Evaluation of Groundwater Quality in the Vicinity of Khurrianwala Industrial Zone, Pakistan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Study Area
2.2. Sampling
2.3. Experimental Methodology
- WQI = water quality index;
- Qi = subindex for the ith water quality parameter;
- Wi = weight associated with the ith water quality parameter;n = number of water quality parameter.
2.4. GIS and Spatial Analysis
2.5. Statistical Analysis
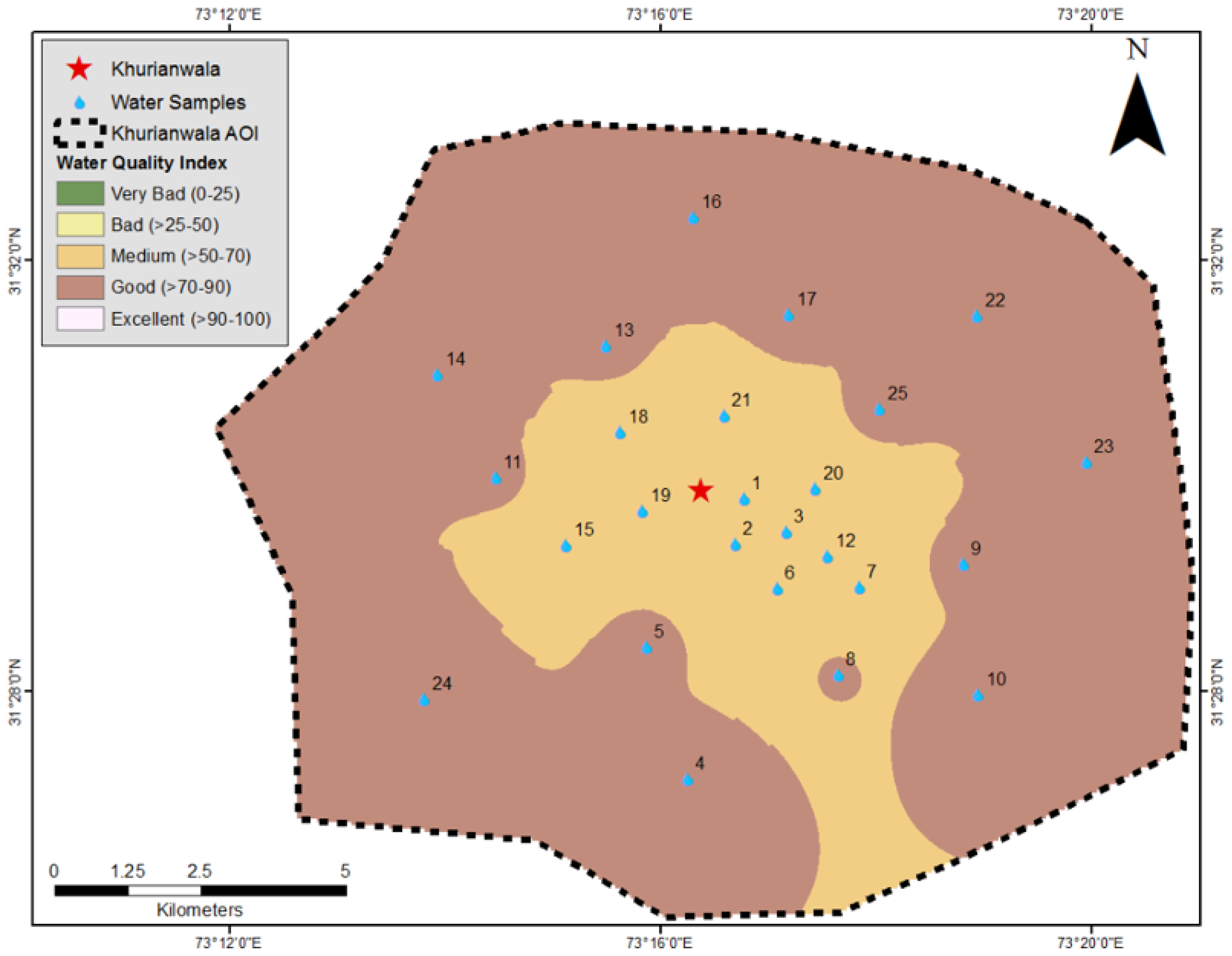
3. Results and Discussion
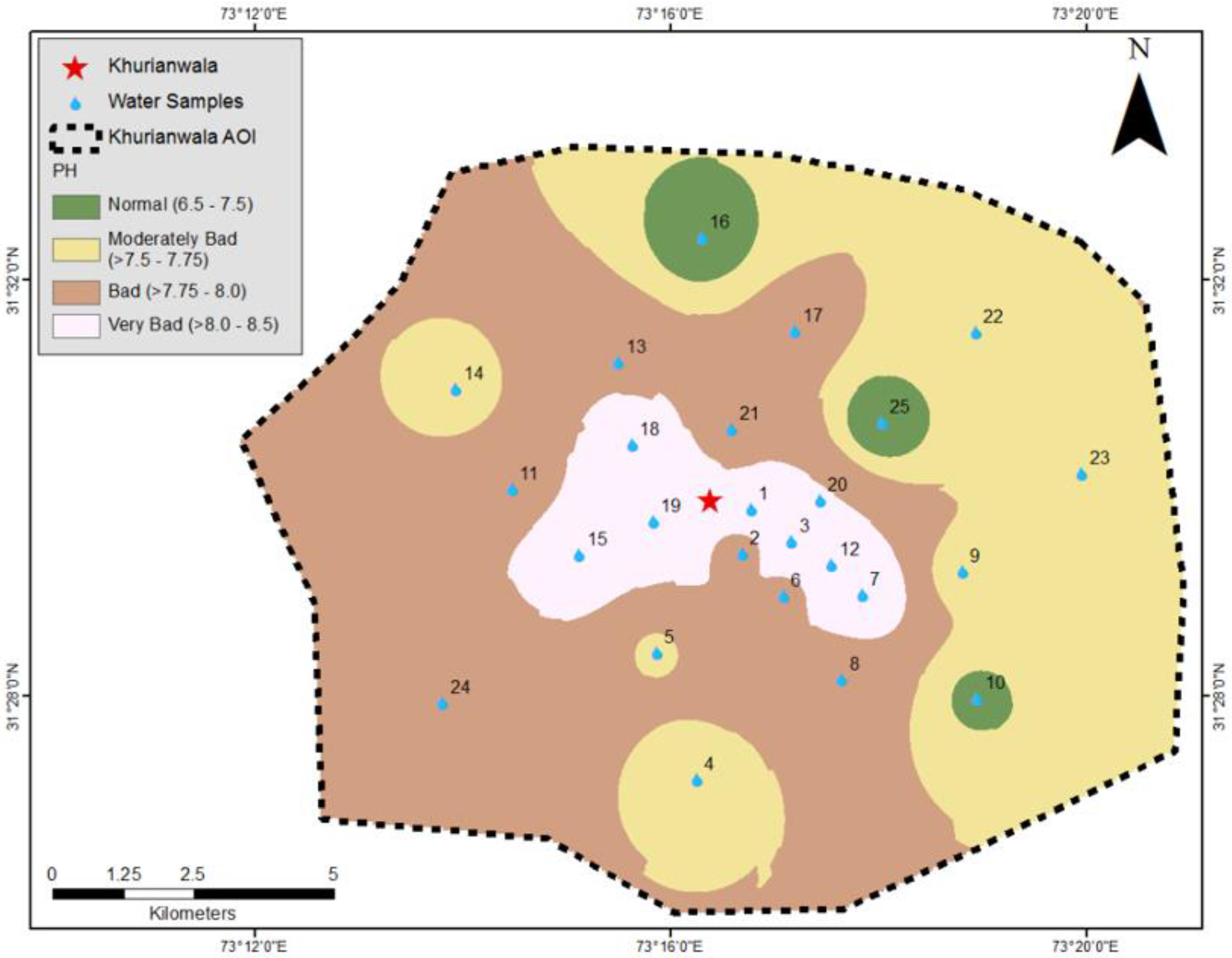
3.1. Drinking Water Quality Analysis
3.2. Bacterial Activity
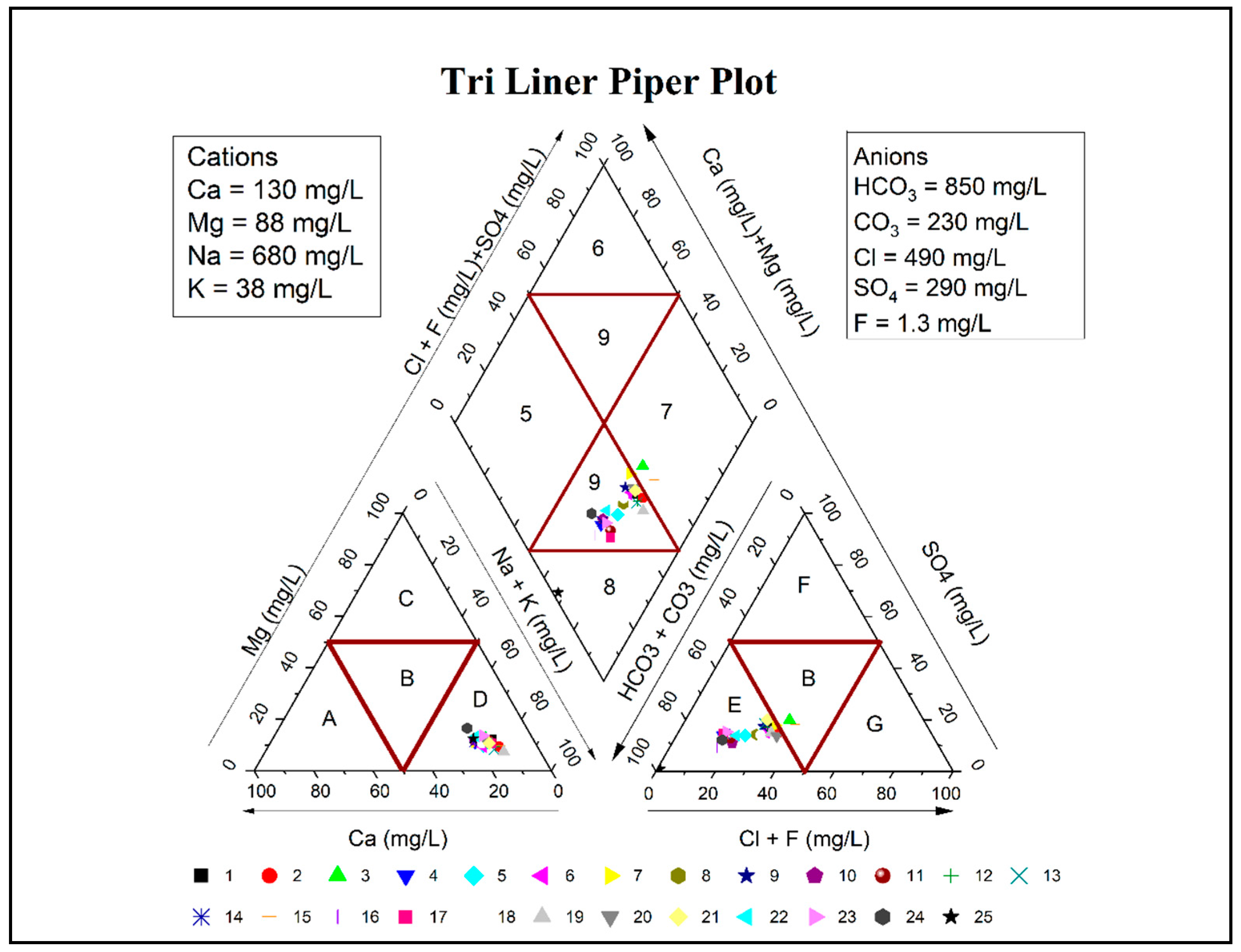
3.3. Cations and Anions
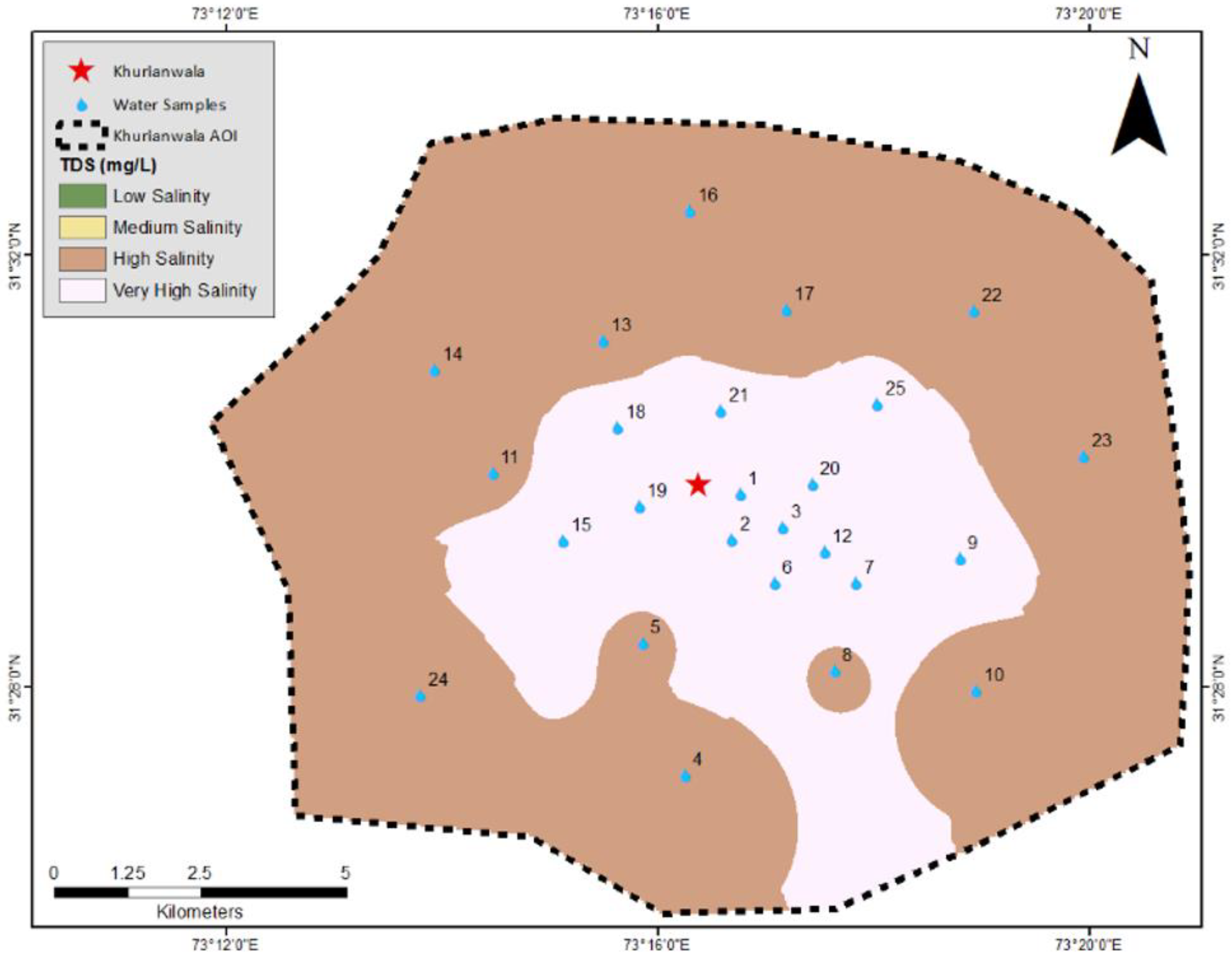
3.4. Irrigation Water Quality Analysis
3.5. Heavy Metal Analysis
3.6. General Survey
4. Conclusions
5. Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APHA | American public health association |
| As | Arsenic |
| B.P. | Blood pressure |
| BOD | Biological oxygen demand |
| Ca | Calcium |
| Cl | Chloride |
| CO3 | Carbonate |
| COD | Chemical oxygen demand |
| Cr | Chromium |
| DO | Dissolved oxygen |
| EDTA | Ethylenediaminetetraacetic acid |
| EC | Electrical conductivity |
| F | Fluoride |
| F. coli | Facial coliform |
| FAO | Food and agriculture organization |
| Fe | Iron |
| GDP | Gross domestic product |
| GIS | Geographic information system |
| GPS | Global positioning system |
| HCO3 | Bi-carbonate |
| IDW | Inverse distance weighted |
