Developing a Biotechnological Tool for Monitoring Water Quality: In Vitro Clone Culture of the Aquatic Moss Fontinalis Antipyretica
Abstract
:1. Introduction
2. Material and Methods
2.1. Propagation of Fontinalis Antipyretica Gametophytes for In Vitro Culture
2.2. In Vitro Cultivation Techniques
2.3. Statistical Analysis
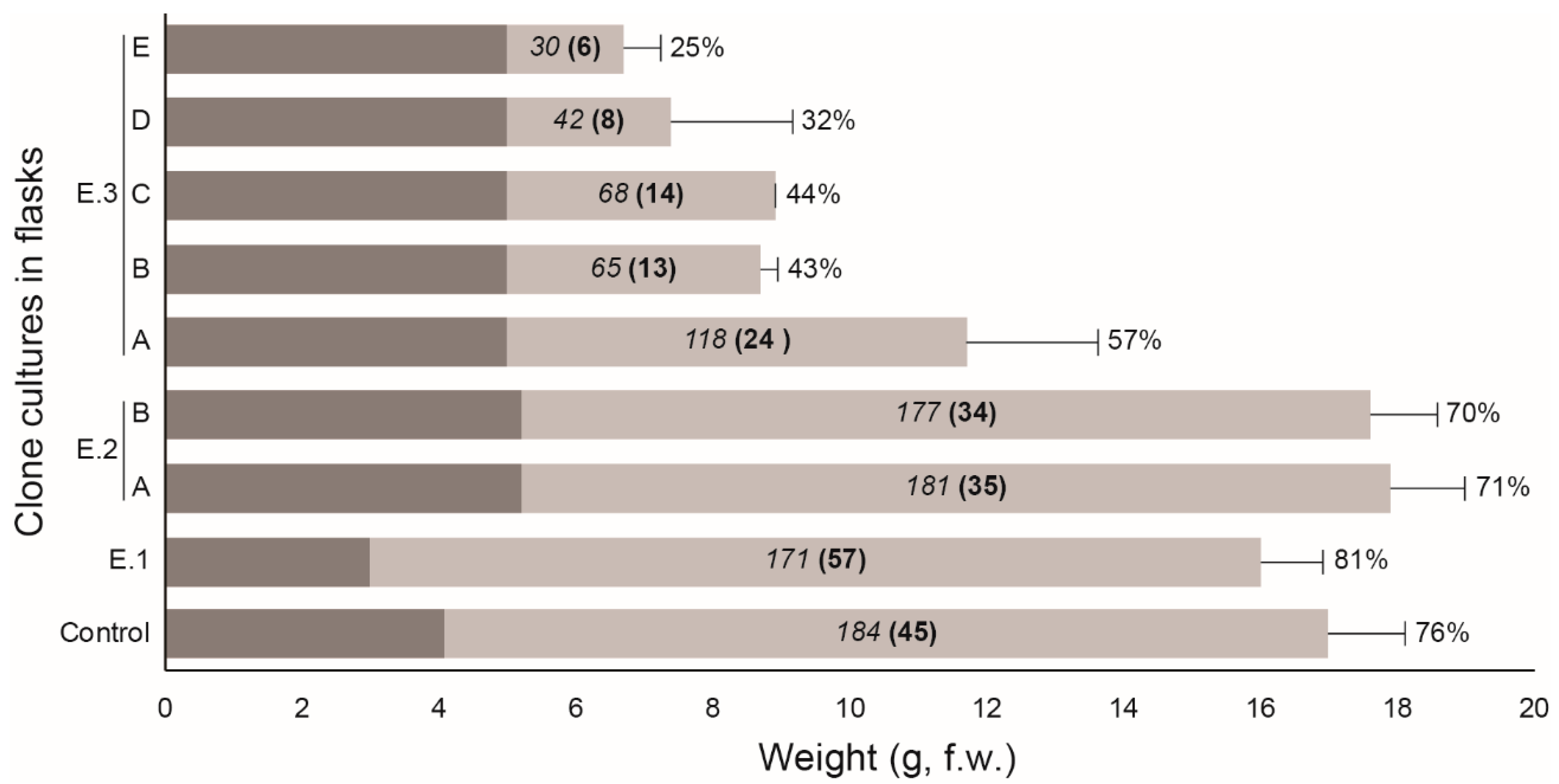
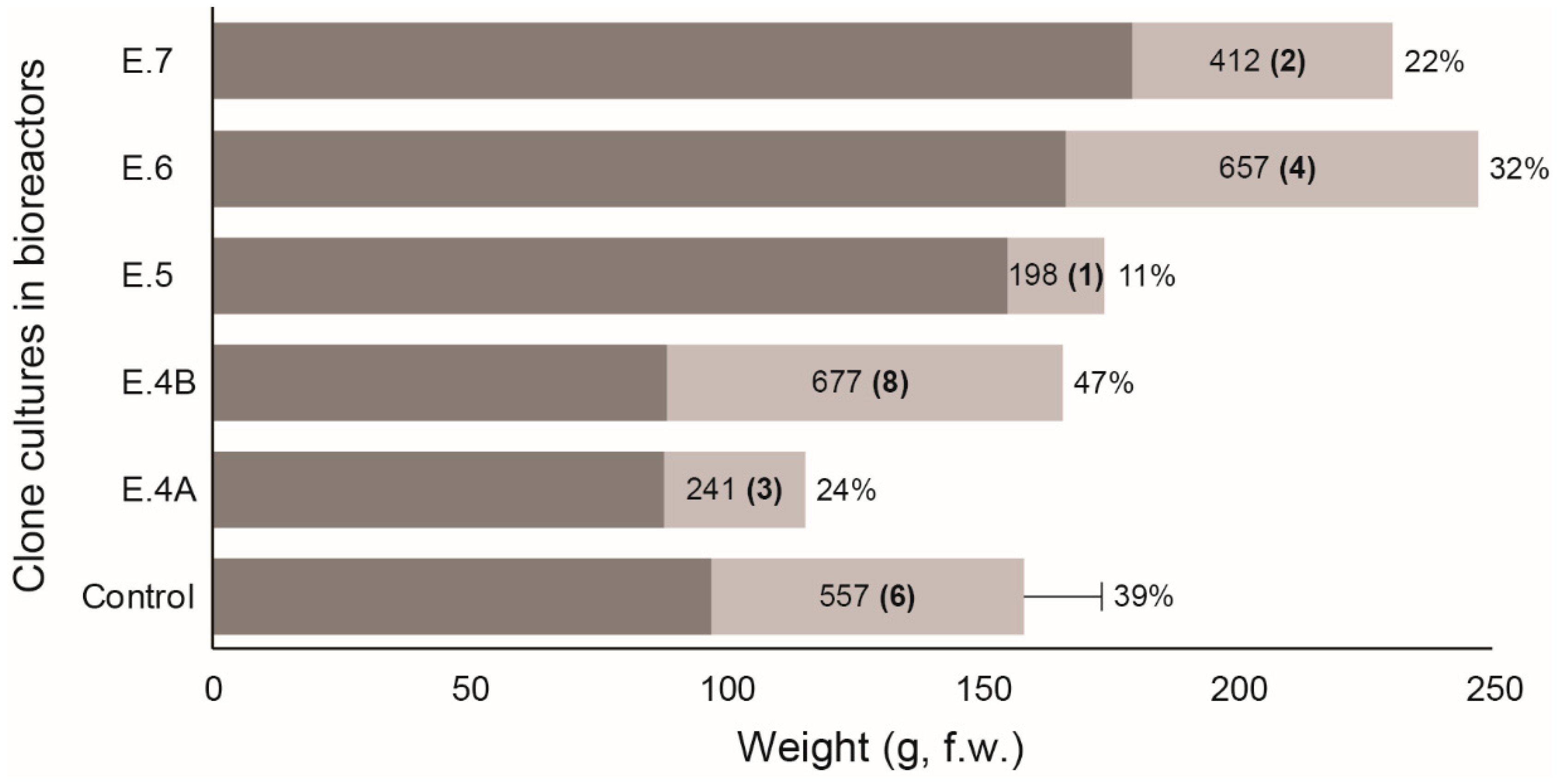
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Debén, S.; Aboal, J.R.; Carballeira, A.; Cesa, M.; Fernández, J.A. Monitoring river water quality with transplanted bryophytes: A methodological review. Ecol. Ind. 2017, 81, 461–470. [Google Scholar] [CrossRef]
- Martínez-Abaigar, J.; García-Álvaro, M.A.; Beaucourt, N.; Núñez-Olivera, E. Combined seasonal and longitudinal variations of element concentrations in two aquatic mosses (Fontinalis antipyretica and F. squamosa). Nova Hedwigia 2002, 74, 349–364. [Google Scholar] [CrossRef]
- Ares, A.; Aboal, J.R.; Carballeira, A.; Giordano, S.; Adamo, P.; Fernández, J.A. Moss bag biomonitoring: A methodological review. Sci. Total Environ. 2012, 432, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Sen, C.K.; Hänninen, O. Monitoring of polycyclic aromatic hydrocarbons using ‘moss bags’: Bioaccumulation and responses of antioxidant enzymes in Fontinalis antipyretica Hedw. Chemosphere 1996, 32, 2305–2315. [Google Scholar] [CrossRef]
- Hongve, D.; Brittain, J.E.; Bjørnstad, H.E. Aquatic mosses as a monitoring tool for 137Cs contamination in streams and rivers-a field study from central southern Norway. J. Environ. Radioact. 2002, 60, 139–147. [Google Scholar] [CrossRef]
- Di Palma, A.; Pardo, D.C.; Spagnuolo, V.; Adamo, P.; Bargagli, R.; Cafasso, D.; Capozzi, F.; Aboal, J.R.; González, A.G.; Pokrovsky, O.; et al. Molecular and chemical characterization of a Sphagnum palustre clone: Key steps towards a standardized and sustainable moss bag technique. Ecol. Ind. 2016, 71, 88–397. [Google Scholar] [CrossRef]
