Bioelectrochemical Systems for Groundwater Remediation: The Development Trend and Research Front Revealed by Bibliometric Analysis
Abstract
:1. Introduction
2. Data Acquisition and Methods
2.1. Data Description
2.2. Scientometrics Analysis Method
3. Results
3.1. Characteristics of the Publications of BESs for Groundwater Remediation
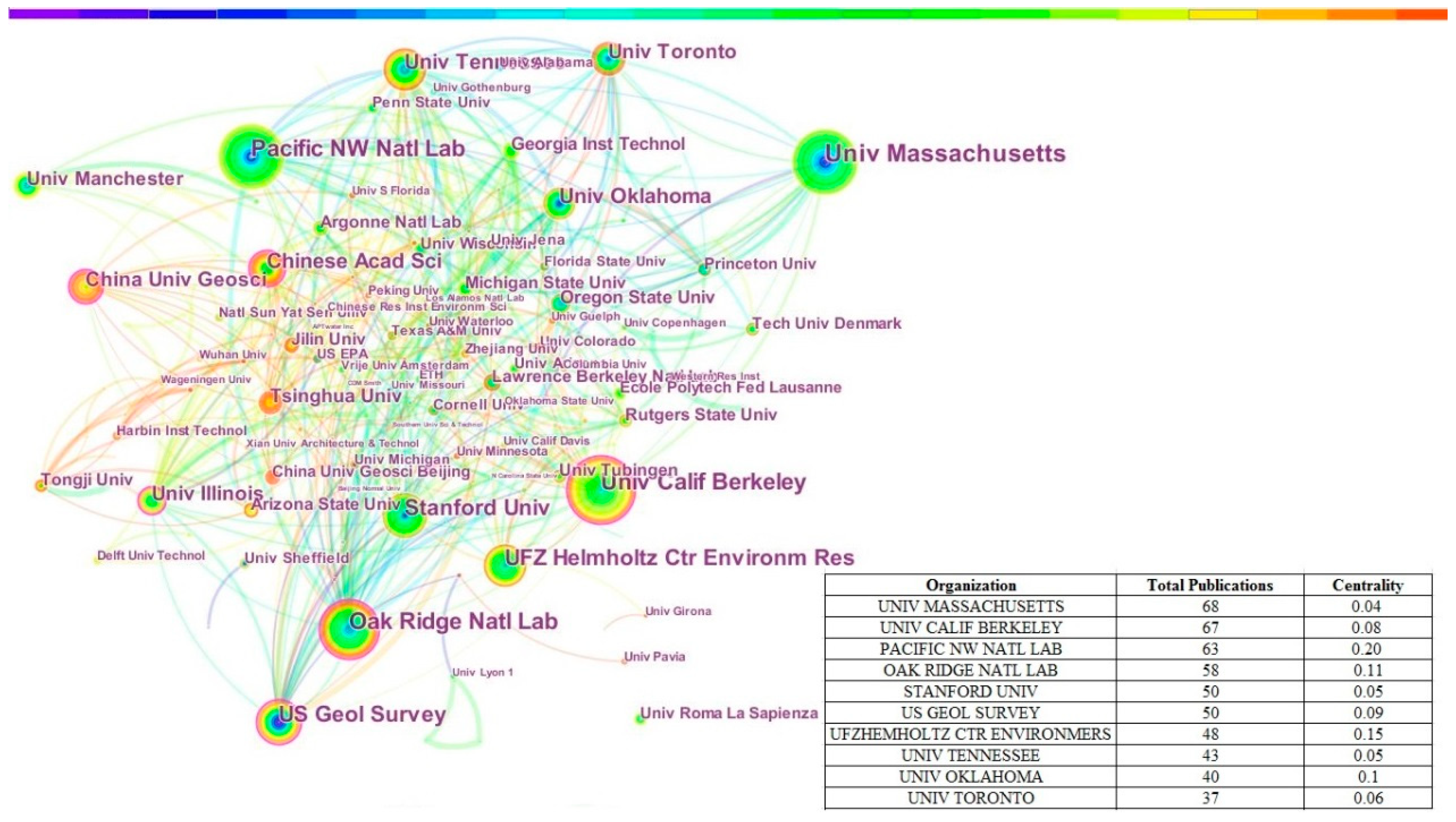
3.2. Published Countries, Organizations and Funding Sources Analysis
3.3. Published Journals and Authors Analysis
3.4. Keywords and Burst Term Detection Analysis
4. Discussion and Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stephen, F.; Daniel, P.L. Non-Renewable Groundwater Resouecrs; United Nations Educational, Scientific and Cultural Organization: Paris, France, 2006; pp. 13–25. [Google Scholar]
- Yao, Y.; Zheng, C.; Andrews, C.; He, X.; Zhang, A.; Liu, J. Integration of groundwater into China’s south-north water transfer strategy. Sci. Total. Environ. 2019, 658, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.J.; Chen, L.; Rene, E.R.; Hu, Q.; Ma, W.F.; Shen, Z.Y. Biological nitrogen removal using soil columns for the reuse of reclaimed water: Performance and microbial community analysis. J. Environ. Manag. 2018, 217, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, M.-y.; Liu, L.-y.; Wang, H.-f.; Yu, S. Groundwater heavy metal levels and associated human health risk in the North China Plain. Arab. J. Geosci. 2015, 8, 10389–10398. [Google Scholar] [CrossRef]
- Gejl, R.N.; Rygaard, M.; Henriksen, H.J.; Rasmussen, J.; Bjerg, P.L. Understanding the impacts of groundwater abstraction through long-term trends in water quality. Water Res. 2019, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Mudiam, M.K.; Pathak, S.P.; Gopal, K.; Murthy, R.C. Studies on urban drinking water quality in a tropical zone. Environ. Monit. Assess. 2012, 184, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Sevda, S.; Sreekishnan, T.R.; Pous, N.; Puig, S.; Pant, D. Bioelectroremediation of perchlorate and nitrate contaminated water: A review. Bioresour. Technol. 2018, 255, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Wakode, H.B.; Baier, K.; Jha, R.; Azzam, R. Impact of urbanization on groundwater recharge and urban water balance for the city of Hyderabad, India. Int. Soil Water Conserv. Res. 2018, 6, 51–62. [Google Scholar] [CrossRef]
- Shrestha, A.; Luo, W. Assessment of Groundwater Nitrate Pollution Potential in Central Valley Aquifer Using Geodetector-Based Frequency Ratio (GFR) and Optimized-DRASTIC Methods. Int. J. Geo-Inf. 2018, 7, 211. [Google Scholar] [CrossRef]
- Bouzourra, H.; Bouhlila, R.; Elango, L.; Slama, F.; Ouslati, N. Characterization of mechanisms and processes of groundwater salinization in irrigated coastal area using statistics, GIS, and hydrogeochemical investigations. Environ. Sci. Pollut. Res. Int. 2015, 22, 2643–2660. [Google Scholar] [CrossRef]
- Liu, F.; Song, X.; Yang, L.; Han, D.; Zhang, Y.; Ma, Y.; Bu, H. The role of anthropogenic and natural factors in shaping the geochemical evolution of groundwater in the Subei Lake basin, Ordos energy base, Northwestern China. Sci. Total. Environ. 2015, 538, 327–340. [Google Scholar] [CrossRef]
- Matiatos, I. Nitrate source identification in groundwater of multiple land-use areas by combining isotopes and multivariate statistical analysis: A case study of Asopos basin (Central Greece). Sci. Total. Environ. 2016, 541, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Cuoco, E.; Darrah, T.H.; Buono, G.; Verrengia, G.; De Francesco, S.; Eymold, W.K.; Tedesco, D. Inorganic contaminants from diffuse pollution in shallow groundwater of the Campanian Plain (Southern Italy). Implications for geochemical survey. Environ. Monit. Assess. 2015, 187, 46. [Google Scholar] [CrossRef] [PubMed]
- Krishna, A.K.; Mohan, K.R. Risk assessment of heavy metals and their source distribution in waters of a contaminated industrial site. Environ. Sci. Pollut. Res. Int. 2014, 21, 3653–3669. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Wu, D.; Chen, W.; Feng, C.; Wei, C. Biocathode denitrification of coke wastewater effluent from an industrial aeration tank: Effect of long-term adaptation. Biochem. Eng. J. 2017, 125, 151–160. [Google Scholar] [CrossRef]
- Belhaj, E.; Lafhaj, Z.; Zakaria, K.; Depelsenaire, G. Formulation of a Permeable Reactive Mixture for Groundwater Remediation Based on an Industrial Co-product: Permeability and Copper Retention Study. Waste and Biomass Valorization 2015, 6, 263–272. [Google Scholar] [CrossRef]
- Pradhan, J.K.; Kumar, S. Informal e-waste recycling: environmental risk assessment of heavy metal contamination in Mandoli industrial area, Delhi, India. Environ. Sci. Pollut. Res. Int. 2014, 21, 7913–7928. [Google Scholar] [CrossRef] [PubMed]
- Krishna, A.K.; Satyanarayanan, M.; Govil, P.K. Assessment of heavy metal pollution in water using multivariate statistical techniques in an industrial area: a case study from Patancheru, Medak District, Andhra Pradesh, India. J. Hazard. Mater. 2009, 167, 366–373. [Google Scholar] [CrossRef]
- Nizami, A.S.; Rehan, M.; Waqas, M.; Naqvi, M.; Ouda, O.K.M.; Shahzad, K.; Miandad, R.; Khan, M.Z.; Syamsiro, M.; Ismail, I.M.I.; et al. Waste biorefineries: Enabling circular economies in developing countries. Bioresour. Technol. 2017, 241, 1101–1117. [Google Scholar] [CrossRef]
- Wang, Y.; Pleasant, S.; Jain, P.; Powell, J.; Townsend, T. Calcium carbonate-based permeable reactive barriers for iron and manganese groundwater remediation at landfills. Waste Manag. 2016, 53, 128–135. [Google Scholar] [CrossRef]
- Aulenta, F.; Di Maio, V.; Ferri, T.; Majone, M. The humic acid analogue antraquinone-2,6-disulfonate (AQDS) serves as an electron shuttle in the electricity-driven microbial dechlorination of trichloroethene to cis-dichloroethene. Bioresour. Technol. 2010, 101, 9728–9733. [Google Scholar] [CrossRef]
