Light Absorption Budget in a Reservoir Cascade System with Widely Differing Optical Properties
Abstract
:1. Introduction
2. Materials and Methods
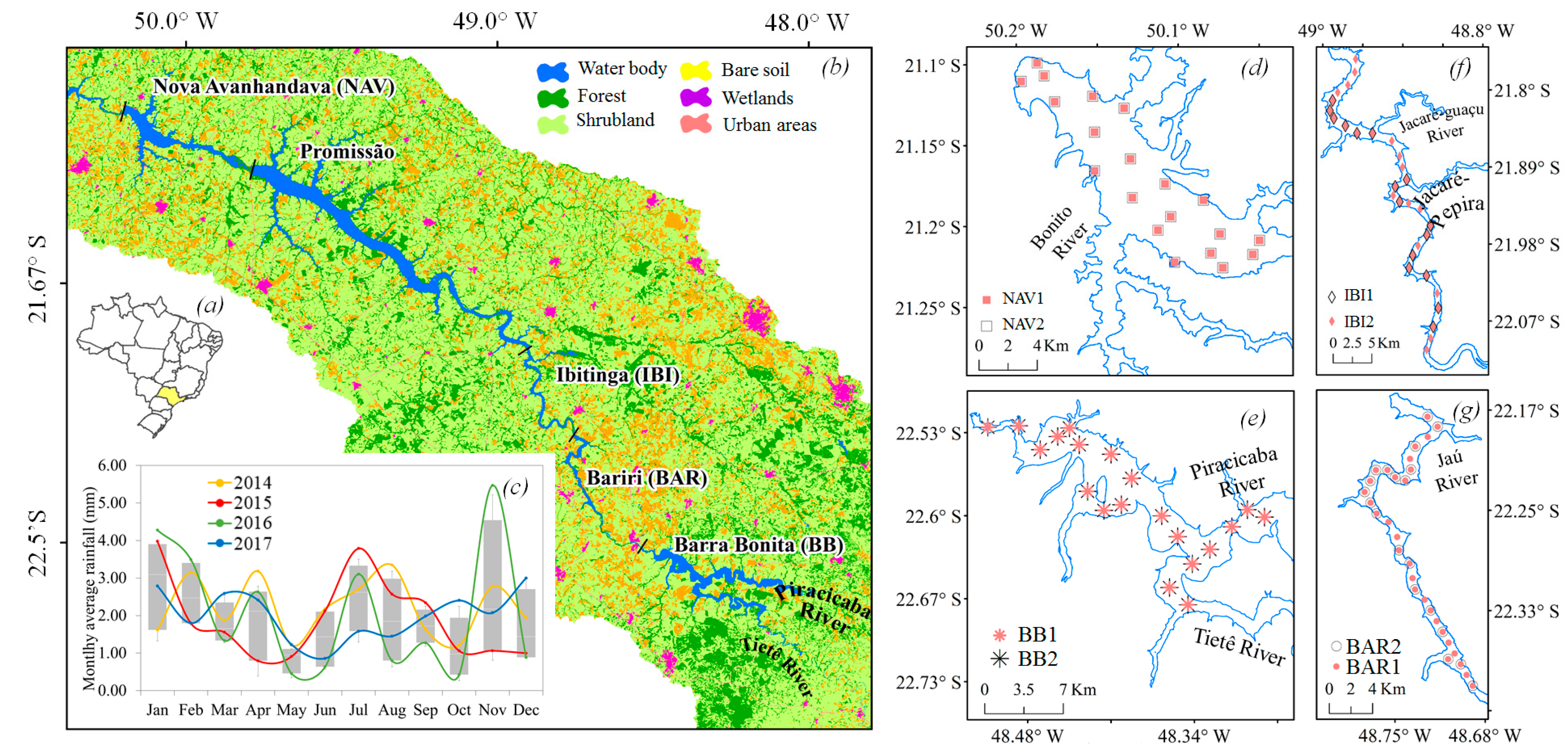
2.1. Study Area
2.2. Field Survey
2.3. Absorption Coefficients
3. Results
3.1. Water Quality Scenery
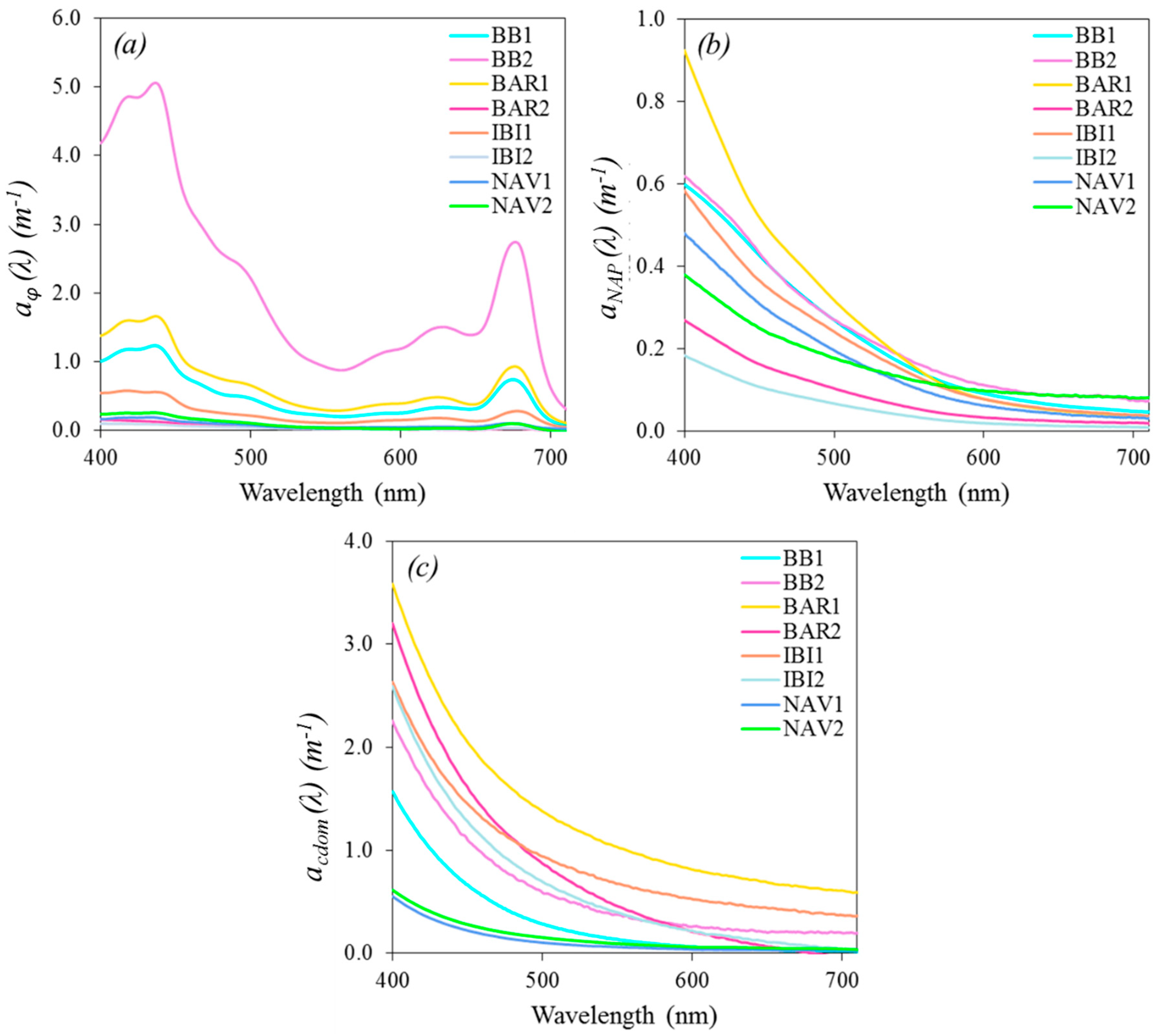
3.2. Absorption Spectra
3.2.1. Absorption by CDOM
3.2.2. Absorption by Particulate Matter
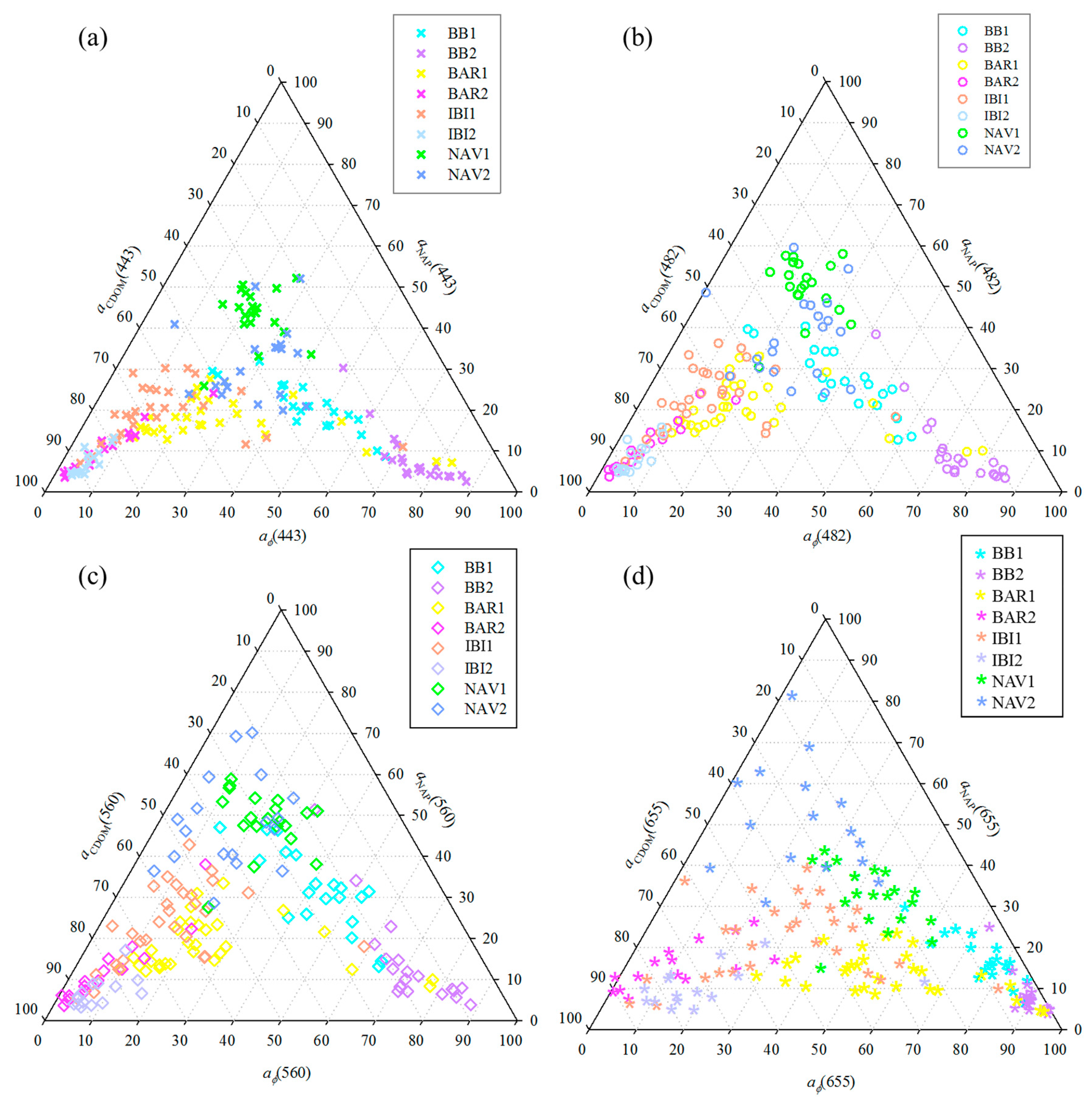
3.3. Relative Contribution of OAC’s Absorptions in TCSR
3.4. Absorption Budget
3.5. Light Absorption Variability from Upstream to Downstream
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, Z.; Carder, K.L.; Arnone, R.A. Deriving inherent optical properties from water color: A multiband quasi-analytical algorithm for optically deep waters. Appl. Opt. 2002, 41, 5755–5772. [Google Scholar] [CrossRef] [PubMed]
- Odermatt, D.; Gitelson, A.; Brando, V.E.; Schaepman, M. Review of constituent retrieval in optically deep and complex waters from satellite imagery. Remote Sens. Environ. 2012, 118, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, T.; Alcântara, E.; Watanabe, F.; Bernardo, N.; Rotta, L.; Imai, N. Spatial and temporal variations of the inherent optical properties in a tropical cascading reservoir system. Model. Earth Syst. Environ. 2016, 2, 86. [Google Scholar] [CrossRef]
- Watanabe, F.; Mishra, D.R.; Astuti, I.; Rodrigues, T.; Alcântara, E.; Imai, N.; Barbosa, C. Parametrization and calibration of a quasi-analytical algorithm for tropical eutrophic waters. ISPRS J. Photogramm. Remote Sens. 2016, 12, 28–47. [Google Scholar] [CrossRef]
- Kirk, J. Light and Photosysnthesis in Aquactic Ecosystems, 2nd ed.; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Bricaud, A.; Morel, A.; Louis, P. Absorption by dissolved organic matter of the sea (yellow substance) in the UV and visible domains. Limnol. Oceanogr. 1981, 26, 43–53. [Google Scholar] [CrossRef]
- Bricaud, A.; Babin, M.; Morel, A.; Claustre, H. Variability in the chlorophyll-specific absorption coefficients of natural phytoplankton: Analysis and parameterization. J. Geophys. Res. 1995, 100, 13321–13332. [Google Scholar] [CrossRef]
- Bricaud, A.; Morel, A.; Babin, M.; Allali, K.; Claustre, H. Variations of light absorption by suspended particles with chlorophyll a concentration in oceanic (case 1) waters: Analysis and implications for bio-optical models. J. Geophys. Res. 1998, 103, 31033–31044. [Google Scholar] [CrossRef]
- Babin, M.; Stramski, D.; Ferrari, G.M.; Claustre, H.; Bricaud, A.; Obolensky, G.; Hoepffner, N. Variations in the light absorption coefficients of phytoplankton, nonalgal particles, and dissolved organic matter in coastal waters around Europe. J. Geophys. Res. 2003, 108. [Google Scholar] [CrossRef] [Green Version]
- Gokul, E.A.; Palanisamy, S.; Sundarabalan, B.; Sahay, A.; Chauhan, P. Modelling the inherent optical properties and estimating the constituents’ concentrations in turbid and eutrophic waters. Cont. Shelf Res. 2014, 84, 120–138. [Google Scholar] [CrossRef]
