pCO2 Dynamics of Stratified Reservoir in Temperate Zone and CO2 Pulse Emissions During Turnover Events
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
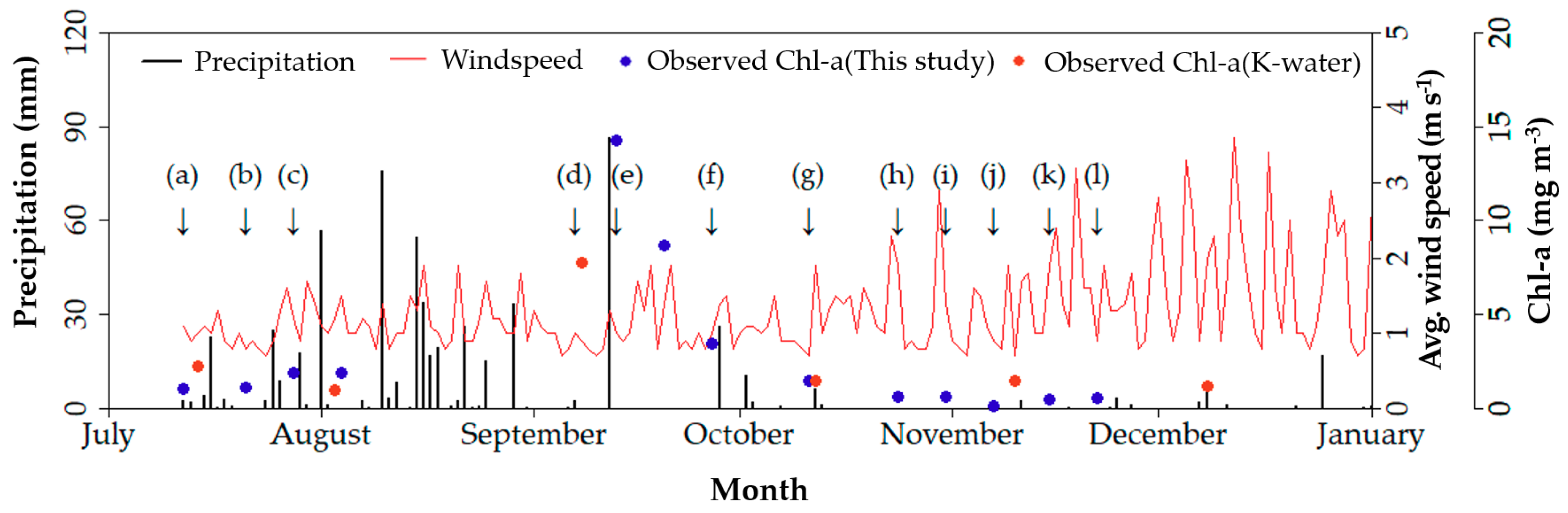
2.2. Sampling and Analysis
2.3. Indirect pCO2 Estimation and Development of Statistical Models
2.4. Analysis of Reservoir Thermal Stratification and Stability
3. Results and Discussion
3.1. Summary Statistics and Correlation Analyses
3.2. Variations in Stratification Structure and Thermal Stability
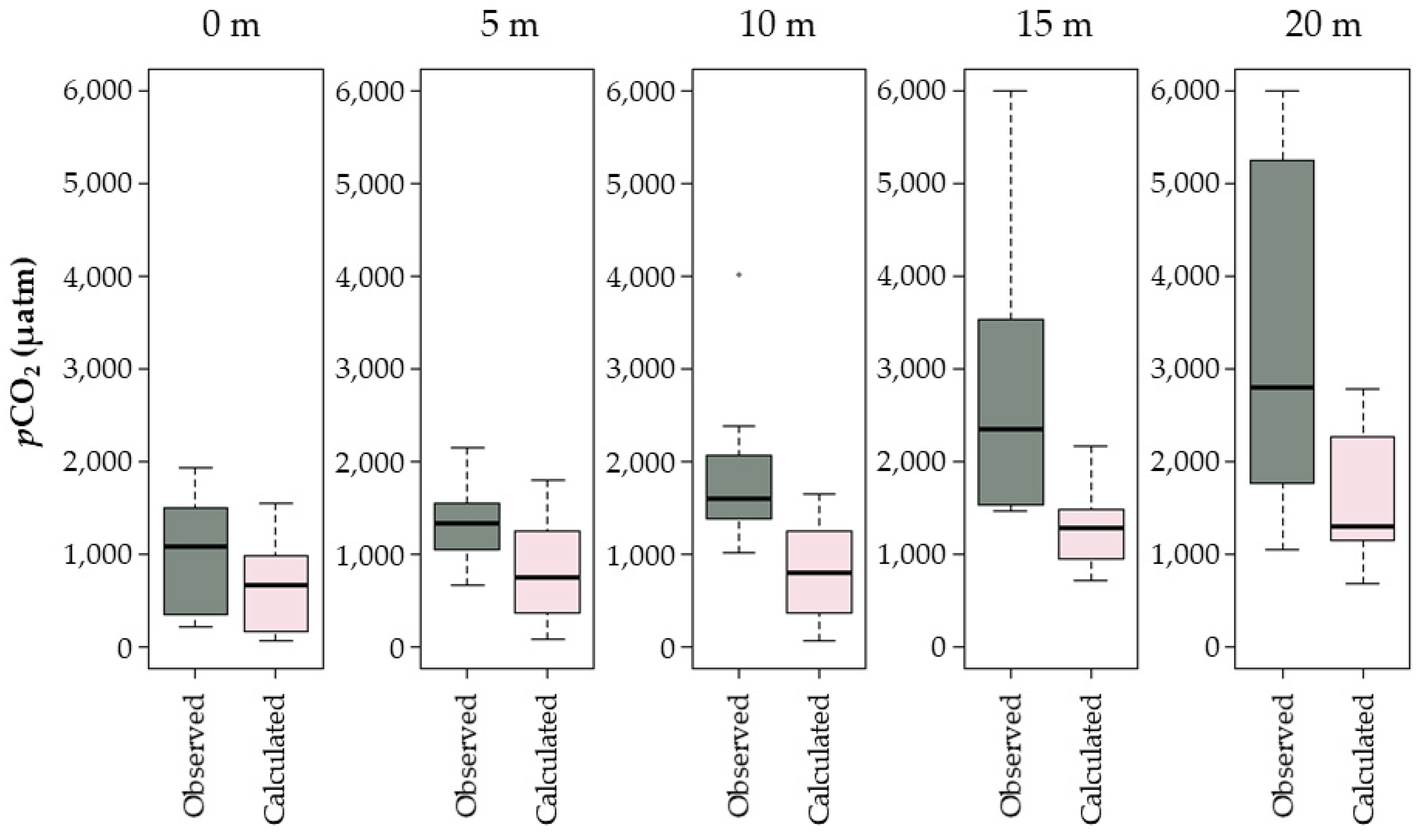
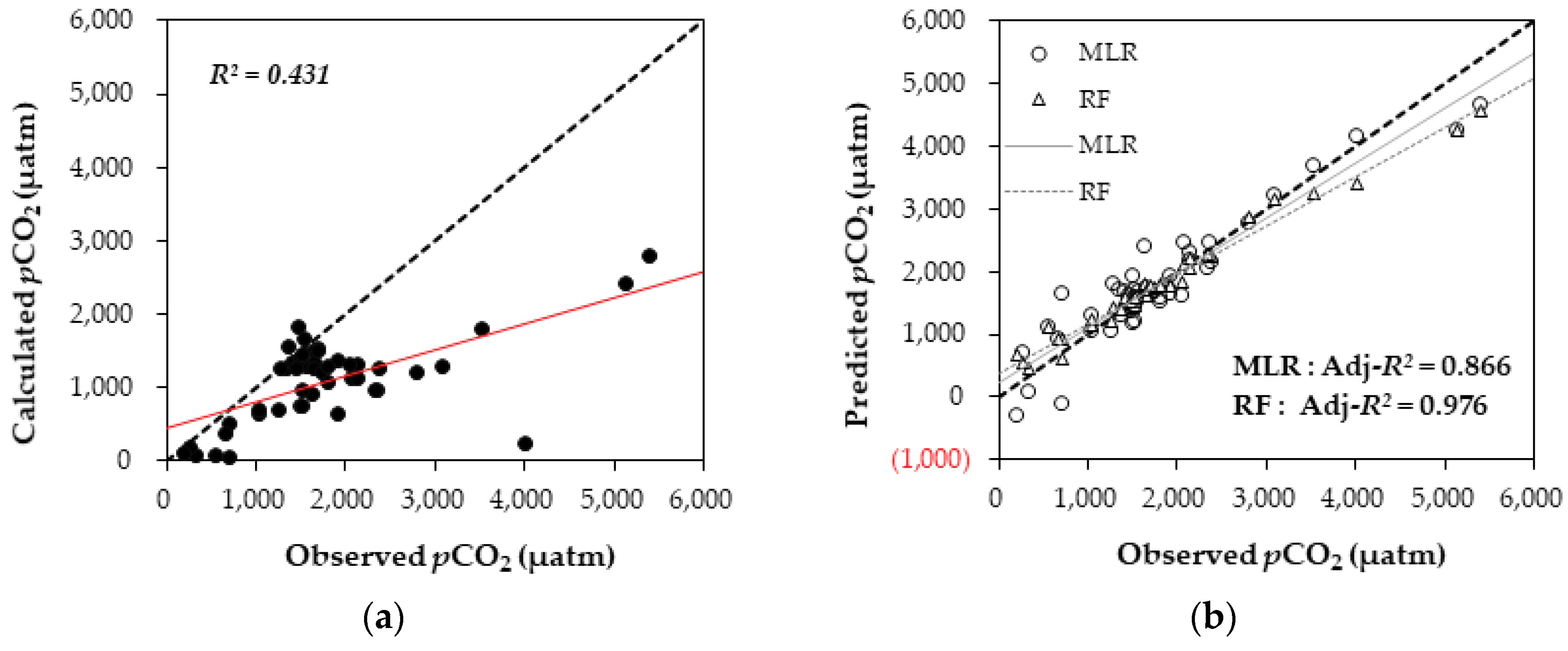
3.3. Comparisons of Measured and Estimated pCO2 Values
3.4. Dynamic Vertical Variations of pCO2 and Water Quality Variables
3.5. Temporal Variations of the CO2 NAF
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IPCC. Climate change, the physical science basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, NY, USA, 2013; ISBN 9781107057999. [Google Scholar]
- IPCC. Climate change, synthesis report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014; ISBN 978-92-9169-143-2. [Google Scholar]
- IPCC National Greenhouse Gas Inventories Programme. IPCC Guidelines for National Greenhouse Gas Inventories; IGES: Hayama, Japan, 2006; ISBN 4-88788-032-4. [Google Scholar]
- Battin, T.J.; Luyssaert, S.; Kaplan, L.A.; Aufdenkampe, A.K.; Richter, A.; Tranvik, L.J. The boundless carbon cycle. Nat. Geosci. 2009, 2, 598–600. [Google Scholar] [CrossRef]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the Global Carbon Cycle: Integrating Inland Waters into the Terrestrial Carbon Budget. Ecosystems 2007, 10, 171–184. [Google Scholar] [CrossRef]
- Golub, M. Controls on Temporal Variation in Ecosystem-Atmosphere Carbon Dioxide Exchange in Lakes and Reservoirs. Ph.D. Thesis, University of Wisconsin-Madison, Madison, WI, USA, November 2016. [Google Scholar]