- Compernolle, T.; Van Passel, S.; Huisman, K.; Kort, P. The option to abandon: stimulating innovative groundwater remediation technologies characterized by technological uncertainty. Sci. Total. Environ. 2014, 496, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ewusi-Mensah, D.; Norgbey, E. Microbial desalination cells technology: a review of the factors affecting the process, performance and efficiency. Desalin. Water Treat. 2017, 87, 140–159. [Google Scholar] [CrossRef]
- Liu, Y.; Mou, H.; Chen, L.; Mirza, Z.A.; Liu, L. Cr(VI)-contaminated groundwater remediation with simulated permeable reactive barrier (PRB) filled with natural pyrite as reactive material: Environmental factors and effectiveness. J. Hazard. Mater. 2015, 298, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Cecconet, D.; Callegari, A.; Capodaglio, A.G. Bioelectrochemical Systems for Removal of Selected Metals and Perchlorate from Groundwater: A Review. Energies 2018, 11, 2643. [Google Scholar] [CrossRef]
- Li, W.; Zhang, M.; Wang, M.; Han, Z.; Liu, J.; Chen, Z.; Liu, B.; Yan, Y.; Liu, Z. Screening of groundwater remedial alternatives for brownfield sites: a comprehensive method integrated MCDA with numerical simulation. Environ. Sci. Pollut. Res. Int. 2018, 25, 15844–15861. [Google Scholar] [CrossRef] [PubMed]
- Sadeghfam, S.; Hassanzadeh, Y.; Khatibi, R.; Nadiri, A.A.; Moazamnia, M. Groundwater Remediation through Pump-Treat-Inject Technology Using Optimum Control by Artificial Intelligence (OCAI). Water Resour. Manag. 2019, 33, 1123–1145. [Google Scholar] [CrossRef]
- Guo, Z.; Brusseau, M.L.; Fogg, G.E. Determining the long-term operational performance of pump and treat and the possibility of closure for a large TCE plume. J. Hazard. Mater. 2019, 365, 796–803. [Google Scholar] [CrossRef]
- Yudao, C.; Yaping, C.; Yaping, J.; Yani, Y.; Zhiteng, L.; Qiling, T.; Zisen, S. Pump-and-treat method to remove nitrate from groundwater with liquor as the carbon source. Environ. Earth Sci. 2017, 76. [Google Scholar] [CrossRef]
- Boal, A.K.; Rhodes, C.; Garcia, S. Pump-and-Treat Groundwater Remediation Using Chlorine/Ultraviolet Advanced Oxidation Processes. Groundw. Monit. Remediat. 2015, 35, 93–100. [Google Scholar] [CrossRef]
- Zhao, P.; Yu, F.; Wang, R.; Ma, Y.; Wu, Y. Sodium alginate/graphene oxide hydrogel beads as permeable reactive barrier material for the remediation of ciprofloxacin-contaminated groundwater. Chemosphere 2018, 200, 612–620. [Google Scholar] [CrossRef]
- McCobb, T.D.; Briggs, M.A.; LeBlanc, D.R.; Day-Lewis, F.D.; Johnson, C.D. Evaluating long-term patterns of decreasing groundwater discharge through a lake-bottom permeable reactive barrier. J. Environ. Manag. 2018, 220, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Stevens, G.W.; Mumford, K.A. The effect of temperature on hydrocarbon adsorption by diphenyldichlorosilane coated zeolite and its application in permeable reactive barriers in cold regions. Cold Reg. Sci. Technol. 2018, 145, 169–176. [Google Scholar] [CrossRef]
- Kornilovych, B.; Wireman, M.; Ubaldini, S.; Guglietta, D.; Koshik, Y.; Caruso, B.; Kovalchuk, I. Uranium Removal from Groundwater by Permeable Reactive Barrier with Zero-Valent Iron and Organic Carbon Mixtures: Laboratory and Field Studies. Metals 2018, 8, 408. [Google Scholar] [CrossRef]
- Gholami, F.; Shavandi, M.; Dastgheib, S.M.M.; Amoozegar, M.A. Naphthalene remediation form groundwater by calcium peroxide (CaO2) nanoparticles in permeable reactive barrier (PRB). Chemosphere 2018, 212, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.C.; Oostrom, M.; Truex, M.J.; Rohay, V.J.; Brusseau, M.L. Assessing performance and closure for soil vapor extraction: integrating vapor discharge and impact to groundwater quality. J. Contam. Hydrol. 2012, 128, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Woo, H.J.; Jeong, K.S. Removal of non-aqueous phase liquids (NAPLs) from TPH-saturated sandy aquifer sediments using in situ air sparging combined with soil vapor extraction. J. Environ. Sci. Health A Tox Hazard Subst. Environ. Eng. 2018, 53, 1253–1266. [Google Scholar] [CrossRef]
- Behar, H.R.; Snyder, E.E.; Marczak, S.; Salazar, L.J.; Rappe, B.; Fordham, G.F.; Chu, S.P.; Strobridge, D.M.; Birdsell, K.H.; Miller, T.A.; et al. An Investigation of Plume Response to Soil Vapor Extraction and Hypothetical Drum Failure. Vadose Zone J. 2019, 18. [Google Scholar] [CrossRef]
- Kawabe, Y.; Komai, T. A Case Study of Natural Attenuation of Chlorinated Solvents Under Unstable Groundwater Conditions in Takahata, Japan. Bull. Environ. Contam. Toxicol. 2019, 102, 280–286. [Google Scholar] [CrossRef]
- Caschetto, M.; Robertson, W.; Petitta, M.; Aravena, R. Partial nitrification enhances natural attenuation of nitrogen in a septic system plume. Sci. Total. Environ. 2018, 625, 801–808. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, D.; Torrento, C.; Palau, J.; Marchesi, M.; Soler, A.; Hunkeler, D.; Domenech, C.; Rosell, M. Unravelling long-term source removal effects and chlorinated methanes natural attenuation processes by C and Cl stable isotopic patterns at a complex field site. Sci. Total. Environ. 2018, 645, 286–296. [Google Scholar] [CrossRef]
- Pous, N.; Dolors Balaguer, M.; Colprim, J.; Puig, S. Opportunities for groundwater microbial electro-remediation. Microb. Biotechnol. 2018, 11, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Pous, N.; Puig, S.; Coma, M.; Balaguer, M.D.; Colprim, J. Bioremediation of nitrate-polluted groundwater in a microbial fuel cell. J. Chem. Technol. Biotechnol. 2013, 88, 1690–1696. [Google Scholar] [CrossRef]
- Hashim, M.A.; Mukhopadhyay, S.; Sahu, J.N.; Sengupta, B. Remediation technologies for heavy metal contaminated groundwater. J Environ. Manag. 2011, 92, 2355–2388. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Aulenta, F.; Mingazzini, M.; Palumbo, M.T.; Papini, M.P.; Verdini, R.; Majone, M. Bioelectrochemical approach for reductive and oxidative dechlorination of chlorinated aliphatic hydrocarbons (CAHs). Chemosphere 2017, 169, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Su, S.G.; Cheng, H.Y.; Zhu, T.T.; Wang, H.C.; Wang, A.J. A novel bioelectrochemical method for real-time nitrate monitoring. Bioelectrochemistry 2019, 125, 33–37. [Google Scholar] [CrossRef]