- Shaju, S.S.; Minu, P.; Srokanth, A.S.; Ashraf, P.M.; Vijayan, A.K.; Meenakumari, B. Decomposition study of in vivo phytoplankton absorption spectra to identify the pigments and phytoplankton group in complex case 2 waters of coastal Arabian Sea. Oceanol. Hydrobiol. Stud. 2017, 44, 282–293. [Google Scholar] [CrossRef]
- Vishnu, P.S.; Shaju, S.S.; Tiwari, S.P.; Menon, N.; Nashad, M.; Joseph, C.A.; Raman, M.; Hatha, M.; Prabhakaran, M.P.; Mohandas, A. Seasonal variability in bio-optical properties along the coastal waters of Cochin. Int. J. Appl. Earth Obs. Geoinf. 2018, 66, 184–195. [Google Scholar] [CrossRef]
- Das, S.; Hazra, S.; Giri, S.; Das, I.; Chanda, A.; Akhand, A.; Maity, S. Light absorption characteristics of chromophoric dissolved organic matter (CDOM) in the coastal waters of northern Bay of Bengal during winter season. Indian J. Geo Mar. Sci. 2017, 46, 884–992. [Google Scholar]
- Ferreira, A.; Ciotti, A.M.; Giannini, M.F.C. Variability in the light absorption coefficients of phytoplankton, non-algae particles, and colored dissolved organic matter in a subtropical bay (Brazil). Estuar. Coast. Shelf Sci. 2014, 139, 127–136. [Google Scholar] [CrossRef]
- Campbell, G.; Phinn, S.R.; Daniel, P. The specific inherent optical properties of three subtropical and tropical water reservoirs in Queensland, Australia. Hydrobiologia 2011, 658, 233–252. [Google Scholar] [CrossRef]
- Matthews, M.W.; Bernard, S. Characterizing the absorption properties for remote sensing of three small optically-diverse South African reservoirs. Remote Sens. 2013, 5, 4370–4404. [Google Scholar] [CrossRef]
- Alcântara, E.; Watanabe, F.; Rodrigues, T.; Bernardo, N.; Rotta, L.; Carmo, A.; Curtarelli, M.; Imai, N. Field measurements of the backscattering coefficient in a cascading reservoir system: First results from Nova Avanhandava and Barra Bonita Reservoirs (São Paulo, Brazil). Remote Sens. Lett. 2016, 7, 417–426. [Google Scholar] [CrossRef]
- Moore, T.; Dowell, M.D.; Bradt, S.; Verdu, A.R. An optical water type framework for selecting and blending retrievals from bio-optical algorithms in lake and coastal waters. Remote Sens. Environ. 2014, 143, 97–111. [Google Scholar] [CrossRef]
- Shen, Q.; Li, J.; Zhang, F.; Li, J.; Li, W.; Zhang, B. Classification of several optically complex waters in China using in situ remote sensing reflectance. Remote Sens. 2015, 7, 429–440. [Google Scholar] [CrossRef]
- Spyrakos, E.; O’Donnell, R.; Hunter, P.D.; Miller, C.; Scott, M.; Simis, S.G.; Neil, C.; Barbosa, C.C.; Binding, C.E.; Bradt, S.; et al. Optical types of inland and coastal waters. Limnol. Oceanogr. 2018, 63, 846–870. [Google Scholar] [CrossRef]
- Pahlevan, N.; Lee, Z.; Wei, J.; Schaaf, C.B.; Schott, J.R.; Berk, A. On-orbit radiometric characterizations of OLI (Landast-8) for applications in aquatic remote sensing. Remote Sens. Environ. 2014, 154, 272–284. [Google Scholar] [CrossRef]