- Wetzel, R.G. Limnology: Lake and River Ecosystems; Academic Press: New York, NY, USA, 2001; ISBN 9780127447605. [Google Scholar]
- Cole, J.J.; Caraco, N.F.; Kling, G.W.; Kratz, T.K. Carbon dioxide supersaturation in the surface waters of lakes. Science 1994, 265, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Algesten, G.S.; Sobek, A.K.; Bergstrom, A.; Agren, L.; Tranvik, J.; Jansson, M. Role of lakes for organic carbon cycling in the boreal zone. Glob. Chang. Biol. 2003, 10, 141–147. [Google Scholar] [CrossRef]
- Roehm, C.L.; Prairie, Y.T.; del Giorgio, P.A. The pCO2 dynamics in lakes in the boreal region of northern Québec, Canada. Glob. Biogeochem. Cycles 2009. [Google Scholar] [CrossRef]
- Einsele, G.; Yan, J.; Hinderer, M. Atmospheric carbon burial in modern lake basins and its significance for the global carbon budget. Glob. Planet. Chang. 2001, 30, 167–195. [Google Scholar] [CrossRef]
- Kortelainen, P.; Rantakari, M.; Huttunen, J.T.; Mattsson, T.; Alm, J.; Juutinen, S.; Larmola, T.; Silvola, J.; Martikainen, P.J. Sediment respiration and lake trophic state are important predictors of large CO2 evasion from small boreal lakes. Glob. Chang. Biol. 2006, 12, 1554–1567. [Google Scholar] [CrossRef]
- Stallard, F.R. Terrestrial sedimentation and the C cycle: Coupling weathering and erosion to carbon storage. Glob. Biogeochem. Cycles 1998, 12, 231–257. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef] [Green Version]
- Downing, J.A.; Cole, J.J.; Middelburg, J.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Prairie, Y.T.; Laube, K.A. Sediment organic carbon burial in agriculturally eutrophic impoundments over the last century. Glob. Biogeochem. Cycles 2008. [Google Scholar] [CrossRef]