| K | Potassium |
| Mg | Magnesium |
| n | Number of water quality parameter |
| Na | Sodium |
| NEQS | National environmental quality standards |
| US-NSFWQI | United States—National Sanitation Foundation Water Quality Index |
| NO3 | Nitrate |
| °C | Degree Celsius |
| PO4 | Phosphate |
| Qi | Sub index for ith water quality parameter |
| RHC | Rural health center |
| R/O | Reverse osmosis |
| RSC | Residual sodium carbonate |
| SAR | Sodium adsorption ratio |
| SO4 | Sulfate |
| TDS | Total dissolved solids |
| TH | Total hardness |
| TP | Total phosphorus |
| UTM | Universal transverse mercator |
| UV/Vis | Ultraviolet–visible |
| WGS-84 | World geodetic system—1984 |
| WHO | World Health Organization |
| Wi | Weight associated with ith water quality parameter |
| WQI | Water quality index |
| Zn | Zinc |
References
- Gorde, S.P.; Jadhav, M.V. Assessment of Water Quality Parameters: A Review. Int. J. Eng. Res. Appl. 2013, 3, 2029–2035. [Google Scholar]
- WHO; UNICEF. Progress on Sanitation and Drinking-Water; WHO: Geneva, Switzerland, 2010; p. 60. [Google Scholar]
- WWAP; UNESCO. The United Nations World Water Development Report 3: Water in a Changing World; UNESCO: Paris, France; Earthscan: London, UK, 2009. [Google Scholar]
- Kavitha, R.E. Ground water quality characteristics at Erode district, Tamilnadu India. Int. J. Environ. Sci. 2010, 1, 145–150. [Google Scholar]
- Pakistan Economic Survey; Ministry of Finance, GoP, Pakistan: Islamabad, Pakistan, 2015; p. 467.