- De Traubenberg, C.R.; Ah-Peng, C. A Procedure to Purify and Culture a Clonal Strain of the Aquatic Moss Fontinalis antipyretica for Use as a Bioindicator of Heavy Metals. Arch. Environ. Contam. Toxicol. 2004, 46, 289–295. [Google Scholar] [CrossRef]
- Capozzi, F.; Giordano, S.; Aboal, J.R.; Adamo, P.; Bargagli, R.; Boquete, T.; Di Palma, A.; Real, C.; Reski, R.; Spagnuolo, V.; et al. Best options for the exposure of traditional and innovative moss bags: A systematic evaluation in three European countries. Environ. Pollut. 2016, 214, 362–473. [Google Scholar] [CrossRef]
- Beike, A.K.; Spagnuolo, V.; Lüth, V.; Steinhart, F.; Ramos-Gómez, J.; Krebs, M.; Adamo, P.; Rey-Asensio, A.I.; Fernández, J.A.; Giordano, S.; et al. Clonal in vitro propagation of peat mosses (Sphagnum L.) as novel green resources for basic and applied research. Plant Cell Tissue Organ Cult. 2015, 120, 1037–1049. [Google Scholar] [CrossRef]
- Ares, A.; Duckett, J.G.; Pressel, S. Asexual reproduction and protonemal development in vitro in Fontinalis antipyretica Hedw. J. Bryol. 2014, 36, 122–133. [Google Scholar] [CrossRef]
- Hohe, A.; Reski, R. Optimisation of a bioreactor culture of the moss Physcomitrella patens for mass production of protoplasts. Plant Sci. 2002, 163, 69–74. [Google Scholar] [CrossRef]
- Schween, G.; Hohe, A.; Koprivova, A.; Reski, R. Effects of nutrients, cell density and culture techniques on protoplast regeneration and early protonema development in a moss, Physcomitrella patens. J. Plant Physiol. 2003, 160, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Bowes, G. Pathways of CO2 fixation by aquatic organisms. In Inorganic Carbon Uptake by Aquatic Photosynthetic Organisms; Lucas, W.J., Berry, J.A., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 1985; pp. 87–210. [Google Scholar]
- Carballeira, A.; Díaz, S.; Vázquez, M.D.; López, J. Inertia and resilience in the responses of the aquatic bryophyte Fontinalis antipyretica Hedw. to thermal stress. Arch. Environ. Contam. Toxicol. 1998, 34, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Peñuelas, J. HCO3− as an exogenous carbon source for aquatic bryophytes Fontinalis antipyretica and Fissidens grandifrons. J. Exp. Bot. 1985, 36, 441–448. [Google Scholar] [CrossRef]