- Callegari, A.; Bolognesi, S.; Cecconet, D. Operation of a 2-Stage Bioelectrochemical System for Groundwater Denitrification. Water 2019, 11, 959. [Google Scholar] [CrossRef]
- Espinoza Tofalos, A.; Daghio, M.; Gonzalez, M.; Papacchini, M.; Franzetti, A.; Seeger, M. Toluene degradation by Cupriavidus metallidurans CH34 in nitrate-reducing conditions and in Bioelectrochemical Systems. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef]
- Cecconet, D.; Zou, S.; Capodaglio, A.G.; He, Z. Evaluation of energy consumption of treating nitrate-contaminated groundwater by bioelectrochemical systems. Sci. Total Environ. 2018, 636, 881–890. [Google Scholar] [CrossRef]
- Hen, D.; Yang, K.; Wei, L.; Wang, H.Y. Microbial community and metabolism activity in a bioelectrochemical denitrification system under long-term presence of p-nitrophenol. Bioresour. Technol. 2016, 218, 189–195. [Google Scholar]
- Tong, Y.; He, Z. Current-driven nitrate migration out of groundwater by using a bioelectrochemical system. Rsc Advances 2014, 4, 10290–10294. [Google Scholar] [CrossRef] [Green Version]
- Wan, D.J.; Liu, H.J.; Qu, J.H.; Lei, P.J.; Mao, S.H.; Hou, Y.N. Using the combined bioelectrochemical and sulfur autotrophic denitrification system for groundwater denitrification. Bioresour. Technol. 2009, 100, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Cast, K.L.; Flora, J.R.V. An evaluation of two cathode materials and the impact of copper on bioelectrochemical denitrification. Water Res. 1998, 32, 63–70. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Yang, K.; Wang, H. Response of a three dimensional bioelectrochemical denitrification system to the long-term presence of graphene oxide. Bioresour. Technol. 2016, 214, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Pous, N.; Puig, S.; Balaguer, M.D.; Colprim, J. Cathode potential and anode electron donor evaluation for a suitable treatment of nitrate-contaminated groundwater in bioelectrochemical systems. Chem. Eng. J. 2015, 263, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Van Khanh, N.; Hong, S.; Park, Y.; Jo, K.; Lee, T. Autotrophic denitrification performance and bacterial community at biocathodes of bioelectrochemical systems with either abiotic or biotic anodes. J. Biosci. Bioeng. 2015, 119, 180–187. [Google Scholar] [CrossRef]
- Cecconet, D.; Devecseri, M.; Callegari, A.; Capodaglio, A.G. Effects of process operating conditions on the autotrophic denitrification of nitrate-contaminated groundwater using bioelectrochemical systems. Sci. Total Environ. 2018, 613, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Adelaja, O.; Keshavarz, T.; Kyazze, G. Treatment of phenanthrene and benzene using microbial fuel cells operated continuously for possible in situ and ex situ applications. Int. Biodeterior. Biodegrad. 2017, 116, 91–103. [Google Scholar] [CrossRef]
- Butler, C.S.; Clauwaert, P.; Green, S.J.; Verstraete, W.; Nerenberg, R. Bioelectrochemical Perchlorate Reduction in a Microbial Fuel Cell. Environ. Sc. Technol. 2010, 44, 4685–4691. [Google Scholar] [CrossRef]
- Clauwaert, P.; Rabaey, K.; Aelterman, P.; De Schamphe, L. Biological Denitrification in Microbial Fuel Cells. Environ. Sci. Technol. 2007, 41, 3354–3360. [Google Scholar] [CrossRef]
- Hennebel, T.; Benner, J.; Clauwaert, P.; Vanhaecke, L.; Aelterman, P.; Callebaut, R.; Boon, N.; Verstraete, W. Dehalogenation of environmental pollutants in microbial electrolysis cells with biogenic palladium nanoparticles. Biotechnol. Lett. 2011, 33, 89–95. [Google Scholar] [CrossRef]
- Chen, X.; Xia, X.; Liang, P.; Cao, X.; Sun, H.; Huang, X. Stacked microbial desalination cells to enhance water desalination efficiency. Environ. Sci. Technol. 2011, 45, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Logan, B.E. Microbial desalination cells for energy production and desalination. Desalination 2013, 308, 122–130. [Google Scholar] [CrossRef]
- Mehanna, M.; Saito, T.; Yan, J.; Hickner, M.; Cao, X.; Huang, X.; Logan, B.E. Using microbial desalination cells to reduce water salinity prior to reverse osmosis. Energy Environ. Sci. 2010, 3. [Google Scholar] [CrossRef]
- Hubbard, C.G.; West, L.J.; Morris, K.; Kulessa, B.; Brookshaw, D.; Lloyd, J.R.; Shaw, S. In search of experimental evidence for the biogeobattery. J. Geophys. Res. 2011, 116. [Google Scholar] [CrossRef]
- Revil, A.; Mendonça, C.A.; Atekwana, E.A.; Kulessa, B.; Hubbard, S.S.; Bohlen, K.J. Understanding biogeobatteries: Where geophysics meets microbiology. J. Geophys. Res. 2010, 115. [Google Scholar] [CrossRef] [Green Version]
- Risgaard-Petersen, N.; Damgaard, L.R.; Revil, A.; Nielsen, L.P. Mapping electron sources and sinks in a marine biogeobattery. J. Geophys. Res. Biogeosci. 2014, 119, 1475–1486. [Google Scholar] [CrossRef]
- Chen, D.; Dai, T.; Wang, H.; Yang, K. Nitrate removal by a combined bioelectrochemical and sulfur autotrophic denitrification (CBSAD) system at low temperatures. Desalin. Water Treat. 2016, 57, 19411–19417. [Google Scholar] [CrossRef]
- Hoseinzadeh, E.; Rezaee, A.; Farzadkia, M. Enhanced biological nitrate removal by alternating electric current bioelectrical reactor: Selectivity and mechanism. J. Mol. Liq. 2017, 246, 93–102. [Google Scholar] [CrossRef]
- Krauter, P.; Daily, B., Jr.; Dibley, V.; Pinkart, H.; Legler, T. Perchlorate and nitrate remediation efficiency and microbial diversity in a containerized wetland bioreactor. Int. J. Phytoremed. 2005, 7, 113–128. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, M.; Zhang, Y.; She, Z.; Ren, Y.; Wang, Z.; Zhao, C. Perchlorate reduction by hydrogen autotrophic bacteria in a bioelectrochemical reactor. J. Environ. Manag. 2014, 142, 10–16. [Google Scholar] [CrossRef]
- Islam, F.; Gault, A.; Boothman, C.; Lloyd, J.R. Role of metal-reducing bacteria in arsenic release from Bengal deltas. Nature 2004, 430, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, J.R.; Oremland, R.S. Microbial Transformations of Arsenic in the Environment- From Soda Lakes to Aquifers. Elements 2006, 2, 85–90. [Google Scholar] [CrossRef]