- Khan, S.; Shahnaz, M.; Jehan, N.; Rehman, S.; Shah, M.T.; Din, I. Drinking water quality and human health risk in Charsadda district, Pakistan. J. Clean. Prod. 2013, 60, 93–101. [Google Scholar] [CrossRef]
- Tahir, M.A.; Akram, M.; Hassan, F.; Farooque, M. Technical Assesment of Water Supply Scheme, Northenran and Central Punjab; 149-2011; PCRWR-Ministry of Sicence and Technology: Islamabad, Pakistan, 2011.
- Aleem, M.; Shun, C.J.; Rashid, H.R.; Aslam, A.M.; Javed, M.F.J.; Sarwar, M.W. Impact assessment of Dijkot branch drain effluent on groundwater quality using GIS technique. J. Glob. Innov. Agric. Soc. Sci. 2017, 5, 20–27. [Google Scholar] [CrossRef]
- Taj, L.; Hussain, S.; Ali, S.; Farid, M.; Haq, M.; Habib, U.; Sajid, S.; Sharif, N. Physico-chemical analysis of ground water contamination caused by industrial waste water in Faisalabad, Pakistan. IJPAES 2013, 3, 140–146. [Google Scholar]
- Babu, B.R.; Parande, A.K.; Raghu, S.; Kumar, T.P. Cotton Textile Processing: Waste Generation and Effluent Treatment. J. Cotton Sci. 2007, 11, 141–153. [Google Scholar]
- Aleem, M.; Nasir, A.; Rashid, H. Characterization and removal of dyeing effluents by adsorption and coagulation methods. J. Agric. Res. 2016, 54, 97–106. [Google Scholar]
- Kahlown, M.A.; Ashraf, M.; Hussain, M.; Salam, H.A.; Bhatti, A.Z. Pakistan Council of Research in Water Resources Khyaban-e-Johar, H-8/1; PCRWR: Islamabad, Pakistan, 2006.