- Rodrigues, T.; Alcântara, E.; Watanabe, F.; Imai, N. Retrieval of Secchi Disk Depth from reservoir using a semi-analytical scheme. Remote Sens. Environ. 2017, 198, 213–228. [Google Scholar] [CrossRef]
- Muow, C.B.; Greb, S.; Aurin, D.; DiGiacomo, P.M.; Lee, Z.; Twardowski, M.T.; Binding, C.; Hu, C.; Ma, R.; Moore, T.; et al. Aquatic color radiometry remote sensing of coastal and inland waters: Challenges and recommendations for future satellite missions. Remote Sens. Environ. 2013, 160, 15–30. [Google Scholar] [CrossRef]
- Palmer, S.C.J.; Kutser, T.; Hunter, P.D. Remote sensing of inland waters: Challenges, progress and future directions. Remote Sens. Environ. 2015, 157, 1–7. [Google Scholar] [CrossRef]
- Shi, L.; Mao, Z.; Wu, J.; Liu, M.; Zhang, Y.; Wang, Z. Variations in spectral absorption properties of phytoplankton, non-algae particles and Chromophoric Dissolved Organic Matter in Lake Qiandaohu. Water 2018, 9, 352. [Google Scholar] [CrossRef]
- Barbosa, F.A.R.; Padisák, J.; Espíndola, E.L.G.; Borics, G.; Rocha, O. The cascading reservoirs continuum concept (CRCC) and its application to the river Tietê-basin, São Paulo State, Brazil. In Theoretical Reservoir Ecology and its Application; Tundisi, J.G., Straskraba, M., Eds.; International Institute of Ecology, Academy of Sciences and Backhuys Publishers: São Carlos, Brazil, 1999; pp. 425–437. [Google Scholar]
- Smith, W.S.; Espíndola, E.L.G.; Rocha, O. Environmental gradients in reservoirs of the medium and low Tietê River: Limnological differences trough the habitat sequence. Acta Limnol. Bras. 2014, 16, 73–88. [Google Scholar] [CrossRef]
- Frascareli, D.; Cardoso-Silva, S.; Mizael, J.D.; Rosa, A.H.; Pompeo, M.L.M.; Doval, J.C.L.; Moschini, C.V. Spatial distribution, bioavailability, and toxicity of metals in surface sediments of tropical reservoirs, Brazil. Environ. Monit. Assess 2018, 190, 199. [Google Scholar] [CrossRef] [PubMed]
- Dellamano-oliveira, M.J.; Vieira, A.A.H.; Rocha, O.; Colombo, V.; Sant’Anna, C.L. Phytoplankton taxonomic composition and temporal changes in a tropical reservoir. Fund Appl. Limnol. 2008, 171, 27–38. [Google Scholar] [CrossRef]
- Londe, L.R.; Novo, E.M.L.M.; Barbosa, C.; Araujo, C.A.S. Water residence time affecting phytoplankton blooms: Study case in Ibitinga Reservoir (São Paulo, Brazil) using Landsat/TM images. Braz. J. Biol. 2016, 76, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Cairo, C.T.; Barbosa, C.C.F.; Novo, E.M.L.M.; Calijuri, M.C. Spatial and seasonal variation in diffuse attenuation coefficients of downward irradiance at Ibitinga Reservoir, São Paulo, Brazil. Hydrobiologia 2017, 784, 265–282. [Google Scholar] [CrossRef]
- Rodrigues, T.W.P.; Guimarães, U.S.; Rotta, L.H.; Watanabe, F.S.; Alcântara, E.; Imai, N.N. Sampling design in reservoirs based on Landsat-8/OLI images: A case study in Nova Avanhandava reservoir (São Paulo State, Brazil). Bol. Ciênc. Geod. 2016, 22, 304–323. [Google Scholar] [CrossRef]