- Upadhyaya, G.; Jackson, J.; Clancy, T.M.; Hyun, S.P.; Brown, J.; Hayes, K.F.; Raskin, L. Simultaneous removal of nitrate and arsenic from drinking water sources utilizing a fixed-bed bioreactor system. Water Res. 2010, 44, 4958–4969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Feng, C.; Ni, J.; Zhang, J.; Huang, W. Simultaneous reduction of vanadium (V) and chromium (VI) with enhanced energy recovery based on microbial fuel cell technology. J. Power Sources 2012, 204, 34–39. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, B.; Tian, C.; Liu, Y.; Shi, C.; Cheng, M.; Feng, C. Enhanced microbial reduction of vanadium (V) in groundwater with bioelectricity from microbial fuel cells. J. Power Sources 2015, 287, 43–49. [Google Scholar] [CrossRef]
- Zhang, B.; Hao, L.; Tian, C.; Yuan, S.; Feng, C.; Ni, J.; Borthwick, A.G. Microbial reduction and precipitation of vanadium (V) in groundwater by immobilized mixed anaerobic culture. Bioresour. Technol. 2015, 192, 410–417. [Google Scholar] [CrossRef]
- Anderson, R.T.; Vrionis, H.A.; Ortiz-Bernad, I.; Resch, C.T.; Long, P.E.; Dayvault, R.; Karp, K.; Marutzky, S.; Metzler, D.R.; Peacock, A.; et al. Stimulating the in situ activity of Geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 2003, 69, 5884–5891. [Google Scholar] [CrossRef]
- Newsome, L.; Morris, K.; Lloyd, J.R. The biogeochemistry and bioremediation of uranium and other priority radionuclides. Chem. Geol. 2014, 363, 164–184. [Google Scholar] [CrossRef]
- Williams, K.H.; Bargar, J.R.; Lloyd, J.R.; Lovley, D.R. Bioremediation of uranium-contaminated groundwater: a systems approach to subsurface biogeochemistry. Curr. Opin. Biotechnol. 2013, 24, 489–497. [Google Scholar] [CrossRef]
- Williams, K.H.; Long, P.E.; Davis, J.A.; Wilkins, M.J.; N’Guessan, A.L.; Steefel, C.I.; Yang, L.; Newcomer, D.; Spane, F.A.; Kerkhof, L.J.; et al. Acetate Availability and its Influence on Sustainable Bioremediation of Uranium-Contaminated Groundwater. Geomicrobiol. J. 2011, 28, 519–539. [Google Scholar] [CrossRef]
- Fan, C.; Gao, Y.; Zhang, Y.; Dong, W.; Lai, M. Remediation of lead and cadmium from simulated groundwater in loess region in northwestern China using permeable reactive barrier filled with environmentally friendly mixed adsorbents. Environ. Sci. Pollut. Res. Int. 2018, 25, 1486–1496. [Google Scholar] [CrossRef] [PubMed]
- Foght, J. Anaerobic biodegradation of aromatic hydrocarbons: pathways and prospects. J. Mol. Microbiol. Biotechnol. 2008, 15, 93–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gannon, S.M.; Nevin, K.P.; Franks, A.E.; Lovley, D.R. Stimulating the anaerobic degradation of aromatic hydrocarbons in contaminated sediments by providing an electrode as the electron acceptor. Environ. Microbiol. 2010, 12, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Li, Z.; Yang, J.; Liang, B.; Huang, C.; Cai, W.; Nan, J.; Wang, A. Electron Fluxes in Biocathode Bioelectrochemical Systems Performing Dechlorination of Chlorinated Aliphatic Hydrocarbons. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liang, P.; Zhang, X.; Huang, X. Bioelectrochemical systems-driven directional ion transport enables low-energy water desalination, pollutant removal, and resource recovery. Bioresour. Technol. 2016, 215, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Pous, N.; Koch, C.; Vila-Rovira, A.; Balaguer, M.D.; Colprim, J.; Muhlenberg, J.; Muller, S.; Harnisch, F.; Puig, S. Monitoring and engineering reactor microbiomes of denitrifying bioelectrochemical systems. Rsc Advances 2015, 5, 68326–68333. [Google Scholar] [CrossRef]
- Morris, J.M.; Jin, S. Feasibility of using microbial fuel cell technology for bioremediation of hydrocarbons in groundwater. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2008, 43, 18–23. [Google Scholar] [CrossRef]
- Liao, H.; Tang, M.; Luo, L.; Li, C.; Chiclana, F.; Zeng, X.-J. A Bibliometric Analysis and Visualization of Medical Big Data Research. Sustainability 2018, 10, 166. [Google Scholar] [CrossRef]
- Ouyang, W.; Wang, Y.; Lin, C.; He, M.; Hao, F.; Liu, H.; Zhu, W. Heavy metal loss from agricultural watershed to aquatic system: A scientometrics review. Sci. Total. Environ. 2018, 637–638, 208–220. [Google Scholar] [CrossRef]
- Chen, C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef]
- Han, J.; Teng, X.; Cai, X. A novel network optimization partner selection method based on collaborative and knowledge networks. Inf. Sci. 2019, 484, 269–285. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Q.; Bai, X.; Deng, J.; Hu, Y. Mapping of Trace Elements in Coal and Ash Research Based on a Bibliometric Analysis Method Spanning 1971–2017. Minerals 2018, 8, 89. [Google Scholar] [CrossRef]
- Lovley, D.R.; Holmes, D.E.; Nevin, K.P. Dissimilatory Fe(III) and Mn(IV) Reduction. Adv. Microb. Physiol. 2004, 49, 219–286. [Google Scholar] [CrossRef] [Green Version]
- Long, P.E.; Williams, K.H.; Davis, J.A.; Fox, P.M.; Wilkins, M.J.; Yabusaki, S.B.; Fang, Y.; Waichler, S.R.; Berman, E.S.F.; Gupta, M.; et al. Bicarbonate impact on U(VI) bioreduction in a shallow alluvial aquifer. Geochimica et Cosmochimica Acta 2015, 150, 106–124. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P.; Gorby, Y.A.; Landa, E.R. Microbial reduction of uranium. Nature 1991, 350, 413–416. [Google Scholar] [CrossRef]
- Lovley, D.R. Anaerobic benzene degradation. Biodegradation 2000, 11, 107–116. [Google Scholar] [CrossRef]
- Xie, X.; Hu, L.; Pasta, M.; Wells, G.F.; Kong, D.; Criddle, C.S.; Cui, Y. Three-dimensional carbon nanotube-textile anode for high-performance microbial fuel cells. Nano Lett 2011, 11, 291–296. [Google Scholar] [CrossRef]
- Cardenas, E.; Wu, W.M.; Leigh, M.B.; Carley, J.; Carroll, S.; Gentry, T.; Luo, J.; Watson, D.; Gu, B.; Ginder-Vogel, M.; et al. Significant association between sulfate-reducing bacteria and uranium-reducing microbial communities as revealed by a combined massively parallel sequencing-indicator species approach. Appl Environ Microbiol 2010, 76, 6778–6786. [Google Scholar] [CrossRef]