- Noreen, M.; Shahid, M.; Iqbal, M.; Nisar, J. Measurement of cytotoxicity and heavy metal load in drains water receiving textile effluents and drinking water in vicinity of drains. Measurement 2017, 109, 88–99. [Google Scholar] [CrossRef]
- Sasayama, K. Report of the 2004 Baseline Survey on Millennium Development Goals in AUICK Associate Cities; International Cooperation NGO: Kobe, Japan, 2005. [Google Scholar]
- Mehdi, S.; Ranjha, A.; Akhtar, S.; Tanvir, M.; Hassan, G. Morphological, chemical and mineralogical Characteristics of a Chronosequence of soils on alluvial deposits in Pakistan. Int. J. Agric. Biol. 2000, 2, 364–368. [Google Scholar]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater; APHA-AWWA-WEF: Washington, DC, USA, 2005. [Google Scholar]
- Nosheen, S.; Nawaz, H.; Rehman, K. Physico-Chemical Characterization of Effluents of Local Textile Industries of Faisalabad–Pakistan. Int. J. Agric. Biol. 2000, 2, 232–233. [Google Scholar]
- Tyagi, S.; Sharma, B.; Singh, P.; Dobhal, R. Water quality assessment in terms of water quality index. Am. J. Water Resour. 2013, 1, 34–38. [Google Scholar]
- Brown, R.M.; Mcclelland, N.I.; Deininger, R.A.; Tozer, R.G. A water quality index: Do we dare? Water Sew. Work. 1970, 117, 339–343. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1994. [Google Scholar]
- Dhv, D.C.; Bv, B.D.H.; Halcrow, T.; CES, ORG & JPS. Hydrology Project Training Module, 28 Major Ions in Water; CES, ORG & JPS: New Delhi, India, 2002. [Google Scholar]
- Mosaferi, M.; Pourakbar, M.; Shakerkhatibi, M.; Fatehifar, E.; Belvasi, M. Quality modeling of drinking groundwater using GIS in rural communities, northwest of Iran. J. Environ. Health Sci. 2014, 12, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; WHO Library Cataloguing-in-Publication Data: Geneva, Switzerland, 2011. [Google Scholar]
- Hasan, M.; Shang, Y.; Akhter, G.; Jin, W. Evaluation of Groundwater Suitability for Drinking and Irrigation Purposes in Toba Tek Singh District, Pakistan. Irrig. Drain. Syst. Eng. 2017, 6, 185. [Google Scholar]
- Ho, K.; Chow, Y.; Yau, J. Chemical and microbiological qualities of The East River (Dongjiang) water, with particular reference to drinking water supply in Hong Kong. Chemosphere 2003, 52, 1441–1450. [Google Scholar] [CrossRef]
- Shankar, B.S.; Balasubramanya, N.; Reddy, M.T.M. Impact of industrialization on groundwater quality–a case study of Peenya industrial area, Bangalore, India. Environ. Monit. Assess. 2008, 142, 263–268. [Google Scholar] [CrossRef] [PubMed]
- NEQS. National Standards for Drinking Water Quality (NSDWQ); NEQS: Islamabad, Pakistan, 2008.
- MWD; USBR. Salinity Management Study–Final Report; Southern California Salinity Coalition: Fountain Valley, CA, USA, 1999. [Google Scholar]
- Basappa, R.M. Status of Ground Water Quality in Bangalore and Its Environment; Department of Mines and Geology: Bangalore, India, 2003.
- Gupta, S.K.; Gupta, R.; Gupta, A.; Seth, A.; Bassin, J.; Gupta, A. Recurrent acute respiratory tract infections in areas with high nitrate concentrations in drinking water. Environ. Health Perspect. 2000, 108, 363. [Google Scholar] [CrossRef] [PubMed]
- Weyer, P.J.; Cerhan, J.R.; Kross, B.C.; Hallberg, G.R.; Kantamneni, J.; Breuer, G.; Jones, M.P.; Zheng, W.; Lynch, C.F. Municipal drinking water nitrate level and cancer risk in older women: The Iowa Women’s Health Study. Epidemiology 2001, 12, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Safdar, S.; Asghar, F.; Jamal, F. Assessment of drinking water quality and its impact on residents health in Bahawalpur city. Int. J. Humanit. Soc. Sci. 2013, 3, 114–128. [Google Scholar]
- Dhanasekarapandian, M.; Chandran, S.; Devi, D.S.; Kumar, V. Spatial and temporal variation of groundwater quality and its suitability for irrigation and drinking purpose using GIS and WQI in an urban fringe. J. Afr. Earth Sci. 2016, 124, 270–288. [Google Scholar] [CrossRef]
- Odonkor, S.T.; Ampofo, J.K. Escherichia coli as an indicator of bacteriological quality of water: An overview. Microbiol. Res. 2013, 4, e2. [Google Scholar] [CrossRef]
