferences from analysis of CCN activity. Atmos. Chem. Phys. 2010, 10, 1585–1597. [Google Scholar] [CrossRef]
- Lu, M.-L.; Conant, W.C.; Jonsson, H.H.; Varutbangkul, V.; Flagan, R.C.; Seinfeld, J.H. The Marine Stratus/Stratocumulus Experiment (MASE): Aerosol-cloud relationships in marine stratocumulus. J. Geophys. Res. D: Atmos. 2007, 112, D10209. [Google Scholar] [CrossRef]
- Marple, V.A.; Chien, C.M. Virtual impactors: A theoretical study. Environ. Sci. Technol. 1980, 14, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Noone, K.J.; Ogren, J.A.; Heintzenberg, J.; Charlson, R.J.; Covert, D.S. Design and calibration of a counterflow virtual impactor for sampling of atmospheric fog and cloud droplets. Aerosol Sci. Technol. 1988, 8, 235–244. [Google Scholar] [CrossRef]
- Roberts, G.C.; Nenes, A. A continuous-flow streamwise thermal-gradient CCN chamber for atmospheric measurements. Aerosol Sci. Technol. 2005, 39, 206–221. [Google Scholar] [CrossRef]
- Lance, S.; Nenes, A.; Medina, J.; Smith, J.N. Mapping the operation of the DMT continuous flow CCN counter. Aerosol Sci. Technol. 2006, 40, 242–254. [Google Scholar] [CrossRef]
- Rose, D.; Gunthe, S.S.; Mikhailov, E.; Frank, G.P.; Dusek, U.; Andreae, M.O.; Pöschl, U. Calibration and measurement uncertainties of a continuous-flow cloud condensation nuclei counter (DMT-CCNC): CCN activation of ammonium sulfate and sodium chloride aerosol particles in theory and experiment. Atmos. Chem. Phys. 2008, 8, 1153–1179. [Google Scholar] [CrossRef]
- Moore, R.H.; Nenes, A.; Medina, J. Scanning mobility CCN analysis—A method for fast measurements of size-resolved CCN distributions and activation kinetics. Aerosol Sci. Technol. 2010, 44, 861–871. [Google Scholar] [CrossRef]
- Asa-Awuku, A.; Sullivan, A.P.; Hennigan, C.J.; Weber, R.J.; Nenes, A. Investigation of molar volume and surfactant characteristics of water-soluble organic compounds in biomass burning aerosol. Atmos. Chem. Phys. 2008, 8, 799–812. [Google Scholar] [CrossRef]
- Brechtel, F.J.; Kreidenweis, S.M. Predicting particle critical supersaturation from hygroscopic growth measurements in the humidified TDMA. Part I: Theory and sensitivity studies. J. Atmos. Sci. 2000, 57, 1854–1871. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. II. liquids.1. J. Am. Chem. Soc. 1917, 39, 1848–1906. [Google Scholar] [CrossRef]
- Taraniuk, I.; Graber, E.R.; Kostinski, A.; Rudich, Y. Surfactant properties of atmospheric and model humic-like substances (HULIS). Geophys. Res. Lett. 2007, 34, L16807. [Google Scholar] [CrossRef]
- Giordano, M.R.; Short, D.Z.; Hosseini, S.; Lichtenberg, W.; Asa-Awuku, A.A. Changes in droplet surface tension affect the observed hygroscopicity of photochemically aged biomass burning aerosol. Environ. Sci. Technol. 2013, 47, 10980–10986. [Google Scholar] [CrossRef] [PubMed]
- Lance, S.; Nenes, A.; Mazzoleni, C.; Dubey, M.K.; Gates, H.; Varutbangkul, V.; Rissman, T.A.; Murphy, S.M.; Sorooshian, A.; Flagan, R.C.; et al. Cloud condensation nuclei activity, closure, and droplet growth kinetics of Houston aerosol during the Gulf of Mexico Atmospheric Composition and Climate Study (GoMACCS). J. Geophys. Res. D: Atmos. 2009, 114, D00F15. [Google Scholar] [CrossRef]
- Asa-Awuku, A.; Moore, R.H.; Nenes, A.; Bahreini, R.; Holloway, J.S.; Brock, C.A.; Middlebrook, A.M.; Ryerson, T.B.; Jimenez, J.L.; DeCarlo, P.F.; et al. Airborne cloud condensation nuclei measurements during the 2006 Texas Air Quality Study. J. Geophys. Res. D: Atmos. 2011, 116, D11201. [Google Scholar] [CrossRef]
- Raatikainen, T.; Moore, R.H.; Lathem, T.L.; Nenes, A. A coupled observation—modeling approach for studying activation kinetics from measurements of CCN activity. Atmos. Chem. Phys. 2012, 12, 4227–4243. [Google Scholar] [CrossRef]