- Lehner, B.; Liermann, C.R.; Revenga, C.; Vörösmarty, C.; Fekete, B.; Crouzet, P.; Döll, P.; Endejan, M.; Freken, K.; Magome, J.; et al. High-resolution mapping of the world’s reservoirs and dams for sustainable river-flow management. Front. Ecol. Environ. 2011, 9, 494–502. [Google Scholar] [CrossRef]
- Winslow, L.A.; Read, J.S.; Hanson, P.C.; Stanley, E.H. Lake shoreline in the contiguous United States: Quantity, distribution and sensitivity to observation resolution. Freshw. Biol. 2013, 59, 213–223. [Google Scholar] [CrossRef]
- Prairie, Y.T.; Alm, J.; Beaulieu, J.; Barros, N.; Battin, T.; Cole, J.; del Giorgio, P.; DelSontro, T.; Guérin, F.; Harby, A.; et al. Greenhouse Gas Emissions from Freshwater Reservoirs: What Does the Atmosphere See? Ecosystems 2018, 21, 1058–1071. [Google Scholar] [CrossRef]
- Deemer, B.R.; Harrison, J.A.; Li, S.; Beaulieu, J.J.; DelSontro, T.; Barros, N.; Bezerra-neto, J.F.; Powers, S.M.; Santos, M.; Vonk, J.A. Greenhouse Gas Emissions from Reservoir Water Surfaces: A New Global Synthesis. BioScience 2016, 66, 949–964. [Google Scholar] [CrossRef] [Green Version]
- Bevelhimer, M.S.; Stewart, A.J.; Fortner, A.M.; Phillips, J.R.; Mosher, J.J. CO2 is Dominant Greenhouse Gas Emitted from Six Hydropower Reservoirs in Southeastern United States during Peak Summer Emissions. Water 2016, 8, 15. [Google Scholar] [CrossRef]
- Denfeld, B.A.; Wallin, M.B.; Sahlee, E.; Sobek, S.; Kokic, J.; Chmiel, H.E.; Weyhenmeyer, G.A. Temporal and spatial carbon dioxide concentration patterns in a small boreal lake in relation to ice-cover dynamics. Boreal Environ. Res. 2015, 20, 679–692. [Google Scholar]
- Laas, A.; Cremona, F.; Meinson, P.; Rõõm, E.; Nõges, T.; Nõges, P. Summer depth distribution profiles of dissolved CO2 and O2 in shallow temperate lakes reveal trophic state and lake type specific differences. Sci. Total Environ. 2016, 566, 63–75. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.P.; Stets, E.G.; Striegl, R.G.; Butman, D. Inorganic carbon loading as a primary driver of dissolved carbon dioxide concentrations in the lakes and reservoirs of the contiguous United States. Glob. Biogeochem. Cycles 2013, 27, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Ballantyne, A.P.; Andres, R.; Houghton, R.; Stocker, B.D.; Wanninkhof, R.; Anderegg, W.; Cooper, L.A.; DeGrandpre, M.; Tans, P.P.; Miller, J.B.; et al. Audit of the global carbon budget: Estimate errors and their impact on uptake uncertainty. Biogeosciences 2015, 12, 2565–2584. [Google Scholar] [CrossRef]
- Wang, F.; Cao, M.; Wang, B.; Fu, J.; Luo, W.; Ma, J. Seasonal variation of CO2 diffusion flux from a large subtropical reservoir in East China. Atmos. Environ. 2015, 103, 129–137. [Google Scholar] [CrossRef]
- Mosher, J.J.; Fortner, A.M.; Phillips, J.M.; Bevelhimer, M.S.; Stewart, A.J.; Troia, M.J. Spatial and temporal correlates of greenhouse gas diffusion from a hydropower reservoir in the Southern United States. Water 2015, 7, 5910–5927. [Google Scholar] [CrossRef]
- Yang, M.; Grace, J.; Geng, X.; Guan, L.; Zhang, Y.; Lei, J.; Lu, C.; Lei, G. Carbon Dioxide Emissions from the Littoral Zone of a Chinese Reservoir. Water 2017, 9, 539. [Google Scholar] [CrossRef]
- Bastien, J.; Demarty, M.; Tremblay, A. CO2 and CH4 diffusive and degassing fluxes from 2003 to 2009 at Eastmain 1 reservoir, Québec, Canada. Inland Waters 2011, 1, 113–123. [Google Scholar] [CrossRef]
- Demarty, M.; Bastien, J.; Tremblay, A. Annual follow-up of gross diffusive carbon dioxide and methane emissions from a boreal reservoir and two nearby lakes in Québec, Canada. Biogeosciences 2011, 8, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, J.J.; Smolenski, R.L.; Nietch, C.T.; Townsend-Small, A.; Elovitz, M.S. High methane emissions from a midlatitude reservoir draining an agricultural watershed. Environ. Sci. Technol. 2014, 48, 11100–11108. [Google Scholar] [CrossRef] [PubMed]