- Mwabi, J.K.; Mamba, B.B.; Momba, M.N. Removal of Escherichia coli and faecal coliforms from surface water and groundwater by household water treatment devices/systems: A sustainable solution for improving water quality in rural communities of the Southern African development community region. Int. J. Environ. Res. Public Health 2012, 9, 139–170. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Mashiatullah, A.; Javed, A.; Khan, M.; Saleem, S. Physicochemical and chemical quality of Mailsi City groundwater. Nucleus 2014, 51, 199–205. [Google Scholar]
- Hassan, A.; Nawaz, M. Microbiological and physicochemical assessments of groundwater quality at Punjab, Pakistan. Afr. J. Microbiol. Res. 2014, 8, 2672–2681. [Google Scholar]
- Khalid, S.; Murtaza, B.; Shaheen, I.; Ahmad, I.; Ullah, M.I.; Abbas, T.; Rehman, F.; Ashraf, M.R.; Khalid, S.; Abbas, S. Assessment and public perception of drinking water quality and safety in district Vehari, Punjab, Pakistan. J. Clean. Prod. 2018, 181, 224–234. [Google Scholar] [CrossRef]
- Schroeder, H.A. Relations between hardness of water and death rates from certain chronic and degenerative diseases in the United States. J. Chronic Dis. 1960, 12, 586–591. [Google Scholar] [CrossRef]
- Muthukumaravel, S.; Padmanabhan, V.; Boopathidoss, P.S.; Sadanandane, C.; Srinivasan, R.; Gunasekaran, K.; Sabesan, S.; Balaraman, K. Invasion of toxic marine cyanobacteria in to the tsunami affected coastal villages of southern India. J. Commun. Dis. 2010, 42, 135–138. [Google Scholar] [PubMed]
- Hussain, M.; Ahmed, S.M.; Abderrahman, W. Cluster analysis and quality assessment of logged water at an irrigation project, eastern Saudi Arabia. J. Environ. Manag. 2008, 86, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Prabha, S.; Kumar, M.; Kumar, A.; Das, P.; Ramanathan, A.L. Impact assessment of textile effluent on groundwater quality in the vicinity of Tirupur industrial area, southern India. Environ. Earth Sci. 2013, 70, 3015–3022. [Google Scholar] [CrossRef]
- Sadashivaiah, C.; Ramakrishnaiah, C.; Ranganna, G. Hydrochemical analysis and evaluation of groundwater quality in Tumkur Taluk, Karnataka State, India. Int. J. Environ. Res. Public Health 2008, 5, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, L. Classification and Use of Irrigation Waters; FAO: Rome, Italy, 1955. [Google Scholar]
- Laluraj, C.; Gopinath, G. Assessment on seasonal variation of groundwater quality of phreatic aquifers—A river basin system. Environ. Monit. Assess. 2006, 117, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.; Farooqi, A.; Xiao, T.; Masood, S.; Kamran, M.A. Elevated levels of arsenic and trace metals in drinking water of Tehsil Mailsi, Punjab, Pakistan. J. Geochem. Explor. 2016, 169, 89–99. [Google Scholar] [CrossRef]
- Vasanthavigar, M.; Srinivasamoorthy, K.; Vijayaragavan, K.; Ganthi, R.R.; Chidambaram, S.; Anandhan, P.; Manivannan, R.; Vasudevan, S. Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ. Monit. Assess. 2010, 171, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, S.; Chidambaram, S.; Prasanna, M.V.; Thivya, C.; Karmegam, U. Hydrochemical characteristics and groundwater quality assessment in Krishnagiri District, Tamilnadu, India. Int. J. Earth Sci. Eng. 2011, 4, 623–632. [Google Scholar]
- Eaton, F.M. Significance of carbonates in irrigation waters. Soil Sci. 1950, 69, 123–134. [Google Scholar] [CrossRef]
- Rao, N.S.; Rao, V.G.; Gupta, C. Groundwater pollution due to discharge of industrial effluents in Venkatapuram area, Visakhapatnam, Andhra Pradesh, India. Environ. Geol. 1998, 33, 289–294. [Google Scholar]
- Offiong, O.; Edet, A. Water quality assessment in Akpabuyo, cross river basin, south-eastern Nigeria. Environ. Geol. 1998, 34, 167–174. [Google Scholar] [CrossRef]
- Sivasankar, V.; Omine, K.; Msagati, T.; Chandramohan, A. Evaluation of groundwater quality in Madurai City, South India for drinking, irrigation and construction purposes. Arab. J. Geosci. 2014, 7, 3093–3107. [Google Scholar] [CrossRef]
- Devendra, D.; Shriram, D.; Atul, K. Analysis of Ground Water Quality Parameters: A Review. Res. J. Eng. Sci. 2014, 3, 26–31. [Google Scholar]