- APHA (American Public Health Association); AWWA (American Water Works Association); WEF (Water Environmental Federation). Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA; AWWA; WEF: Washington, DC, USA, 1998. [Google Scholar]
- Golterman, H.L. Developments in Water Science 2. Physiological Limnology: An Approach to the Physiology of Lake Ecosystems; Elsevier: Amsterdam, The Netherlands, 1975. [Google Scholar]
- Smith, R.C.; Baker, K.S. Optical properties of the clearest natural waters (200–800 nm). Appl. Opt. 1981, 20, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Tassan, S.; Ferrari, G.M. An alternative approach to absorption measurement of aquatic particles retained on filters. Limnol. Oceanogr. 1995, 40, 1358–1368. [Google Scholar] [CrossRef]
- Tassan, S.; Ferrari, G.M. Measurement of light absorption by aquatic particles retained on filters: Determination of the optical path length amplification by the ‘transmittance-reflectance’ method. J. Plankton Res. 1998, 20, 1699–1709. [Google Scholar] [CrossRef]
- Twardowski, M.S.; Boss, E.; Sullivan, J.M.; Donaghay, P.L. Modeling the spectral shape of absorption by chromophoric dissolved organic matter. Mar. Chem. 2004, 89, 69–88. [Google Scholar] [CrossRef]
- Alcântara, E.; Bernardo, N.; Watanabe, F.; Rodrigues, T.; Rotta, L.; Carmo, A.; Shimabukuro, M.; Gonçalves, S.; Imai, N. Estimating the CDOM absorption coefficient in tropical inland waters using OLI/Landsat-8 images. Remote Sens. Lett. 2016, 7, 661–670. [Google Scholar] [CrossRef]
- Cetinic, I. Phytoplankton: Optical Constituents of the Ocean. Available online: http://www.oceanopticsbook.info/view/optical_constituents_of_the_ocean/_phytoplankton#searchResult1 (accessed on 5 September 2018).
- Massicote, P.; Asmala, E.; Stedmon, C.; Markager, S. Global distribution of dissolved organic matter along the aquatic continuum: Across rivers, lakes and oceans. Sci. Total Environ. 2017, 609, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Straškraba, M. Retention time as a key variable of reservoir limnology. In Theoretical Reservoir Ecology and Its Applications; Tundisi, J.G., Straškraba, M., Eds.; International Institute of Ecology, Brazilian Academy of Sciences and Backhuys Publishers: São Carlos, Brazil, 1999; pp. 385–410. [Google Scholar]
- Bukata, R.P.; Jerome, J.H.; Kondratyev, A.S.; Pozdnyakov, D.V. Optical Properties and Remote Sensing of Inland and Coastal Waters; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Tundisi, J.G.; Matsumura-Tundisi, T. Limnology and eutrophication of Barra Bonita reservoir, S. Paulo State, Southern Brazil. Arch. Hydrobiol. Beih. 1990, 33, 661–676. [Google Scholar]
- Periotto, N.A.; Tundisi, J.G. A characterization of ecosystems services, drives and values of two watersheds in São Paulo State, Brazil. Braz. J. Biol. 2018, 78, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Massicotte, P.; Frenette, J.-J. Spatial connectivity in a large river system: Resolving the sources and fate of dissolved organic matter. Ecol. Appl. 2011, 21, 2600–2617. [Google Scholar] [CrossRef]