- Decker, E.L.; Reski, R. Current achievements in the production of complex biopharmaceuticals with moss bioreactors. Bioprocess Biosyst. Eng. 2008, 31, 3–9. [Google Scholar] [CrossRef]
- Maberly, S.C. Photosynthesis by Fontinalis antipyretica I. IInteraction between photon irradiance concentration of carbon dioxide and temperature. New Phytol. 1985, 100, 127–140. [Google Scholar] [CrossRef]
- Maberly, S.C. Photosynthesis by Fontinalis antipyretica II. Assessment of environmental factors limiting photosynthesis and production. New Phytol. 1985, 100, 141–155. [Google Scholar] [CrossRef]
- Jarvie, H.P.; Whitton, B.A.; Neal, C. Nitrogen and phosphorus in east coast British rivers: Speciation, sources and biological significance. Sci. Total Environ. 1998, 210/211, 79–109. [Google Scholar] [CrossRef]
- Carroll, J.A.; Caporn, S.J.M.; Johnson, D.; Morecroft, M.D.; Lee, J.A. The interactions between plant growth, vegetation structure and soil processes in semi-natural acidic and calcareous grasslands receiving long-term inputs of simulated pollutant nitrogen deposition. Environ. Pollut. 2003, 121, 363–376. [Google Scholar] [CrossRef]
- Phoenix, G.K.; Booth, R.E.; Leake, J.R.; Read, D.J.; Grime, J.P.; Lee, J.A. Effects of enhanced nitrogen deposition and phosphorus limitation on nitrogen budgets of semi-natural grasslands. Glob. Chang. Biol. 2003, 9, 1309–1321. [Google Scholar] [CrossRef]
- Jauhiainen, J.; Vasander, H.; Silvola, J. Response of Sphagnum fuscum to N deposition and increased CO2. J. Bryol. 1994, 18, 83–95. [Google Scholar] [CrossRef]
- Kooijman, A.M.; Bakker, C. Species replacement in the bryophyte layer in mires: The role of water type, nutrient supply and interspecific interactions. J. Ecol. 1995, 83, 1–8. [Google Scholar] [CrossRef]