- K-Water. Available online: http://english.kwater.or.kr/ (accessed on 31 July 2018).
- UNESCO/IHA. GHG Measurement Guidelines for Freshwater Reservoirs; International Hydropower Association: London, UK, 2010; ISBN 978-0-9566228-0-8. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005; ISBN 9780875530475. [Google Scholar]
- Lewis, E.; Wallace, D. Program Developed for CO2 System Calculations; Department of Applied Science Brookhaven National Laboratory: Upton, NY, USA, 1998. [Google Scholar] [CrossRef]
- Raymond, P.A.; Caraco, N.F.; Cole, J.J. Carbon dioxide concentration and atmospheric flux in the Hudson River. Estuaries Coasts 1997, 20, 381–390. [Google Scholar] [CrossRef]
- Wang, Z.A.; Bienvenu, D.J.; Mann, P.J.; Hoering, K.A.; Poulsen, J.R.; Spencer, R.G.M. Holmes, R.M. Inorganic carbon speciation and fluxes in the Congo River. Geophys. Res. Lett. 2013, 40, 511–516. [Google Scholar] [CrossRef]
- Chung, S.W.; Park, H.S.; Yoo, J.S. Variability of pCO2 in surface waters and development of prediction model. Sci. Total Environ. 2018, 622–623, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Hebbali, A. Tools for Teaching and Learning OLS Regression. 2017. Available online: https://rsquaredacademy.github.io/olsrr/ (accessed on 31 July 2018).
- Liaw, A.; Wiener, M. Classification and regression by random forest. R News. 2002, 2, 18–22. Available online: https://www.r-project.org/doc/Rnews/Rnews_2002-3.pdf (accessed on 31 December 2002).
- Winslow, L.A.; Read, J.S.; Woolway, R.; Brentrup, J.; Leach, T.; Zwart, J.; Albers, S.; Collinge, D. Lake Physics Tools. 2017. Available online: https://github.com/GLEON/rLakeAnalyzer (accessed on 31 July 2018).