- Gu, B.H.; Wu, W.M.; Ginder-Vogel, M.A.; Yan, H. Bioreduction of Uranium in a Contaminated Soil. Environ. Sci. Technol. 2005, 39, 4841–4847. [Google Scholar] [CrossRef]
- Wu, W.M.; Carley, J.; Fienen, M.; Mehlhorn, T. Pilot-Scale in Situ Bioremediation of Uranium in a Highly Contaminated Aquifer. 1. Conditioning of a Treatment Zone. Environ. Sci. Technol. 2006, 40, 3978–3985. [Google Scholar] [CrossRef]
- Wu, W.M.; Carley, J.; Luo, J.; Ginder-Vogel, M.A. In Situ Bioreduction of Uranium (VI) to Submicromolar Levels and Reoxidation by Dissolved Oxygen. Environ. Sci. Technol. 2007, 41, 5716–5723. [Google Scholar] [CrossRef] [PubMed]
- Witt, M.E.; Dybas, M.J.; Mark, W.R.; Criddle, C.S. Motility-Enhanced Bioremediation of Carbon Tetrachloride-Contaminated Aquifer Sediments. Environ. Sci. Technol. 1999, 33, 2958–2964. [Google Scholar] [CrossRef]
- Aulenta, F.; Majone, M.; Tandoi, V. Enhanced anaerobic bioremediation of chlorinated solvents: environmental factors influencing microbial activity and their relevance under field conditions. J. Chem. Technol. Biotechnol. 2006, 81, 1463–1474. [Google Scholar] [CrossRef]
- Aulenta, F.; Tocca, L.; Reale, P.; Rossetti, S.; Majone, M. Bioelectrochemical dechlorination of trichloroethene: From electron transfer mechanisms to process scale-up. J. Biotechnol. 2010, 150, S34–S35. [Google Scholar] [CrossRef]
- Aulenta, F.; Tocca, L.; Verdini, R.; Reale, P.; Majone, M. Dechlorination of Trichloroethene in a Continuous-Flow Bioelectrochemical Reactor: Effect of Cathode Potential on Rate, Selectivity, and Electron Transfer Mechanisms. Environ. Sci. Technol. 2011, 45, 8444–8451. [Google Scholar] [CrossRef] [PubMed]
- Aulenta, F.; Verdini, R.; Zeppilli, M.; Zanaroli, G.; Fava, F.; Rossetti, S.; Majone, M. Electrochemical stimulation of microbial cis-dichloroethene (cis-DCE) oxidation by an ethene-assimilating culture. New Biotechnol. 2013, 30, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.; Verdini, R.; Aulenta, F.; Majone, M. Influence of nitrate and sulfate reduction in the bioelectrochemically assisted dechlorination of cis-DCE. Chemosphere 2015, 125, 147–154. [Google Scholar] [CrossRef]
- Lai, A.; Verdini, R.; Simone, M.; Aulenta, F.; Majone, M. Bioelectrochemical CAHs dechlorination. New Biotechnol. 2016, 33, S132–S133. [Google Scholar] [CrossRef]
- Leitao, P.; Rossetti, S.; Danko, A.S.; Nouws, H.; Aulenta, F. Enrichment of Dehalococcoides mccartyi spp. from a municipal activated sludge during AQDS-mediated bioelectrochemical dechlorination of 1,2-dichloroethane to ethene. Bioresour. Technol. 2016, 214, 426–431. [Google Scholar] [CrossRef]
- Leitao, P.; Nouws, H.; Danko, A.S.; Aulenta, F. Bioelectrochemical Dechlorination of 1,2-DCAwith an AQDS-Functionalized Cathode Serving as Electron Donor. Fuel Cells 2017, 17, 612–617. [Google Scholar] [CrossRef]
- Leitao, P.; Rossetti, S.; Nouws, H.P.A.; Danko, A.S.; Majone, M.; Aulenta, F. Bioelectrochemically-assisted reductive dechlorination of 1,2-dichloroethane by a Dehalococcoides-enriched microbial culture. Bioresour. Technol. 2015, 195, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Palma, E.; Daghio, M.; Tofalos, A.E.; Franzetti, A.; Viggi, C.C.; Fazi, S.; Papini, M.P.; Aulenta, F. Anaerobic electrogenic oxidation of toluene in a continuous-flow bioelectrochemical reactor: process performance, microbial community analysis, and biodegradation pathways. Environ. Sci-Water Res. Technol. 2018, 4, 2136–2145. [Google Scholar] [CrossRef]
- Verdini, R.; Aulenta, F.; de Tora, F.; Lai, A.; Majone, M. Relative contribution of set cathode potential and external mass transport on TCE dechlorination in a continuous-flow bioelectrochemical reactor. Chemosphere 2015, 136, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Feng, C.; Wang, Q.; Yang, Y.; Zhang, Z.; Sugiura, N. Nitrate removal from groundwater by cooperating heterotrophic with autotrophic denitrification in a biofilm-electrode reactor. J. Hazard. Mater. 2011, 192, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, B.; Feng, C.; Huang, F.; Zhang, P.; Zhang, Z.; Yang, Y.; Sugiura, N. Behavior of autotrophic denitrification and heterotrophic denitrification in an intensified biofilm-electrode reactor for nitrate-contaminated drinking water treatment. Bioresour. Technol. 2012, 107, 159–165. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, B.; Cheng, M.; Feng, C. Effects of various organic carbon sources on simultaneous V(V) reduction and bioelectricity generation in single chamber microbial fuel cells. Bioresour. Technol. 2016, 201, 105–110. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Tong, S.; Zheng, M.; Zhao, Y.; Tian, C.; Liu, H.; Feng, C. Enhancement of bacterial denitrification for nitrate removal in groundwater with electrical stimulation from microbial fuel cells. J. Power Sources 2014, 268, 423–429. [Google Scholar] [CrossRef]
- Tong, S.; Zhang, B.; Feng, C.; Zhao, Y.; Chen, N.; Hao, C.; Pu, J.; Zhao, L. Characteristics of heterotrophic/biofilm-electrode autotrophic denitrification for nitrate removal from groundwater. Bioresour. Technol. 2013, 148, 121–127. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B.G.; Borthwick, A.G.L.; Feng, C.P. Utilization of single-chamber microbial fuel cells as renewable power sources for electrochemical degradation of nitrogen-containing organic compounds. Chem. Eng. J. 2015, 280, 99–105. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Zhou, X.; Shi, C.; Guo, H.; Feng, C. Electrochemical decolorization of methyl orange powered by bioelectricity from single-chamber microbial fuel cells. Bioresour. Technol. 2015, 181, 360–362. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Renshaw, J.C. Bioremediation of radioactive waste: radionuclide-microbe interactions in laboratory and field-scale studies. Curr. Opin. Biotechnol. 2005, 16, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Morris, K.; Livens, F.R.; Charnock, J.M.; Burke, I.T.; McBeth, J.M.; Begg, J.D.C.; Boothman, C.; Lloyd, J.R. An X-ray absorption study of the fate of technetium in reduced and reoxidised sediments and mineral phases. Appl. Geochem. 2008, 23, 603–617. [Google Scholar] [CrossRef]