- Lambert, T.; Teodoru, C.R.; Nyoni, F.C.; Bouillon, S.; Darchambeau, F.; Massicotte, P.; Borges, A.V. Along-stream transport and transformation of dissolved organic matter in a large tropical river. Biogeosciences 2016, 13, 2727–2741. [Google Scholar] [CrossRef] [Green Version]
- Martins, S.; Chokmani, K.; Alcântara, E.; Ogashawara, I.; El-Alem, A. Mapping the coloured dissolved organic matter absorption coefficient in a eutrophic reservoir using remotely sensed images. Inland Waters 2018, 8, 488–504. [Google Scholar] [CrossRef]
- Vähätalo, A.V.; Wetzel, R.G. Photochemical and microbial decomposition of chromophoric dissolved organic matter during long (months–years) exposures. Mar. Chem. 2004, 89, 313–326. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.; Wang, X.; Li, J.; Feng, S.; Zhao, Q.; Liu, M.; Qin, B. A study of absorption characteristics of chromophoric dissolved organic matter and particles in Lake Taihu, China. Hydrobiologia 2007, 592, 105–120. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, J.; Dong, Q.; Song, Q.; Ding, J. Retrieval of total suspended matter concentration in the Yellow and East China Seas from MODIS imagery. Remote Sens. Envron. 2010, 114, 392–403. [Google Scholar] [CrossRef]
- Riddick, C.A.L.; Hunter, P.D.; Tyler, A.N.; Vicente, V.M.; Horváth, H.; Kovács, A.W.; Vörös, L.; Preston, T.; Présing, M. Spatial Variability of absorption coefficient over a biogeochemical gradient in a large and optically complex shallow lake. J. Geophys. Res. 2015, 120, 7040–7066. [Google Scholar] [CrossRef]
- Carder, K.L.; Steward, R.G.; Harvey, G.R.; Ortner, P.B. Marine humic and fulvic acids: Their effects on remote sensing of ocean chlorophyll. Limnol. Oceanogr. 1989, 34, 68–81. [Google Scholar] [CrossRef] [Green Version]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr 2008, 53, 955–969. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, A.; Hooker, S.B.; Bricaud, A.; Babin, M. Estimating absorption coefficients of colored dissolved organic matter (CDOM) using a semi-analytical algorithm for southern Beaufort Sea Waters: Application to deriving concentrations of dissolved organic carbon from space. Biogeosciences 2013, 10, 917–927. [Google Scholar] [CrossRef]
- Tilstone, G.H.; Lotliker, A.A.; Miller, P.I.; Ashraf, P.M.; Kumar, T.S.; Suresh, T.; Ragavan, B.R.; Menon, H.B. Assessment of MODIS-Aqua chlorophyll-a algorithms in coastal and shelf waters of the eastern Arabian Sea. Cont. Shelf Res. 2013, 65, 14–26. [Google Scholar] [CrossRef]
- Sathyendranath, S.; Lazzara, L.; Prieur, L. Variations in the spectral values of specific absorption of phytoplankton. Limnol. Oceanogr. 1987, 32, 403–415. [Google Scholar] [CrossRef] [Green Version]
- Mobley, C.D. Light and Water: Radiative Transfer in Natural Water; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Richardson, L.L. Remote sensing of algal bloom dynamics: A new research fuses remote sensing of aquatic ecosystems with algal accessory pigment analysis. Bioscience 1996, 46, 492–501. [Google Scholar] [CrossRef]