- Mosaferi, M.; Yunesian, M.; Dastgiri, S.; Mesdaghinia, A.; Esmailnasab, N. Prevalence of skin lesions and exposure to arsenic in drinking water in Iran. Sci. Total Environ. 2008, 390, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Doğan, M.; Soylak, M.; Elçi, L.; von Bohlen, A. Application of total reflection X-ray fluorescence spectrometry in the textile industry. Microchim. Acta 2002, 138, 77–82. [Google Scholar] [CrossRef]
- Sungur, Ş.; Gülmez, F. Determination of metal contents of various fibers used in textile industry by MP-AES. J. Spectrosc. 2015, 2015, 640271. [Google Scholar] [CrossRef]
- Nickson, R.T.; McArthur, J.M.; Shrestha, B.; Kyaw-Myint, T.O.; Lowry, D. Arsenic and other drinking water quality issues, Muzaffargarh District, Pakistan. Appl. Geochem. 2005, 20, 55–68. [Google Scholar] [CrossRef]




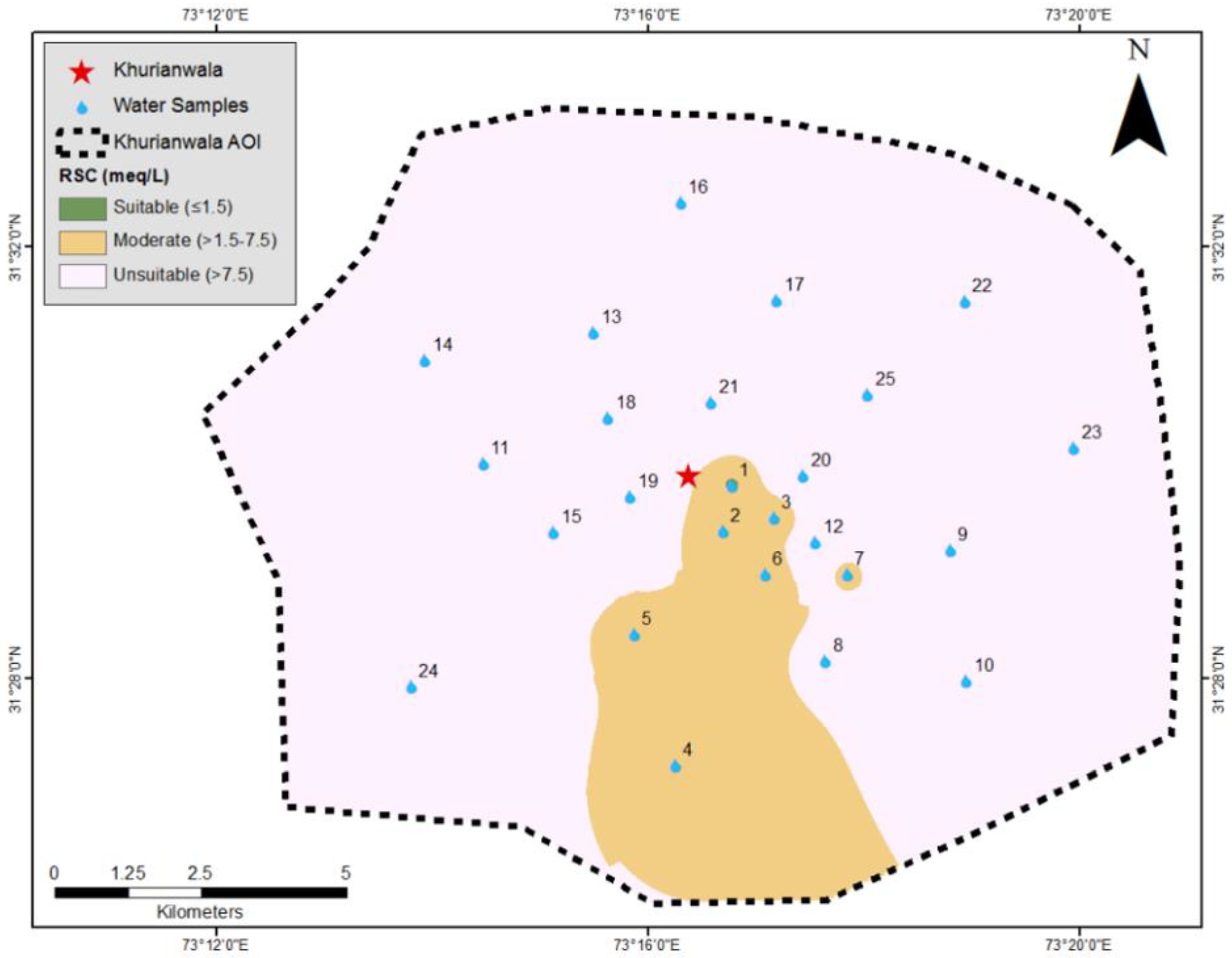

| SAR | RSC | Remarks (Irrigation Water Quality) |
|---|---|---|
| Range (meq/L) | Range (meq/L) | |
| <3.00 | <1.50 | Suitable |
| 3.00–9.00 | 1.50–7.50 | Moderate |
| >9.00 | >7.50 | Not suitable |
| Samples | Latitude | Longitude | pH | Turbidity | TDS | (PO4) | (NO3) | DO | F-Coli | Na | Ca | Mg | K | HCO3 | CO3 | SO4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Units | N | E | -- | NTU | mg/L | mg/L | mg/L | % | col/100 mL | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L |
| 1 | 31.49649 | 73.27959 | 8.10 | 4.10 | 1845 | 1.02 | 69.0 | 09.0 | 11.0 | 545 | 111 | 98.0 | 44.0 | 982 | 243 | 350 |
| 2 | 31.48949 | 73.27823 | 7.90 | 3.80 | 1548 | 1.00 | 53.0 | 08.0 | 09.0 | 613 | 107 | 78.0 | 32.0 | 870 | 250 | 323 |
| 3 | 31.49141 | 73.28613 | 8.40 | 4.00 | 1643 | 0.67 | 58.0 | 09.0 | 12.0 | 459 | 110 | 88.0 | 38.0 | 892 | 219 | 340 |
| 4 | 31.45318 | 73.27089 | 7.50 | 1.10 | 1237 | 0.18 | 23.0 | 12.0 | 04.0 | 219 | 87.0 | 47.0 | 77.0 | 652 | 120 | 189 |
| 5 | 31.47354 | 73.26450 | 7.70 | 2.40 | 1400 | 0.34 | 36.0 | 11.0 | 06.0 | 332 | 98.0 | 53.0 | 65.0 | 760 | 189 | 231 |
| 6 | 31.48272 | 73.28478 | 7.90 | 3.70 | 2114 | 0.89 | 47.0 | 10.0 | 09.0 | 498 | 139 | 72.0 | 49.0 | 834 | 220 | 298 |
| 7 | 31.48278 | 73.29746 | 8.30 | 2.90 | 1821 | 0.67 | 42.0 | 08.0 | 07.0 | 471 | 154 | 86.0 | 54.0 | 897 | 204 | 340 |
| 8 | 31.46933 | 73.29414 | 7.80 | 2.70 | 1404 | 0.56 | 32.0 | 11.0 | 06.0 | 387 | 102 | 72.0 | 68.0 | 724 | 250 | 232 |
| 9 | 31.48650 | 73.31352 | 7.60 | 2.00 | 1612 | 0.50 | 35.0 | 10.0 | 04.0 | 299 | 98.0 | 55.0 | 54.0 | 760 | 198 | 248 |
| 10 | 31.46622 | 73.31588 | 7.40 | 0.50 | 1231 | 0.12 | 28.0 | 12.0 | 03.0 | 180 | 78.4 | 46.0 | 89.0 | 688 | 102 | 140 |
| 11 | 31.49982 | 73.24126 | 7.90 | 3.40 | 1402 | 0.34 | 36.0 | 09.0 | 07.0 | 303 | 76.0 | 59.0 | 65.0 | 698 | 187 | 201 |
| 12 | 31.48762 | 73.29250 | 8.10 | 3.30 | 1689 | 0.76 | 43.0 | 07.0 | 08.0 | 681 | 132 | 88.0 | 38.0 | 850 | 229 | 288 |
| 13 | 31.52018 | 73.25823 | 8.00 | 2.10 | 1312 | 0.45 | 38.0 | 11.0 | 04.0 | 432 | 98.0 | 54.0 | 69.0 | 696 | 210 | 265 |
| 14 | 31.51579 | 73.23220 | 7.60 | 0.90 | 1002 | 0.23 | 30.0 | 11.0 | 03.0 | 148 | 77.0 | 42.0 | 112 | 598 | 182 | 178 |
| 15 | 31.48930 | 73.25206 | 8.20 | 1.10 | 1912 | 0.76 | 51.0 | 09.0 | 07.0 | 389 | 69.0 | 54.0 | 45.0 | 689 | 170 | 306 |
| 16 | 31.54017 | 73.27171 | 7.20 | 1.10 | 1098 | 0.30 | 22.0 | 10.0 | 02.0 | 202 | 89.0 | 48.0 | 98.0 | 602 | 142 | 120 |
| 17 | 31.52505 | 73.28654 | 7.90 | 1.90 | 1208 | 0.36 | 31.0 | 10.0 | 08.0 | 321 | 78.0 | 56.0 | 72.0 | 780 | 200 | 268 |
| 18 | 31.50687 | 73.26046 | 8.10 | 2.80 | 1701 | 0.71 | 39.0 | 09.0 | 10.0 | 402 | 112 | 66.0 | 40.0 | 960 | 311 | 298 |
| 19 | 31.49465 | 73.26387 | 8.10 | 2.90 | 1650 | 0.59 | 47.0 | 09.0 | 10.0 | 601 | 98.0 | 59.0 | 37.0 | 921 | 308 | 320 |
| 20 | 31.49805 | 73.29063 | 8.00 | 3.10 | 1921 | 0.82 | 38.0 | 07.0 | 08.0 | 512 | 124 | 87.0 | 47.0 | 898 | 248 | 289 |
| 21 | 31.50936 | 73.27647 | 7.90 | 2.10 | 1499 | 0.43 | 55.0 | 08.0 | 11.0 | 455 | 106 | 73.0 | 47.0 | 912 | 198 | 354 |
| 22 | 31.52488 | 73.31560 | 7.70 | 1.00 | 1258 | 0.21 | 21.0 | 12.0 | 02.0 | 174 | 78.0 | 56.0 | 110 | 721 | 210 | 164 |
| 23 | 31.50230 | 73.33255 | 7.70 | 0.60 | 987 | 0.32 | 26.0 | 12.0 | 03.0 | 190 | 71.0 | 60.0 | 118 | 597 | 186 | 178 |
| 24 | 31.46548 | 73.23006 | 7.90 | 0.45 | 1308 | 0.17 | 24.0 | 13.0 | 03.0 | 121 | 69.0 | 57.0 | 98.0 | 606 | 198 | 156 |
| 25 | 31.51048 | 73.30060 | 7.10 | 0.90 | 1521 | 0.38 | 34.0 | 11.0 | 05.0 | 240 | 94.0 | 59.0 | 78.0 | 712 | 210 | 198 |