- Burke, I.T.; Boothman, C.; Lloyd, J.R.; MORTIMER, R.J.G. Effects of Progressive Anoxia on the Solubility of Technetium in Sediments. Environ. Sci. Technol. 2005, 39, 4109–4116. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, C.L.; Law, G.T.W.; Lloyd, J.R.; Williams, H.A.; Atherton, N.; Morris, K. Quantifying Technetium and Strontium Bioremediation Potential in Flowing Sediment Columns. Environ. Sci. Technol. 2017, 51, 12104–12113. [Google Scholar] [CrossRef] [PubMed]
- Molognoni, D.; Devecseri, M.; Cecconet, D.; Capodaglio, A.G. Cathodic groundwater denitrification with a bioelectrochemical system. J. Water Process Eng. 2017, 19, 67–73. [Google Scholar] [CrossRef]
- Cecconet, D.; Bolognesi, S.; Callegari, A.; Capodaglio, A.G. Controlled sequential biocathodic denitrification for contaminated groundwater bioremediation. Sci. Total. Environ. 2019, 651, 3107–3116. [Google Scholar] [CrossRef] [PubMed]
- Cecconet, D.; Bolognesi, S.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Influence of reactor’s hydrodynamics on the performance of microbial fuel cells. J. Water Process Eng. 2018, 26, 281–288. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Cecconet, D.; Molognoni, D. An Integrated Mathematical Model of Microbial Fuel Cell Processes: Bioelectrochemical and Microbiologic Aspects. Processes 2017, 5, 73. [Google Scholar] [CrossRef]
- Chen, D.; Yang, K.; Wang, H.; Lv, B. Nitrate removal from groundwater by hydrogen-fed autotrophic denitrification in a bio-ceramsite reactor. Water Sci. Technol. 2014, 69, 2417–2422. [Google Scholar] [CrossRef]
- Chen, D.; Wang, H.; Ji, B.; Yang, K.; Wei, L.; Jiang, Y. A high-throughput sequencing study of bacterial communities in an autohydrogenotrophic denitrifying bio-ceramsite reactor. Process Biochem. 2015, 50, 1904–1910. [Google Scholar] [CrossRef]
- Chen, D.; Wang, H.; Yang, K.; Ma, F. Performance and microbial communities in a combined bioelectrochemical and sulfur autotrophic denitrification system at low temperature. Chemosphere 2018, 193, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wei, L.; Zou, Z.; Yang, K.; Wang, H. Bacterial communities in a novel three-dimensional bioelectrochemical denitrification system: the effects of pH. Appl. Microbiol. Biotechnol. 2016, 100, 6805–6813. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.E.; Finneran, K.T.; O’Neil, R.A.; Lovley, D.R. Enrichment of members of the family Geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. Appl.Environ. Microbiol. 2002, 68, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Yun, S.T.; Mayer, B.; Kim, K.H. Sources and biogeochemical behavior of nitrate and sulfate in an alluvial aquifer: hydrochemical and stable isotope approaches. Appl. Geochem. 2011, 26, 1249–1260. [Google Scholar] [CrossRef]
- Kumar, N.; Couture, R.M.; Millot, R.; Battaglia-Brunet, F.; Rose, J. Microbial Sulfate Reduction Enhances Arsenic Mobility Downstream of Zerovalent-Iron-Based Permeable Reactive Barrier. Environ. Sci. Technol. 2016, 50, 7610–7617. [Google Scholar] [CrossRef]
- Van Khanh, N.; Park, Y.; Yang, H.; Yu, J.; Lee, T. Effect of the cathode potential and sulfate ions on nitrate reduction in a microbial electrochemical denitrification system. J. Ind. Microbiol. Biotechnol. 2016, 43, 783–793. [Google Scholar] [CrossRef]
- Florez, C.; Park, Y.; Valles-Rosales, D.; Lara, A.; Rivera, E. Removal of Uranium from Contaminated Water by Clay Ceramics in Flow-Through Columns. Water 2017, 9, 761. [Google Scholar] [CrossRef]
- Pous, N.; Koch, C.; Colprim, J.; Puig, S.; Harnisch, F. Extracellular electron transfer of biocathodes: Revealing the potentials for nitrate and nitrite reduction of denitrifying microbiomes dominated by Thiobacillus sp. Electrochem. Commun. 2014, 49, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Reguera, G.; McCarthy, K.D.; Mehta, T.; Nicoll, J.S.; Tuominen, M.T.; Lovley, D.R. Extracellular electron transfer via microbial nanowires. Nature 2005, 435, 1098–1101. [Google Scholar] [CrossRef]
- Gregory, K.B.; Bond, D.R.; Lovley, D.R. Graphite electrodes as electron donors for anaerobic respiration. Environ. Microbiol. 2004, 6, 596–604. [Google Scholar] [CrossRef]
- Gregory, K.B.; Lovley, D.R. Remediation and recovery of uranium from contaminanted subsurface environments with electrodes. Environ. Sci. Technol. 2005, 39, 8943–8947. [Google Scholar] [CrossRef] [PubMed]
- Winderl, C.; Anneser, B.; Griebler, C.; Meckenstock, R.U.; Lueders, T. Depth-resolved quantification of anaerobic toluene degraders and aquifer microbial community patterns in distinct redox zones of a tar oil contaminant plume. Appl. Environ. Microbiol. 2008, 74, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.B.; Yarbrough, H.F. Preliminary Experiments on a Microbial Fuel Cell. Science 1962, 137, 615–616. [Google Scholar] [CrossRef] [PubMed]
- Puig, S.; Coma, M.; Desloover, J.; Boon, N.; Colprim, J.; Balaguer, M.D. Autotrophic Denitrification in Microbial Fuel Cells Treating Low Ionic Strength Waters. Environ. Sci. Technol. 2012, 46, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Guan, L.; Taylor, D.P.; Kuhn, D.; He, Z. Nitrogen removal from water of recirculating aquaculture system by a microbial fuel cell. Aquaculture 2018, 497, 74–81. [Google Scholar] [CrossRef]
- Vrionis, H.A.; Anderson, R.T.; Ortiz-Bernad, I.; O’Neill, K.R.; Resch, C.T.; Peacock, A.D.; Dayvault, R.; White, D.C.; Long, P.E.; Lovley, D.R. Microbiological and geochemical heterogeneity in an in situ uranium bioremediation field site. Appl. Environ. Microbiol. 2005, 71, 6308–6318. [Google Scholar] [CrossRef] [PubMed]
- Rooney-varga, J.; Anderson, R.T.; Fraga, J.; Ringelberg, D. Microbial Communities Associated with Anaerobic Benzene Degradation in a Petroleum Contaminated Aquifer. Appl. Environ. Microbiol. 1999, 65, 3056–3063. [Google Scholar]
- Harrison, I.; Williams, G.; Higgo, J.; Leader, R. Microcosm studies of microbial degradation in a coal tar distillate plume. J. Contam. Hydrol. 2001, 53, 319–340. [Google Scholar] [CrossRef]