- Gerdol, R.; Bonora, A.; Marchesini, R.; Gualandri, R.; Pancaldi, S. Growth response of Sphagnum capillifolium to nighttime temperature and nutrient level: Mechanisms and implications for global change. Arctic Alpine Res. 1998, 30, 388–395. [Google Scholar] [CrossRef]
- Gunnarsson, U.; Rydin, H. Nitrogen fertilization reduces Sphagnum production in bog communities. New Phytol. 2000, 147, 527–537. [Google Scholar] [CrossRef] [Green Version]
- Glime, J.M.; Carr, R.E. Temperature survival of Fontinalis novae-angliae Sull. Bryologist 1974, 17–22. [Google Scholar] [CrossRef]
- Glime, J.M.; Acton, D.W. Temperature effects on assimilation and respiration in the Fontinalis duriaei -Periphyton Association. Bryologist 1979, 82, 382–392. [Google Scholar] [CrossRef]
- Dilks, T.J.K.; Proctor, M.C.F. Comparative experiments on temperature responses of bryophytes: Assimilation, respiration and freezing damage. J. Bryol. 1975, 8, 317–336. [Google Scholar] [CrossRef]
- Glime, J.M.; Raeymaekers, G. Temperature effects on branch and rhizoid production in six species of Fontinalis. J. Bryol. 1987, 14, 779–790. [Google Scholar] [CrossRef]
- Glime, J.M.; Vitt, D.H. The physiological adaptations of aquatic music. Lindbergia 1984, 10, 41–52. [Google Scholar]
- Tani, A.; Akita, M.; Murase, H.; Kimbara, K. Culturable bacteria in hydroponic cultures of moss Racomitrium japonicum and their potential as biofertilizers for moss production. J. Biosci. Bioeng. 2011, 112, 32–39. [Google Scholar] [CrossRef]
- Moeller, R.E. The temperature-determined growing season of a submerged hydrophyte: Tissue chemistry and biomass turnover of Utricularia purpurea. Freshw. Biol. 1980, 10, 391–400. [Google Scholar] [CrossRef]


| Type of Culture | Exp. | Treatm. | n | Time (days) | T (°C) | Volume (L) | Photoperiod (h light/dark) | Sucrose (%) | N:P Ratio | P (mg·L−1) | CO2 | NH4 (g·L−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Flasks | Control | 13 | 57–76 | 15 | 0.5 | 16/8 | 0.3 | 2.1 | 250 | - | - | |
| E.1 | 7 | 76 | 15 | 0.5 | 24/0 | 0.3 | 2.1 | 250 | - | - | ||