- Mishra, S.; Mishra, D.R.; Lee, Z. Bio-optical inversion in highly turbid and cyanobacteria-dominated waters. IEEE Trans. Geosci. Remote 2014, 52, 375–388. [Google Scholar] [CrossRef]
- Perkins, M.G.; Effler, S.W.; Strait, C.M. Phytoplankton absorption and the chlorophyll a-specific absorption coefficient in dynamic Onondaga Lake. Inland Waters 2014, 4, 133–146. [Google Scholar] [CrossRef]
- Watanabe, F.S.Y.; Alcântara, E.H.; Rodrigues, T.W.P.; Bernardo, N.M.R.; Rotta, L.H.S.; Imai, N.N. Phytoplankton community dynamic detection from the chlorophyll-specific absorption coefficient in productive inland waters. Acta Limnol. Bras. 2017, 29, s2179–s2975. [Google Scholar] [CrossRef]
- Estapa, M.L.; Boss, E.; Mayer, L.M.; Roesler, C.S. Role of iron and organic carbon in mass-specific light absorption by particulate matter from Louisiana coastal waters. Limnol. Oceanogr 2012, 57, 97–112. [Google Scholar] [CrossRef]
- Souza, A.D.G.; Tundisi, J.G. Hidrogeochemical comparative study of the Jaú and Jacaré-Guaçu River watersheds, São Paulo, Brazil. (English). Rev. Bras. Biol. 2000, 60, 563–570. [Google Scholar] [CrossRef]
- Tundisi, J.D.; Matsumura-Tundisi, T.; Pareschi, D.C.; Luzia, A.P.; von Haeling, P.H.; Frollini, E. The Tietê/Jacaré watershed: A case study in research and management. Estud. Av. 2008, 22, 159–172. [Google Scholar] [CrossRef]
- Woźniak, S.B.; Meler, J.; Lednicka, B.; Zdun, A.; Stoń-Egiert, J. Inherent optical properties of suspended particulate matter in the southern Baltic Sea. Oceanologia 2011, 53, 691–729. [Google Scholar] [CrossRef]
- Meler, J.; Ostrowska, M.; Ston-Egiert, J.; Zablocka, M. Seasonal and spatial variability of light absorption by suspended particles in the southern Baltic: A mathematical description. J. Mar. Syst. 2017, 170, 68–87. [Google Scholar] [CrossRef]
- Prieur, L.; Sathyendranath, S. An optical classification of coastal and oceanic waters based on specific spectral absorption curves of phytoplankton pigments, dissolved organic matter, and other particulate materials. Limnol. Oceanogr. 1981, 26, 671–689. [Google Scholar] [CrossRef]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; Melack, J. Plumbing the Global Carbon Cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 175–185. [Google Scholar] [CrossRef]
- Zhu, W.; Yu, Q.; Tian, Y.Q.; Becker, B.L.; Zheng, T.; Carrick, H.J. An assessment of remote sensing algorithms for colored dissolved organic matter in complex freshwater environments. Remote Sens. Environ. 2014, 140, 776–778. [Google Scholar] [CrossRef]




| Zsd (m) | Turbidity (NTU) | pH | Chl-a (mg·m−³) | SPM (mg·L−1) | PIM (mg·L−1) | POM (mg·L−1) | Temp. (°C) | |
|---|---|---|---|---|---|---|---|---|
| Barra Bonita 1 (n = 20)—BB1 | ||||||||
| Min | 0.80 | 1.66 | 7.18 | 17.75 | 3.60 | 0.20 | 2.80 | 24.5 |