- Gómez, M.A.; Hontoria, E.; González-López, J. Effect of dissolved oxygen concentration on nitrate removal from groundwater using a denitrifying submerged filter. J. Hazard. Mater. 2002, B90, 267–278. [Google Scholar] [CrossRef]
- Tobler, N.B.; Hofstetter, T.B.; Schwarzenbach, R. Carbon and Hydrogen Isotope Fractionation during Anaerobic Toluene Oxidation by Geobacter metallireducens with Different Fe(III) Phases as Terminal Electron Acceptors. Environ. Sci. Technol. 2008, 42, 7786–7792. [Google Scholar] [CrossRef]
- Geets, J.; Vanbroekhoven, K.; Borremans, B.; Vangronsveld, J.; Diels, L.; van der Lelie, D. Column experiments to assess the effects of electron donors on the efficiency of in situ precipitation of Zn, Cd, Co and Ni in contaminated groundwater applying the biological sulfate removal technology. Environ. Sci. Pollut. Res. 2005, 13, 362–378. [Google Scholar] [CrossRef]
- Ma, X.; Novak, P.J.; Semmens, M.J.; Clapp, L.W.; Hozalski, R.M. Comparison of pulsed and continuous addition of H2 gas via membranes for stimulating PCE biodegradation in soil columns. Water Res. 2006, 40, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Foo, K.Y.; Hameed, B.H. Detoxification of pesticide waste via activated carbon adsorption process. J. Hazard. Mater. 2010, 175, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Feleke, Z.; Sakakibara, Y. A bio-electrochemical reactor coupled with adsorber for the removal of nitrate and inhibitory pesticide. Water Res. 2002, 36, 3092–3102. [Google Scholar] [CrossRef]
- Nguyen, V.K.; Park, Y.; Yu, J.; Lee, T. Bioelectrochemical denitrification on biocathode buried in simulated aquifer saturated with nitrate-contaminated groundwater. Environ. Sci. Pollut. Res. 2016, 23, 15443–15451. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ramnarayanan, R.; Logan, B.E. Production of Electricity during Wastewater Treatment Using a Single Chamber Microbial Fuel Cell. Environ. Sci. Technol. 2004, 38, 2281–2285. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Minteer, S.D.; Angenent, L.T. Electricity Generation from Artificial Wastewater Using an Upflow Microbial Fuel Cell. Environ. Sci. Technol. 2005, 39, 5262–5267. [Google Scholar] [CrossRef] [PubMed]
- Aelterman, P.; Rabaey, K.; Clauwaert, P.; Verstraete, W. Microbial fuel cells for wastewater treatment. Water Sci. Technol. 2006, 54, 9–15. [Google Scholar] [CrossRef] [Green Version]
- Capodaglio, A.G.; Molognoni, D.; Puig, S.; Balaguer, M.D.; Colprim, J. Role of Operating Conditions on Energetic Pathways in a Microbial Fuel Cell. Energy Procedia 2015, 74, 728–735. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Logan, B.E. Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem. Commun. 2007, 9, 492–496. [Google Scholar] [CrossRef]
- Xu, H.; Tong, N.; Huang, S.; Hayat, W.; Fazal, S.; Li, J.; Li, S.; Yan, J.; Zhang, Y. Simultaneous autotrophic removal of sulphate and nitrate at different voltages in a bioelectrochemical reactor (BER): Evaluation of degradation efficiency and characterization of microbial communities. Bioresour. Technol. 2018, 265, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Aulenta, F.; Reale, P.; Canosa, A.; Rossetti, S.; Panero, S.; Majone, M. Characterization of an electro-active biocathode capable of dechlorinating trichloroethene and cis-dichloroethene to ethene. Biosens. Bioelectron. 2010, 25, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Callegari, A.; Cecconet, D.; Molognoni, D.; Capodaglio, A.G. Sustainable processing of dairy wastewater: Long-term pilot application of a bio-electrochemical system. J. Clean Prod. 2018, 189, 563–569. [Google Scholar] [CrossRef]
- Clauwaert, P.; Desloover, J.; Shea, C.; Nerenberg, R.; Boon, N.; Verstraete, W. Enhanced nitrogen removal in bio-electrochemical systems by pH control. Biotechnol. Lett. 2009, 31, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Ng, G.H.; Bekins, B.A.; Cozzarelli, I.M.; Baedecker, M.J.; Bennett, P.C.; Amos, R.T. A mass balance approach to investigating geochemical controls on secondary water quality impacts at a crude oil spill site near Bemidji, MN. J. Contam. Hydrol. 2014, 164, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Y.; He, Z. Nitrate removal from groundwater driven by electricity generation and heterotrophic denitrification in a bioelectrochemical system. J. Hazard. Mater. 2013, 262, 614–619. [Google Scholar] [CrossRef]
- Mao, D.; Lu, L.; Revil, A.; Zuo, Y.; Hinton, J.; Ren, Z.J. Geophysical Monitoring of Hydrocarbon-Contaminated Soils Remediated with a Bioelectrochemical System. Environ. Sci. Technol. 2016, 50, 8205–8213. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, L.; Ma, H.; Yu, K.; Martin, J.D. Hydrochemical characterization and pollution sources identification of groundwater in Salawusu aquifer system of Ordos Basin, China. Environ. Pollut. 2016, 216, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Yu, H.; Li, C.; Ren, Y.; Wei, C.; Feng, C. Competitive microbial reduction of perchlorate and nitrate with a cathode directly serving as the electron donor. Electrochimica Acta 2014, 133, 217–223. [Google Scholar] [CrossRef]
- Kong, X.; Han, Z.; Zhang, W.; Song, L.; Li, H. Synthesis of zeolite-supported microscale zero-valent iron for the removal of Cr(6+) and Cd(2+) from aqueous solution. J. Environ. Manage. 2016, 169, 84–90. [Google Scholar] [CrossRef]
- Liu, T.; Yang, X.; Wang, Z.L.; Yan, X. Enhanced chitosan beads-supported Fe(0)-nanoparticles for removal of heavy metals from electroplating wastewater in permeable reactive barriers. Water Res. 2013, 47, 6691–6700. [Google Scholar] [CrossRef] [PubMed]
- Kokabian, B.; Gude, V.G. Biodiesel produced wastewater treatment combined with desalination in bioelectrochemical systems. Abstracts of Papers of the American Chemical Society 2013, 245. [Google Scholar]
- Morris, J.M.; Jin, S.; Crimi, B.; Pruden, A. Microbial fuel cell in enhancing anaerobic biodegradation of diesel. Chem. Eng. J. 2009, 146, 161–167. [Google Scholar] [CrossRef]