- Raatikainen, T.; Nenes, A.; Seinfeld, J.H.; Morales, R.; Moore, R.H.; Lathem, T.L.; Lance, S.; Padró, L.T.; Lin, J.J.; Cerully, K.M.; et al. Worldwide data sets constrain the water vapor uptake coefficient in cloud formation. Proc. Natl. Acad. Sci. USA 2013, 110, 3760–3764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelhart, G.J.; Asa-Awuku, A.; Nenes, A.; Pandis, S.N. CCN activity and droplet growth kinetics of fresh and aged monoterpene secondary organic aerosol. Atmos. Chem. Phys. 2008, 8, 3937–3949. [Google Scholar] [CrossRef]
- Lathem, T.L.; Nenes, A. Water vapor depletion in the DMT continuous-flow CCN chamber: Effects on supersaturation and droplet growth. Aerosol Sci. Technol. 2011, 45, 604–615. [Google Scholar] [CrossRef]
- Cavalli, F.; Facchini, M.C.; Decesari, S.; Mircea, M.; Emblico, L.; Fuzzi, S.; Ceburnis, D.; Yoon, Y.J.; O’Dowd, C.D.; Putaud, J.-P.; et al. Advances in characterization of size-resolved organic matter in marine aerosol over the North Atlantic. J. Geophys. Res. D: Atmos. 2004, 109, D24215. [Google Scholar] [CrossRef]
- Kiss, G.; Tombácz, E.; Hansson, H.-C. Surface tension effects of humic-like substances in the aqueous extract of tropospheric fine aerosol. J. Atmos. Chem. 2005, 50, 279–294. [Google Scholar] [CrossRef]
- Hallberg, A.; Ogren, J.A.; Noone, K.J.; Okada, K.; Heintzenberg, J.; Svenningsson, I.B. The Influence of Aerosol Particle Composition on Cloud Droplet Formation. In The Kleiner Feldberg Cloud Experiment 1990; Springer Netherlands: Houten, the Netherlands, 1994; pp. 153–171. [Google Scholar]
- Fountoukis, C.; Nenes, A. ISORROPIA II: A computationally efficient thermodynamic equilibrium model for K+-Ca2+-Mg2+-NH4+-Na+-SO42−-NO3−-Cl−-H2O aerosols. Atmos. Chem. Phys. 2007, 7, 4639–4659. [Google Scholar] [CrossRef]
- Turpin, B.J.; Lim, H.-J. Species contributions to PM2.5 mass concentrations: Revisiting common assumptions for estimating organic MASS. Aerosol Sci. Technol. 2001, 35, 602–610. [Google Scholar] [CrossRef]
- Oppo, C.; Bellandi, S.; Degli Innocenti, N.; Stortini, A.M.; Loglio, G.; Schiavuta, E.; Cini, R. Surfactant components of marine organic matter as agents for biogeochemical fractionation and pollutant transport via marine aerosols. Mar. Chem. 1999, 63, 235–253. [Google Scholar] [CrossRef] [Green Version]
- Hansell, D.A.; Carlson, C.A. Biogeochemistry of Marine Dissolved Organic Matter; Academic Press: London, UK, 2002. [Google Scholar]
- Redfield, A.C.; Ketchum, B.H.; Richards, F.A. The influence of organisms on the composition of sea-water. Sea 1963, 2, 26–77. [Google Scholar]
- Schulz, H.D.; Zabel, M. Marine Geochemistry; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Asa-Awuku, A.; Engelhart, G.J.; Lee, B.H.; Pandis, S.N.; Nenes, A. Relating CCN activity, volatility, and droplet growth kinetics of β-caryophyllene secondary organic aerosol. Atmos. Chem. Phys. Disc. 2008, 8, 10105–10151. [Google Scholar] [CrossRef]
- Wonaschütz, A.; Coggon, M.; Sorooshian, A.; Modini, R.; Frossard, A.A.; Ahlm, L.; Mülmenstädt, J.; Roberts, G.C.; Russell, L.M.; Dey, S.; et al. Hygroscopic properties of smoke-generated organic aerosol particles emitted in the marine atmosphere. Atmos. Chem. Phys. 2013, 13, 9819–9835. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asa-Awuku, A.; Sorooshian, A.; Flagan, R.C.; Seinfeld, J.H.; Nenes, A. CCN Properties of Organic Aerosol Collected Below and within Marine Stratocumulus Clouds near Monterey, California. Atmosphere 2015, 6, 1590-1607. https://doi.org/10.3390/atmos6111590
Asa-Awuku A, Sorooshian A, Flagan RC, Seinfeld JH, Nenes A. CCN Properties of Organic Aerosol Collected Below and within Marine Stratocumulus Clouds near Monterey, California. Atmosphere. 2015; 6(11):1590-1607. https://doi.org/10.3390/atmos6111590
Chicago/Turabian StyleAsa-Awuku, Akua, Armin Sorooshian, Richard C. Flagan, John H. Seinfeld, and Athanasios Nenes. 2015. "CCN Properties of Organic Aerosol Collected Below and within Marine Stratocumulus Clouds near Monterey, California" Atmosphere 6, no. 11: 1590-1607. https://doi.org/10.3390/atmos6111590