| Max | 2.30 | 12.50 | 9.25 | 279.86 | 16.30 | 4.40 | 14.7 | 26.9 |
| Mean | 1.49 | 5.17 | 8.36 | 120.44 | 7.21 | 1.10 | 6.10 | 25.6 |
| CV | 28.9 | 47.0 | 8.32 | 58.4 | 45.21 | 78.8 | 52.0 | 2.8 |
| Barra Bonita 2 (n = 20)—BB2 | ||||||||
| Min | 0.37 | 11.60 | 7.12 | 263.20 | 10.80 | 0.60 | 10.2 | 24.5 |
| Max | 0.78 | 33.20 | 10.1 | 797.80 | 44.00 | 3.80 | 30.4 | 32.1 |
| Mean | 0.57 | 18.64 | 9.28 | 428.72 | 21.97 | 2.60 | 18.2 | 28.1 |
| CV | 17.18 | 28.26 | 9.44 | 36.03 | 32.05 | 37.30 | 26.2 | 7.8 |
| Bariri (n = 30)—BAR1 | ||||||||
| Min | 0.50 | 7.80 | 6.10 | 25.67 | 3.60 | 0.90 | 1.4 | 21.1 |
| Max | 1.60 | 80.90 | 9.90 | 709.89 | 40.33 | 4.00 | 36.3 | 39.4 |
| Mean | 1.16 | 16.60 | 7.90 | 119.76 | 8.28 | 2.30 | 5.9 | 24.3 |
| CV | 20.03 | 45.82 | 10.5 | 80.52 | 54.76 | 21.4 | 75.1 | 15.4 |
| Bariri (n = 18)—BAR2 | ||||||||
| Min | 1.60 | 3.50 | 6.83 | 3.80 | 0.20 | 0.20 | 0.40 | 22.0 |
| Max | 3.20 | 8.80 | 7.28 | 19.0 | 2.60 | 1.30 | 1.60 | 23.9 |
| Mean | 2.20 | 5.7 | 6.97 | 8.00 | 1.60 | 0.60 | 1.10 | 22.8 |
| CV | 10.9 | 21.9 | 1.90 | 40.9 | 27.90 | 42.4 | 28.8 | 1.90 |
| Ibitinga (n = 30)—IBI1 | ||||||||
| Min | 1.60 | 2.82 | 5.50 | 1.37 | 1.00 | 0.30 | 0.50 | 21.2 |
| Max | 3.20 | 8.87 | 7.00 | 119.04 | 8.10 | 2.60 | 6.00 | 30.1 |
| Mean | 2.23 | 4.29 | 6.10 | 21.75 | 2.61 | 0.80 | 1.80 | 23.7 |
| CV | 10.91 | 17.90 | 6.80 | 85.97 | 39.20 | 35.3 | 49.6 | 9.50 |
| Ibitinga (n = 16)—IBI2 | ||||||||
| Min | 1.90 | 1.85 | 6.50 | 2.50 | 0.20 | 0.20 | 0.30 | 21.40 |
| Max | 3.80 | 3.60 | 6.96 | 13.7 | 2.20 | 1.00 | 1.90 | 24.00 |
| Mean | 2.90 | 2.47 | 6.78 | 6.64 | 1.06 | 0.40 | 0.93 | 22.82 |
| CV | 19.5 | 21.1 | 1.77 | 67.2 | 53.5 | 61.8 | 49.8 | 3.80 |
| Nova Avanhandava 1 (n = 20)—NAV1 | ||||||||
| Min | 2.29 | 1.01 | 8.50 | 2.46 | 0.10 | 0.10 | 0.20 | 25.1 |
| Max | 4.80 | 2.47 | 8.90 | 12.56 | 2.60 | 2.20 | 0.90 | 26.3 |
| Mean | 3.15 | 1.66 | 8.60 | 6.21 | 1.01 | 0.70 | 0.93 | 26.0 |
| CV | 19.95 | 25.40 | 1.39 | 40.0 | 61.7 | 76.7 | 40.8 | 1.12 |
| Nova Avanhandava 2 (n = 20)—NAV2 | ||||||||
| Min | 2.45 | 1.01 | 7.60 | 4.51 | 0.50 | 0.14 | 0.30 | 23.8 |
| Max | 4.65 | 2.56 | 8.30 | 20.5 | 2.80 | 2.00 | 1.10 | 25.6 |
| Mean | 3.41 | 1.73 | 8.10 | 9.01 | 1.00 | 0.50 | 0.50 | 24.6 |
| CV | 14.1 | 18.98 | 2.20 | 34.9 | 37.6 | 65.8 | 26.5 | 1.90 |
| Entire Dataset (n = 174 *) | ||||||||
| Min | 0.37 | 1.01 | 5.50 | 1.37 | 0.10 | 0.08 | 0.20 | 21.1 |
| Max | 4.80 | 80.9 | 10.1 | 797.80 | 44.00 | 4.40 | 43.00 | 39.4 |
| Mean | 1.15 | 13.31 | 8.36 | 197.50 | 11.34 | 1.94 | 9.40 | 25.8 |
| CV | 88.7 | 67.0 | 13.9 | 80.0 | 69.7 | 53.1 | 81.0 | 10.8 |
| Field Sites | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| BB1 | BB2 | BAR1 | BAR2 | IBI1 | IBI2 | NAV1 | NAV2 | ||
| ap(443) | Min | 0.69 | 03.12 | 0.70 | 0.16 | 0.23 | 0.12 | 0.10 | 0.24 |
| Max | 2.94 | 11.2 | 9.98 | 0.42 | 3.94 | 0.34 | 0.54 | 1.20 | |
| Mean | 1.67 | 5.15 | 1.99 | 0.30 | 0.80 | 0.21 | 0.22 | 0.52 | |
| CV | 38.90 | 27.10 | 64.70 | 25.90 | 54.10 | 38.40 | 60.40 | 25.60 | |