| E.2 | A | 3 | 70 | 15 | 0.5 | 16/8 | 2 | 2.1 | 250 | - | - | |
| B | 3 | 70 | 15 | 0.5 | 16/8 | 4 | 2.1 | 250 | - | - | ||
| E.3 | A | 3 | 57 | 15 | 0.5 | 16/8 | 0.3 | 23 | 250 | - | - | |
| B | 3 | 57 | 15 | 0.5 | 16/8 | 0.3 | 46 | 250 | - | - | ||
| C | 3 | 57 | 15 | 0.5 | 16/8 | 0.3 | 2.1 | 5200 | - | - | ||
| D | 3 | 57 | 15 | 0.5 | 16/8 | 0.3 | 23 | 500 | - | - | ||
| E | 3 | 57 | 15 | 0.5 | 16/8 | 0.3 | 46 | 500 | - | - | ||
| Bioreactors | Control | 4 | 97–135 | 15 | 12 | 24/0 | 0.3 | 2.1 | 250 | - | - | |
| E.4 | A | 1 | 113 | 10 | 12 | 24/0 | 0.3 | 2.1 | 250 | - | - | |
| B | 1 | 114 | 20 | 12 | 24/0 | 0.3 | 2.1 | 250 | - | - | ||
| E.5 | 1 | 97 | 15 + 20 | 4 + 8 | 24/0 | 0.3 | 2.1 | 250 | - | - | ||
| E.6 | 1 | 122 | 15 | 12 | 24/0 | 0.3 | 2.1 | 250 | 0.1 | - | ||
| E.7 | 1 | 123 | 15 | 12 | 24/0 | 0.3 | 2.1 | 250 | - | 0.1 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debén, S.; Aboal, J.R.; Giráldez, P.; Varela, Z.; Fernández, J.Á. Developing a Biotechnological Tool for Monitoring Water Quality: In Vitro Clone Culture of the Aquatic Moss Fontinalis Antipyretica. Water 2019, 11, 145. https://doi.org/10.3390/w11010145
Debén S, Aboal JR, Giráldez P, Varela Z, Fernández JÁ. Developing a Biotechnological Tool for Monitoring Water Quality: In Vitro Clone Culture of the Aquatic Moss Fontinalis Antipyretica. Water. 2019; 11(1):145. https://doi.org/10.3390/w11010145
Chicago/Turabian StyleDebén, Sofía, Jesús Ramón Aboal, Pablo Giráldez, Zulema Varela, and Jose Ángel Fernández. 2019. "Developing a Biotechnological Tool for Monitoring Water Quality: In Vitro Clone Culture of the Aquatic Moss Fontinalis Antipyretica" Water 11, no. 1: 145. https://doi.org/10.3390/w11010145
APA StyleDebén, S., Aboal, J. R., Giráldez, P., Varela, Z., & Fernández, J. Á. (2019). Developing a Biotechnological Tool for Monitoring Water Quality: In Vitro Clone Culture of the Aquatic Moss Fontinalis Antipyretica. Water, 11(1), 145. https://doi.org/10.3390/w11010145