- Idso, S.B. On the concept of lake stability. Limnol. Oceanogr. 1973, 18, 681–683. [Google Scholar] [CrossRef] [Green Version]
- Imberger, J.; Patterson, J.C. Physical limnology. Adv. App. Mech. 1989, 27, 303–475. [Google Scholar] [CrossRef]
- Amaral, J.H.; Borges, A.V.; Melack, J.M.; Sarmento, H.; Barbosa, P.M.; Kasper, D.; Melo, M.L.; Fex-Wolf, D.D.; Silva, J.S.; Forsberg, B.R. Influence of plankton metabolism and mixing depth on CO2 dynamics in an Amazon floodplain lake. Sci. Total Environ. 2018, 630, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, M.A.; Rosa, L.P.; Sikar, B.; Sikar, E.; dos Santos, E.O. Gross greenhouse gas fluxes from hydro-power reservoir compared to thermos-power plants. Energy Policy 2006, 34, 481–488. [Google Scholar] [CrossRef]
- Tadonléké, R.D.; Marty, J.; Planas, D. Assessing factors underlying variation of CO2 emissions in boreal lakes vs. reservoirs. FEMS Microbiol. Ecol. 2012, 79, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, F.S.; Roland, F.; Downing, J.A. Eutrophication reverses whole-lake carbon budgets. Inland Waters 2013, 4, 41–48. [Google Scholar] [CrossRef]
- Hanson, P.C.; Pace, M.L.; Carpenter, S.R.; Cole, J.J.; Stanley, E.H. Integrating landscape carbon cycling: Research needs for resolving organic carbon budgets of lakes. Ecosystems 2015, 18, 363–375. [Google Scholar] [CrossRef]
- Jonsson, A.; Karlsson, J.; Jansson, M. Sources of carbon dioxide supersaturation in clearwater and humic lakes in northern Sweden. Ecosystems 2003, 6, 224–235. [Google Scholar] [CrossRef]