| NEQS | ---- | ---- | 6.50–8.00 | 5.00 | 1000 | ---- | 50.0 | n/a | 0.00 | ---- | ---- | ---- | ---- | ---- | ---- | 250 |
| WHO | ---- | ---- | 6.50–8.00 | 5.00 | 500 | ---- | 50.0 | ---- | 0.00 | 20.0 | ---- | ---- | ---- | n/a | ---- | 250 |
| Samples | Latitude | Longitude | Cl | F | TH | EC | Fe | Cr | As | Zn | TP | SAR | RSC | %Age Ion Bal. | % Na | WQI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Units | N | E | mg/L | mg/L | mg/L | uS/cm | mg/L | mg/L | ppb | mg/L | mg/L | meq/L | meq/L | meq/L | meq/L | |
| 1 | 31.49649 | 73.27959 | 616 | 1.60 | 378 | 2976 | 5.02 | 0.80 | 0.07 | 0.08 | 0.33 | 12.8 | 10.5 | 11.8 | 68.3 | 65.3 |
| 2 | 31.48949 | 73.27823 | 732 | 1.50 | 438 | 2497 | 3.12 | 0.92 | 0.08 | 0.08 | 0.33 | 15.5 | 10.7 | 11.9 | 73.9 | 63.6 |
| 3 | 31.49141 | 73.28613 | 598 | 1.60 | 392 | 2650 | 4.83 | 0.65 | 0.10 | 0.06 | 0.22 | 11.1 | 9.09 | 15.2 | 66.0 | 63.4 |
| 4 | 31.45318 | 73.27089 | 205 | 0.40 | 150 | 1995 | 0.88 | 0.23 | 0.00 | 0.01 | 0.06 | 6.6 | 6.42 | 10.5 | 50.9 | 74.6 |
| 5 | 31.47354 | 73.26450 | 387 | 0.70 | 189 | 2258 | 2.10 | 0.43 | 0.03 | 0.08 | 0.11 | 9.5 | 9.44 | 15.1 | 60.6 | 72.4 |
| 6 | 31.48272 | 73.28478 | 590 | 0.80 | 320 | 3410 | 3.42 | 0.52 | 0.05 | 0.01 | 0.29 | 12.0 | 8.06 | 10.0 | 65.7 | 67.0 |
| 7 | 31.48278 | 73.29746 | 598 | 0.90 | 388 | 2937 | 2.66 | 0.87 | 0.08 | 0.07 | 0.22 | 10.6 | 6.64 | 10.6 | 61.6 | 65.6 |
| 8 | 31.46933 | 73.29414 | 432 | 1.10 | 300 | 2265 | 1.22 | 0.25 | 0.04 | 0.08 | 0.18 | 10.1 | 9.10 | 11.3 | 61.5 | 70.6 |
| 9 | 31.48650 | 73.31352 | 394 | 0.50 | 289 | 2600 | 1.21 | 0.42 | 0.04 | 0.03 | 0.16 | 8.4 | 9.58 | 19.4 | 59.1 | 72.3 |
| 10 | 31.46622 | 73.31588 | 259 | 0.30 | 187 | 1985 | 0.56 | 0.12 | 0.00 | 0.01 | 0.04 | 5.6 | 6.93 | 16.4 | 45.7 | 74.7 |
| 11 | 31.49982 | 73.24126 | 294 | 0.80 | 298 | 2261 | 2.50 | 0.19 | 0.02 | 0.01 | 0.11 | 8.9 | 8.96 | 12.3 | 60.2 | 70.6 |
| 12 | 31.48762 | 73.29250 | 489 | 1.30 | 304 | 2724 | 3.22 | 0.74 | 0.06 | 0.06 | 0.25 | 15.8 | 7.63 | 3.70 | 72.5 | 64.8 |
| 13 | 31.52018 | 73.25823 | 394 | 0.60 | 298 | 2116 | 2.26 | 0.43 | 0.03 | 0.05 | 0.15 | 12.3 | 9.01 | 7.81 | 66.2 | 71.2 |
| 14 | 31.51579 | 73.23220 | 198 | 0.30 | 220 | 1616 | 1.00 | 0.24 | 0.01 | 0.03 | 0.07 | 4.7 | 8.52 | 20.3 | 39.1 | 74.9 |
| 15 | 31.48930 | 73.25206 | 636 | 1.40 | 400 | 3084 | 2.98 | 0.43 | 0.04 | 0.09 | 0.25 | 12.0 | 9.01 | 22.6 | 69.8 | 66.9 |
| 16 | 31.54017 | 73.27171 | 210 | 0.50 | 165 | 1771 | 1.40 | 0.76 | 0.00 | 0.08 | 0.10 | 6.04 | 6.15 | 7.65 | 46.2 | 76.1 |
| 17 | 31.52505 | 73.28654 | 278 | 0.60 | 278 | 1948 | 2.42 | 0.26 | 0.00 | 0.07 | 0.12 | 9.54 | 10.9 | 14.8 | 60.9 | 72.1 |
| 18 | 31.50687 | 73.26046 | 598 | 0.80 | 320 | 2744 | 3.23 | 0.44 | 0.03 | 0.10 | 0.23 | 10.5 | 15.0 | 24.8 | 64.8 | 68.2 |
| 19 | 31.49465 | 73.26387 | 666 | 1.30 | 288 | 2661 | 1.80 | 0.86 | 0.09 | 0.09 | 0.19 | 16.7 | 15.5 | 15.8 | 75.6 | 67.7 |
| 20 | 31.49805 | 73.29063 | 712 | 1.20 | 392 | 3098 | 2.42 | 0.58 | 0.10 | 0.09 | 0.27 | 12.1 | 9.54 | 14.1 | 66.5 | 63.6 |
| 21 | 31.50936 | 73.27647 | 486 | 1.60 | 332 | 2418 | 2.06 | 0.94 | 0.06 | 0.07 | 0.14 | 11.7 | 10.2 | 13.7 | 66.8 | 67.8 |
| 22 | 31.52488 | 73.31560 | 240 | 0.50 | 250 | 2029 | 0.66 | 0.32 | 0.01 | 0.04 | 0.07 | 5.2 | 10.2 | 20.9 | 41.6 | 75.3 |
| 23 | 31.50230 | 73.33255 | 190 | 0.30 | 200 | 1592 | 1.02 | 0.32 | 0.01 | 0.02 | 0.10 | 5.65 | 7.44 | 11.6 | 43.3 | 73.6 |
| 24 | 31.46548 | 73.23006 | 210 | 0.30 | 242 | 2110 | 0.98 | 0.28 | 0.00 | 0.01 | 0.06 | 3.67 | 8.33 | 23.3 | 35.1 | 73.6 |
| 25 | 31.51048 | 73.30060 | 254 | 0.60 | 288 | 2453 | 1.50 | 0.42 | 0.04 | 0.04 | 0.12 | 6.73 | 9.06 | 15.2 | 50.9 | 72.4 |
| NEQS | ---- | ---- | 250 | 1.50 | <300 | ---- | ---- | 0.10 | 0.05 | 5.00 | ---- | Table 1 | Table 1 | <10.0 | Table 7 | NSFWQI |
| WHO | ---- | ---- | 250 | 1.50 | <300 | ---- | 0.30 | 0.05 | 0.01 | 3.00 | ---- |
| Na | Ca | Mg | K | |
|---|---|---|---|---|
| HCO3 | 0.95 | 0.98 | 0.98 | 0.87 |
| CO3 | 0.95 | 0.97 | 0.97 | 0.86 |
| Cl | 0.98 | 0.95 | 0.95 | 0.73 |
| SO4 | 0.97 | 0.97 | 0.98 | 0.81 |
| Triangle | Diamond | ||
|---|---|---|---|
| Water Class | Remarks | Water Class | Remarks |
| A | Calcium type | 5 | Mg(HCO3)2 type |
| B | No Dominant type | 6 | CaCl2 type |
| C | Magnesium type | 7 | NaCl type |