| aϕ(443) | Min | 0.29 | 2.77 | 0.34 | 0.04 | 0.06 | 0.05 | 0.02 | 0.06 |
| Max | 2.62 | 10.9 | 9.19 | 0.20 | 3.41 | 0.14 | 0.18 | 0.44 | |
| Mean | 1.21 | 4.67 | 1.41 | 0.12 | 0.42 | 0.09 | 0.06 | 0.25 | |
| CV | 52.30 | 31.90 | 64.70 | 28.20 | 89.50 | 33.30 | 72.80 | 31.80 | |
| anap(443) | Min | 0.32 | 0.23 | 0.34 | 0.09 | 0.15 | 0.06 | 0.03 | 0.14 |
| Max | 0.80 | 1.70 | 0.84 | 0.26 | 0.63 | 0.20 | 0.38 | 0.78 | |
| Mean | 0.47 | 0.49 | 0.58 | 0.18 | 0.39 | 0.12 | 0.12 | 0.27 | |
| CV | 25.30 | 76.50 | 22.70 | 25.90 | 27.40 | 48.30 | 71.20 | 34.00 | |
| acdom(443) | Min | 0.45 | 0.77 | 1.12 | 0.32 | 0.72 | 0.77 | 0.26 | 0.24 |
| Max | 0.97 | 1.35 | 2.46 | 3.17 | 2.23 | 2.29 | 0.54 | 0.47 | |
| Mean | 0.83 | 1.05 | 1.71 | 1.84 | 1.29 | 1.35 | 0.26 | 0.32 | |
| CV | 13.2 | 16.6 | 20.2 | 33.1 | 17.3 | 38.1 | 8.5 | 12.6 | |
| Scdom | Min | 0.016 | 0.004 | 0.010 | 0.009 | 0.006 | 0.011 | 0.014 | 0.016 |
| Max | 0.018 | 0.016 | 0.015 | 0.019 | 0.013 | 0.015 | 0.017 | 0.020 | |
| Mean | 0.017 | 0.012 | 0.012 | 0.013 | 0.009 | 0.013 | 0.015 | 0.018 | |
| CV | 3.2 | 19.6 | 10.2 | 10.9 | 23.8 | 8.8 | 4.5 | 5.7 | |
| Snap | Min | 0.008 | 0.006 | 0.010 | 0.009 | 0.008 | 0.005 | 0.008 | 0.003 |
| Max | 0.011 | 0.009 | 0.012 | 0.011 | 0.014 | 0.022 | 0.011 | 0.007 | |
| Mean | 0.009 | 0.008 | 0.011 | 0.010 | 0.010 | 0.013 | 0.009 | 0.005 | |
| CV | 7.90 | 9.40 | 3.90 | 4.10 | 11.60 | 38.60 | 6.70 | 16.70 | |
| Field Sites | ||||||||
|---|---|---|---|---|---|---|---|---|
| BB1 | BB2 | BAR1 | BAR2 | IBI1 | IBI2 | NAV1 | NAV2 | |
| acdom | 26.1% | 19.0% | 40.5% | 72.6% | 49.0% | 72.1% | 22.8% | 18.6% |
| aϕ | 43.0% | 68.9% | 37.4% | 5.2% | 21.3% | 4.7% | 16.7% | 10.9% |
| anap | 18.3% | 7.4% | 14.1% | 8.1% | 16.8% | 6.3% | 31.2% | 29.8% |
| aw | 12.6% | 4.7% | 8.0% | 14.1% | 12.9% | 16.9% | 29.3% | 40.7% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardo, N.; Alcântara, E.; Watanabe, F.; Rodrigues, T.; Carmo, A.d.; Gomes, A.C.C.; Andrade, C. Light Absorption Budget in a Reservoir Cascade System with Widely Differing Optical Properties. Water 2019, 11, 229. https://doi.org/10.3390/w11020229
Bernardo N, Alcântara E, Watanabe F, Rodrigues T, Carmo Ad, Gomes ACC, Andrade C. Light Absorption Budget in a Reservoir Cascade System with Widely Differing Optical Properties. Water. 2019; 11(2):229. https://doi.org/10.3390/w11020229
Chicago/Turabian StyleBernardo, Nariane, Enner Alcântara, Fernanda Watanabe, Thanan Rodrigues, Alisson do Carmo, Ana Carolina Campos Gomes, and Caroline Andrade. 2019. "Light Absorption Budget in a Reservoir Cascade System with Widely Differing Optical Properties" Water 11, no. 2: 229. https://doi.org/10.3390/w11020229
APA StyleBernardo, N., Alcântara, E., Watanabe, F., Rodrigues, T., Carmo, A. d., Gomes, A. C. C., & Andrade, C. (2019). Light Absorption Budget in a Reservoir Cascade System with Widely Differing Optical Properties. Water, 11(2), 229. https://doi.org/10.3390/w11020229