- Jones, S.E.; Kratz, T.K.; Chiu, C.Y.; McMahon, K.D. Influence of typhoons on annual CO2 flux from a subtropical, humic lake. Glob. Chang. Biol. 2009, 15, 243–254. [Google Scholar] [CrossRef]
- Marotta, H.; Duarte, C.M.; Pinho, L.; Enrich-Prast, A. Rainfall leads to increased pCO2 in Brazilian coastal lakes. Biogeosciences 2010, 7, 1607–1614. [Google Scholar] [CrossRef] [Green Version]
- Pquay, F.S.; Mackenzie, F.T.; Borges, A.V. Carbon dioxide dynamics in rivers and coastal waters of the “big island” of Hawaii, USA, during baseline and heavy rain conditions. Aquat. Geochem. 2007, 13, 1–18. [Google Scholar] [CrossRef]
- McManus, J.; Heinen, E.A.; Baehr, M.M. Hypolimnetic oxidation rates in Lake Superior: Role of dissolved organic material on the lake’s carbon budget. Limnol. Oceanogr. 2003, 48, 1624–1632. [Google Scholar] [CrossRef]
- Liss, P.; Slater, P. Fluxes of gases across the air-sea interface. Nature 1974, 247, 181–184. [Google Scholar] [CrossRef]
- Cole, J.; Prairie, Y.T. Dissolved CO2. In Encyclopedia of Inland Waters; Elsevier Science & Technology: Amsterdam, The Netherlands, 2009; pp. 30–34. [Google Scholar] [CrossRef]
- Wanninkhof, R.; Knox, M. Chemical enhancement of CO2 exchange in natural waters. Limnol. Oceanogr. 1996, 41, 689–697. [Google Scholar] [CrossRef]
- Louis, V.L.; Kelly, C.A.; Duchemin, É.; Rudd, J.W.M.; Rosenberg, D.M. Reservoir Surfaces as Sources of Greenhouse Gases to the Atmosphere: A Global Estimate: Reservoirs are sources of greenhouse gases to the atmosphere, and their surface areas have increased to the point where they should be included in global inventories of anthropogenic emissions of greenhouse gases. BioScience 2000, 50, 766–775. [Google Scholar] [CrossRef]