| D | Na + K type | 8 | NaHCO3 type |
| E | Bicarbonate type | 9 | Mixed Type |
| F | Sulphate type | 1 = 5 + 9 + 6 | Alkaline earths exceed alkalies |
| G | Chloride type | 2 = 7 + 9 + 8 | Alkalies exceed alkaline earths |
| 3 = 5 + 9 + 8 | Weak acids exceed strong acids | ||
| 4 = 7 + 9 + 6 | Strong acids exceed weak acids | ||
| Samples | Latitude | Longitude | Cations | Anions | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | Ca | Mg | K | HCO3 | CO3 | SO4 | Cl | F | |||
| N | E | meq/L | meq/L | meq/L | meq/L | meq/L | meq/L | meq/L | meq/L | meq/L | |
| 1 | 31.4965 | 73.2796 | 23.70 | 5.55 | 8.17 | 1.13 | 16.10 | 8.10 | 7.29 | 17.35 | 0.084 |
| 2 | 31.4895 | 73.2782 | 26.65 | 5.35 | 6.50 | 0.82 | 14.26 | 8.33 | 6.73 | 20.62 | 0.079 |
| 3 | 31.4914 | 73.2861 | 19.96 | 5.50 | 7.33 | 0.97 | 14.62 | 7.30 | 7.08 | 16.85 | 0.084 |
| 4 | 31.4532 | 73.2709 | 9.52 | 4.35 | 3.92 | 1.97 | 10.69 | 4.00 | 3.94 | 5.77 | 0.042 |
| 5 | 31.4735 | 73.2645 | 14.43 | 4.90 | 4.42 | 1.67 | 12.46 | 6.30 | 4.81 | 10.90 | 0.063 |
| 6 | 31.4827 | 73.2848 | 21.65 | 6.95 | 6.00 | 1.26 | 13.67 | 7.33 | 6.21 | 16.62 | 0.042 |
| 7 | 31.4828 | 73.2975 | 20.48 | 7.70 | 7.17 | 1.38 | 14.70 | 6.80 | 7.08 | 16.85 | 0.047 |
| 8 | 31.4693 | 73.2941 | 16.83 | 5.10 | 6.00 | 1.74 | 11.87 | 8.33 | 4.83 | 12.17 | 0.058 |
| 9 | 31.4865 | 73.3135 | 13.00 | 4.90 | 4.58 | 1.38 | 12.46 | 6.60 | 5.17 | 11.10 | 0.026 |
| 10 | 31.4662 | 73.3159 | 7.83 | 3.92 | 3.83 | 2.28 | 11.28 | 3.40 | 2.92 | 7.30 | 0.016 |
| 11 | 31.4998 | 73.2413 | 13.17 | 3.80 | 4.92 | 1.67 | 11.44 | 6.23 | 4.19 | 8.28 | 0.042 |
| 12 | 31.4876 | 73.2925 | 29.61 | 6.60 | 7.33 | 0.97 | 13.93 | 7.63 | 6.00 | 13.77 | 0.068 |
| 13 | 31.5202 | 73.2582 | 18.78 | 4.90 | 4.50 | 1.77 | 11.41 | 7.00 | 5.52 | 11.10 | 0.032 |
| 14 | 31.5158 | 73.2322 | 6.43 | 3.85 | 3.50 | 2.87 | 9.80 | 6.07 | 3.71 | 5.58 | 0.037 |
| 15 | 31.4893 | 73.2521 | 16.91 | 3.45 | 4.50 | 1.15 | 11.30 | 5.67 | 6.38 | 17.92 | 0.074 |
| 16 | 31.5402 | 73.2717 | 8.78 | 4.45 | 4.00 | 2.51 | 9.87 | 4.73 | 2.50 | 5.92 | 0.032 |
| 17 | 31.5251 | 73.2865 | 13.96 | 3.90 | 4.67 | 1.85 | 12.79 | 6.67 | 5.58 | 7.83 | 0.058 |
| 18 | 31.5069 | 73.2605 | 17.48 | 5.60 | 5.50 | 1.03 | 15.74 | 10.37 | 6.21 | 16.85 | 0.089 |
| 19 | 31.4946 | 73.2639 | 26.13 | 4.90 | 4.92 | 0.95 | 15.10 | 10.27 | 6.67 | 18.76 | 0.079 |
| 20 | 31.4981 | 73.2906 | 22.26 | 6.20 | 7.25 | 1.21 | 14.72 | 8.27 | 6.02 | 20.06 | 0.079 |
| 21 | 31.5094 | 73.2765 | 19.78 | 5.30 | 6.08 | 1.21 | 14.95 | 6.60 | 7.38 | 13.69 | 0.084 |
| 22 | 31.5249 | 73.3156 | 7.57 | 3.90 | 4.67 | 2.82 | 11.82 | 7.00 | 3.42 | 6.76 | 0.026 |
| 23 | 31.5023 | 73.3326 | 8.26 | 3.55 | 5.00 | 3.03 | 9.79 | 6.20 | 3.71 | 5.35 | 0.016 |
| 24 | 31.4655 | 73.2301 | 5.26 | 3.45 | 4.75 | 2.51 | 9.93 | 6.60 | 3.25 | 5.92 | 0.016 |
| 25 | 31.5105 | 73.3006 | 10.43 | 4.70 | 4.92 | 2.00 | 11.67 | 7.00 | 4.13 | 7.15 | 0.032 |
| EC | Na % | ||||
|---|---|---|---|---|---|
| Range | Water Class | No. of Samples | Range | Water Class | No. of Samples |
| <250 | Low saline | -- | <20 | Excellent | -- |
| 250–750 | Medium saline | -- | 20–40 | Good | 2 |
| 750–2250 | High saline | 15 | 40–60 | Permissible | 7 |
| 2250–5000 | Very high saline | 10 | 60–80 | Doubtful | 17 |
| 80> | Unsuitable | -- | |||
| Na | EC | RSC | |
|---|---|---|---|
| EC | 0.99 | ||
| RSC | 0.97 | 0.96 | |
| SAR | 0.98 | 0.96 | 0.95 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleem, M.; Shun, C.J.; Li, C.; Aslam, A.M.; Yang, W.; Nawaz, M.I.; Ahmed, W.S.; Buttar, N.A. Evaluation of Groundwater Quality in the Vicinity of Khurrianwala Industrial Zone, Pakistan. Water 2018, 10, 1321. https://doi.org/10.3390/w10101321
Aleem M, Shun CJ, Li C, Aslam AM, Yang W, Nawaz MI, Ahmed WS, Buttar NA. Evaluation of Groundwater Quality in the Vicinity of Khurrianwala Industrial Zone, Pakistan. Water. 2018; 10(10):1321. https://doi.org/10.3390/w10101321
Chicago/Turabian StyleAleem, Muhammad, Cao Jia Shun, Chao Li, Arslan Muhammad Aslam, Wu Yang, Muhammad Imran Nawaz, Wasif Saeed Ahmed, and Noman Ali Buttar. 2018. "Evaluation of Groundwater Quality in the Vicinity of Khurrianwala Industrial Zone, Pakistan" Water 10, no. 10: 1321. https://doi.org/10.3390/w10101321