- Feleke, Z.; Sakakibara, Y. Nitrate and pesticide removal by a combined bioelectrochemical/adsorption process. Water Sci. Technol. 2001, 43, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Obiri-Nyarko, F.; Grajales-Mesa, S.J.; Malina, G. An overview of permeable reactive barriers for in situ sustainable groundwater remediation. Chemosphere 2014, 111, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Feng, H.; Wang, M.; Li, N.; Cong, Y.; Shen, D. The effect of C/N ratio on nitrogen removal in a bioelectrochemical system. Bioresour. Technol. 2013, 132, 91–98. [Google Scholar] [CrossRef]






| No. | Journal | Total Publications | Sum of Times Cited | Journal IF (2018)/Quartile in Category |
|---|---|---|---|---|
| 1 | ENVIRON. SCI. TECHNOL. | 163 | 8004 | 7.149/Q1 |
| 2 | APPL. ENVIRON. MICROB. | 85 | 5650 | 4.077/Q1 |
| 3 | WATER RES. | 71 | 3143 | 7.913/Q1 |
| 4 | J. CONTAM. HYDROL. | 53 | 2051 | 2.65/Q2 |
| 5 | BIORESOUR. TECHNOL. | 52 | 1115 | 6.669/Q1 |
| 6 | GEOCHIM. COSMOCHIM. ACTA | 45 | 2273 | 4.258/Q1 |
| 7 | GEOMICROBIOL. J. | 44 | 1421 | 1.609/Q3 |
| 8 | CHEMOSPHERE | 38 | 774 | 5.108/Q1 |
| 9 | J. HAZARD. MATER. | 38 | 1519 | 7.65/Q1 |
| 10 | FEMS MICROBIOL. ECOL. | 33 | 1072 | 4.098/Q2 |
| Group No. | Authors | Research Topics in this Field |
|---|---|---|
| 1 | Lovley D.R., Williams K.H. and Long P.E. et al. | Dissimilatory Fe(III) and Mn(IV) reduction [94]; Uranium(VI) bioreduction [80,81,95,96]; Anaerobic benzene degradation [97]; |
| 2 | Criddle C.S., Wu W.M. and Hazen T.C. et al. | Microbial fuel cells [98]; Uranium(VI) bioremediation [99,100,101,102]; Carbon tetrachloride bioremediation [103]; |
| 3 | Aulenta F., Majone M. and Papini M.P. et al. | Microbial dechlorination [21,45,104,105,106,107,108,109,110,111,112,113,114]; |
| 4 | Feng C.P., Zhang B.G., and Liu Y. et al. | Biofilm electrode reactor denitrification [115,116]; Vanadium (V) bioremediation with microbial fuel cell [75,76,117]; Nitrate removal with microbial fuel cell [118,119]; Pyridine and methyl orange removal with microbial fuel cell [120,121]; |
| 5 | Lloyd J.R., Morris K. and Boothman C. et al. | Metal-reducing bacteria [72]; Bioremediation of uranium [79,80,122]; Detection and bioremediation of technetium [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125]; |
| 6 | Cecconet D. and Capodaglio A.G. | Groundwater denitrification [47,126,127]; Energy consumption [49]; Metals and perchlorate removal [25]; Microbial fuel cell [49,128,129]; |
| 7 | Wang H.Y. and Yang K. | Autohydrogenotrophic denitrification [130,131]; Bioelectrochemical denitrification [54,132,133]; |
| Cited Frequency | Author | Title | Published Year | Published Journal |
|---|---|---|---|---|
| 102 | Anderson R.T. et al. | Stimulating the in situ activity of geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. | 2003 | APPL. ENVIRON. MICROB. |
| 61 | Holmes D.E. et al. | Enrichment of members of the family geobacteraceae associated with stimulation of dissimilatory metal reduction in uranium-contaminated aquifer sediments. | 2002 | APPL. ENVIRON. MICROB. |
| 49 | Istok J.D. et al. | In situ bioreduction of technetium and uranium in a nitrate-contaminated aquifer. | 2004 | ENVIRON. SCI. TECHNOL. |
| 45 | Rooney-Varga J.N. et al. | Microbial communities associated with anaerobic benzene degradation in a petroleum-contaminated aquifer. | 1999 | APPL. ENVIRON. MICROB. |
| 44 | Wu W.M. et al. | Pilot-scale in situ bioremedation of uranium in a highly contaminated aquifer. 2. reduction of U(VI) and geochemical control of U(VI) bioavailability. | 2006 | ENVIRON. SCI. TECHNOL. |
| 43 | North N.N. et al. | Change in bacterial community structure during in situ biostimulation of subsurface sediment co-contaminated with uranium and nitrate. | 2004 | APPL. ENVIRON. MICROB. |
| 41 | Vrionis H.A. et al. | Microbiological and geochemical heterogeneity in an in situ uranium bioremediation field site. | 2005 | APPL. ENVIRON. MICROB. |
| 40 | Lovley D.R. et al. | Dissimilatory Fe(III) and Mn(IV) reduction. | 2004 | ADV. MICROB. PHYSIOL. |
| 40 | Caporaso J.P. et al. | QIIME allows analysis of high-throughput community sequencing data. | 2010 | NAT. METHODS |
| 37 | Reguera G. et al. | Extracellular electron transfer via microbial nanowires. | 2005 | NATURE |
| Time Slice/Keywords Catalogs | Reactive Materials/Contaminants | Reactions/Processes | Experimental Apparatus/Microorganism |
|---|---|---|---|
| 1999–2003 | phenol | electron accepting process | biofilm reactor |
| monoaromatic | biomineralization | biofilter | |
| hydrocarbon | anaerobic benzene oxidation | ||
| petroleum hydrocarbon | / | / | |
| 2004–2008 | hydrous ferric oxide | in situ hybridization | pseudomonas putida |
| benzylsuccinate synthase | dissimilatory sulfite reductase | hollow fiber membrane | |
| 2009–2013 | activated carbon | in situ biostimulation | / |
| 2014–2018 | methane | electricity generation | biofilm electrode reactor |
| hydrogen peroxide | fermentation | biocathode | |
| perchlorate | / | / |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Chen, X.; Xie, L.; Liu, Z.; Xiong, X. Bioelectrochemical Systems for Groundwater Remediation: The Development Trend and Research Front Revealed by Bibliometric Analysis. Water 2019, 11, 1532. https://doi.org/10.3390/w11081532
Li W, Chen X, Xie L, Liu Z, Xiong X. Bioelectrochemical Systems for Groundwater Remediation: The Development Trend and Research Front Revealed by Bibliometric Analysis. Water. 2019; 11(8):1532. https://doi.org/10.3390/w11081532
Chicago/Turabian StyleLi, Wei, Xiaohong Chen, Linshen Xie, Zhao Liu, and Xiangyun Xiong. 2019. "Bioelectrochemical Systems for Groundwater Remediation: The Development Trend and Research Front Revealed by Bibliometric Analysis" Water 11, no. 8: 1532. https://doi.org/10.3390/w11081532
APA StyleLi, W., Chen, X., Xie, L., Liu, Z., & Xiong, X. (2019). Bioelectrochemical Systems for Groundwater Remediation: The Development Trend and Research Front Revealed by Bibliometric Analysis. Water, 11(8), 1532. https://doi.org/10.3390/w11081532