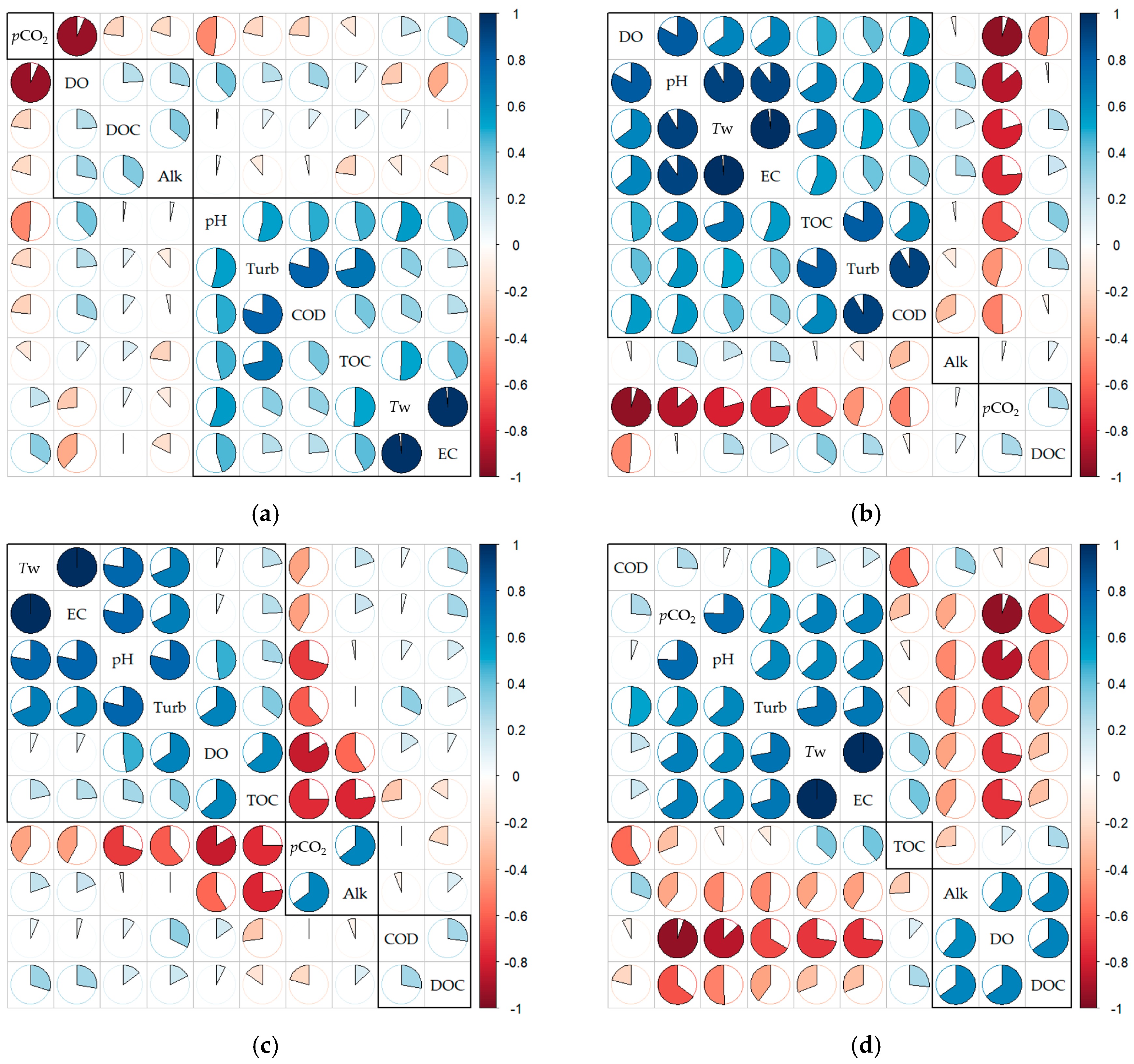
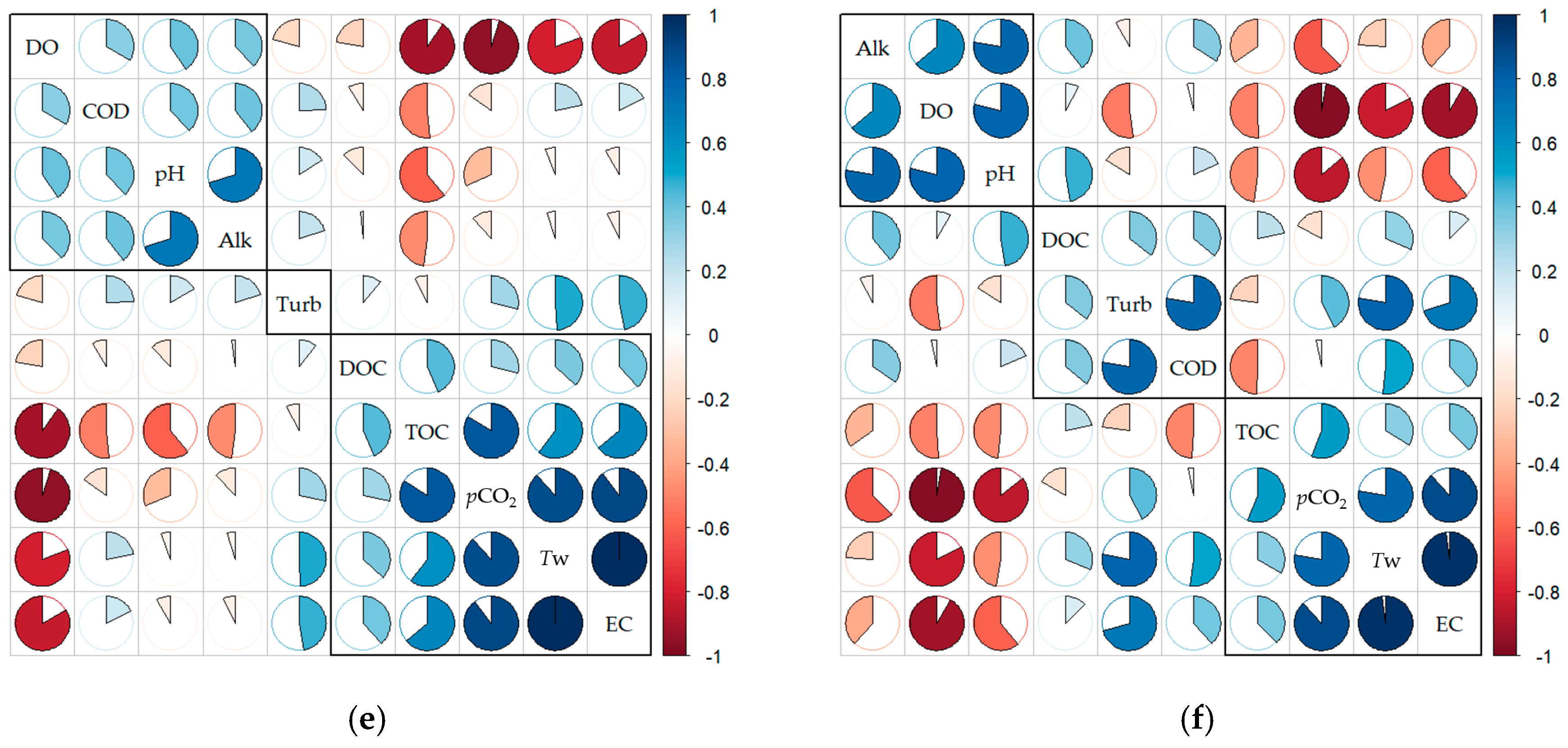



| Index | pCO21 (µatm) | Tw 2 (°C) | Cond 3 (μS·cm−1) | DO 4 (mg·L−1) | pH | Turb 5 (NTU) | COD 6 (mg·L−1) | TOC 7 (mg·L−1) | DOC 8 (mg·L−1) | Chl-a 9 (mg·m−3) | Alk 10 (mg·L−1 as CaCO3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| nobs11 | 58 | 200 | 200 | 200 | 200 | 160 | 200 | 200 | 200 | 94 | 200 |
| Min | 215 | 7.2 | 130 | 1.6 | 6.9 | 0.4 | 0.2 | 1.6 | 1.6 | 0.1 | 30.0 |
| Max | 6000 | 30.5 | 187 | 13.1 | 8.7 | 2.9 | 10.3 | 11.9 | 8.3 | 19.9 | 51.4 |
| median | 1538 | 21.4 | 157 | 7.1 | 7.4 | 0.8 | 2.5 | 2.5 | 2.6 | 1.1 | 42.0 |
| Mean | 2085 | 21.3 | 154.4 | 6.7 | 7.5 | 1.0 | 2.6 | 2.7 | 2.7 | 3.1 | 42.2 |
| std dev | 1529 | 4.7 | 14.1 | 2.2 | 0.4 | 0.6 | 1.3 | 1.5 | 0.7 | 4.9 | 3.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Chung, S. pCO2 Dynamics of Stratified Reservoir in Temperate Zone and CO2 Pulse Emissions During Turnover Events. Water 2018, 10, 1347. https://doi.org/10.3390/w10101347
Park H, Chung S. pCO2 Dynamics of Stratified Reservoir in Temperate Zone and CO2 Pulse Emissions During Turnover Events. Water. 2018; 10(10):1347. https://doi.org/10.3390/w10101347
Chicago/Turabian StylePark, Hyungseok, and Sewoong Chung. 2018. "pCO2 Dynamics of Stratified Reservoir in Temperate Zone and CO2 Pulse Emissions During Turnover Events" Water 10, no. 10: 1347. https://doi.org/10.3390/w10101347






