Research on the Migration of the Total Manganese during the Process of Water Icing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Natural Icing Experiment
2.2. Simulated Icing Experiment
2.2.1. Icing Simulation Device
2.2.2. Experimental Method
3. Results and Discussion
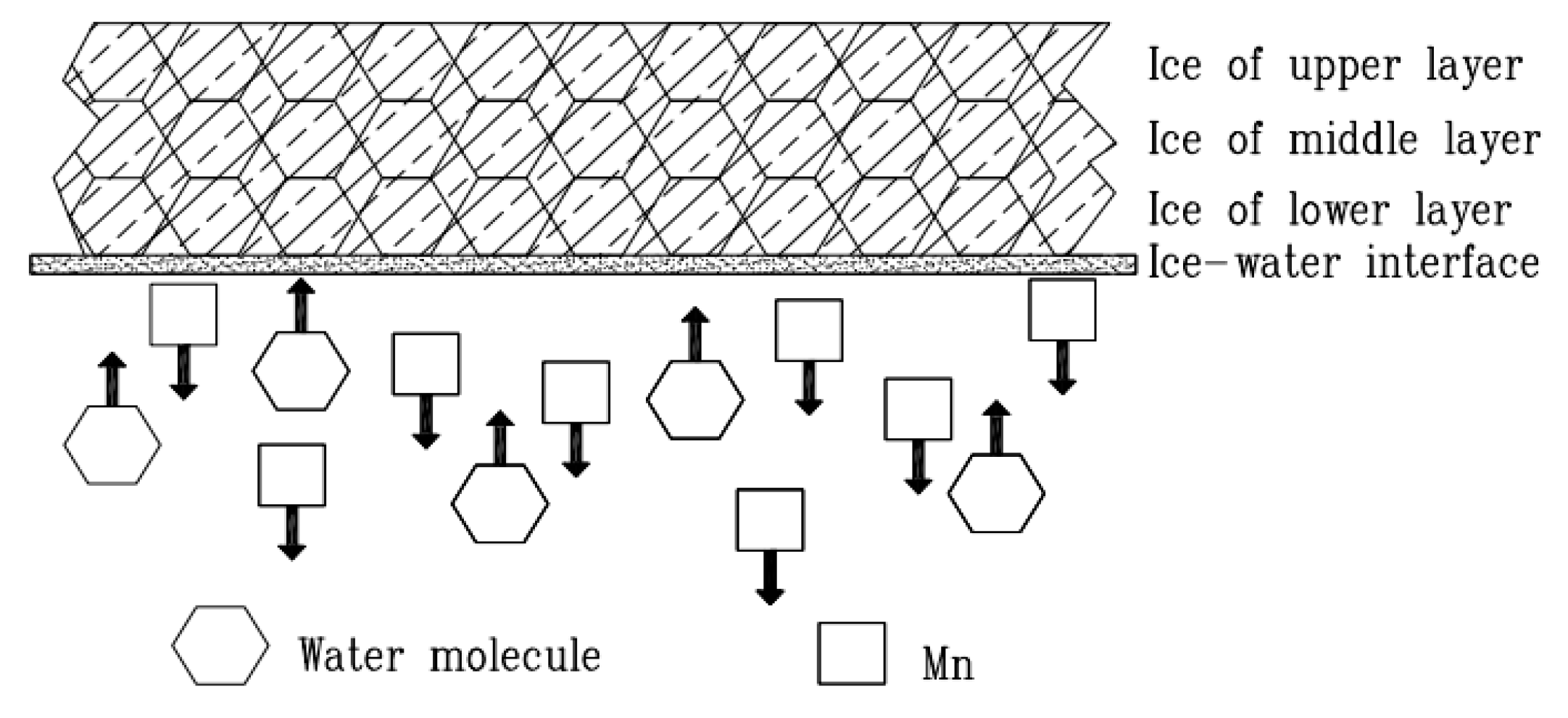
3.1. Natural Icing Experiment
3.2. Simulated Icing Experiment
3.2.1. Effects of Ice Thickness on the Migration of TMn
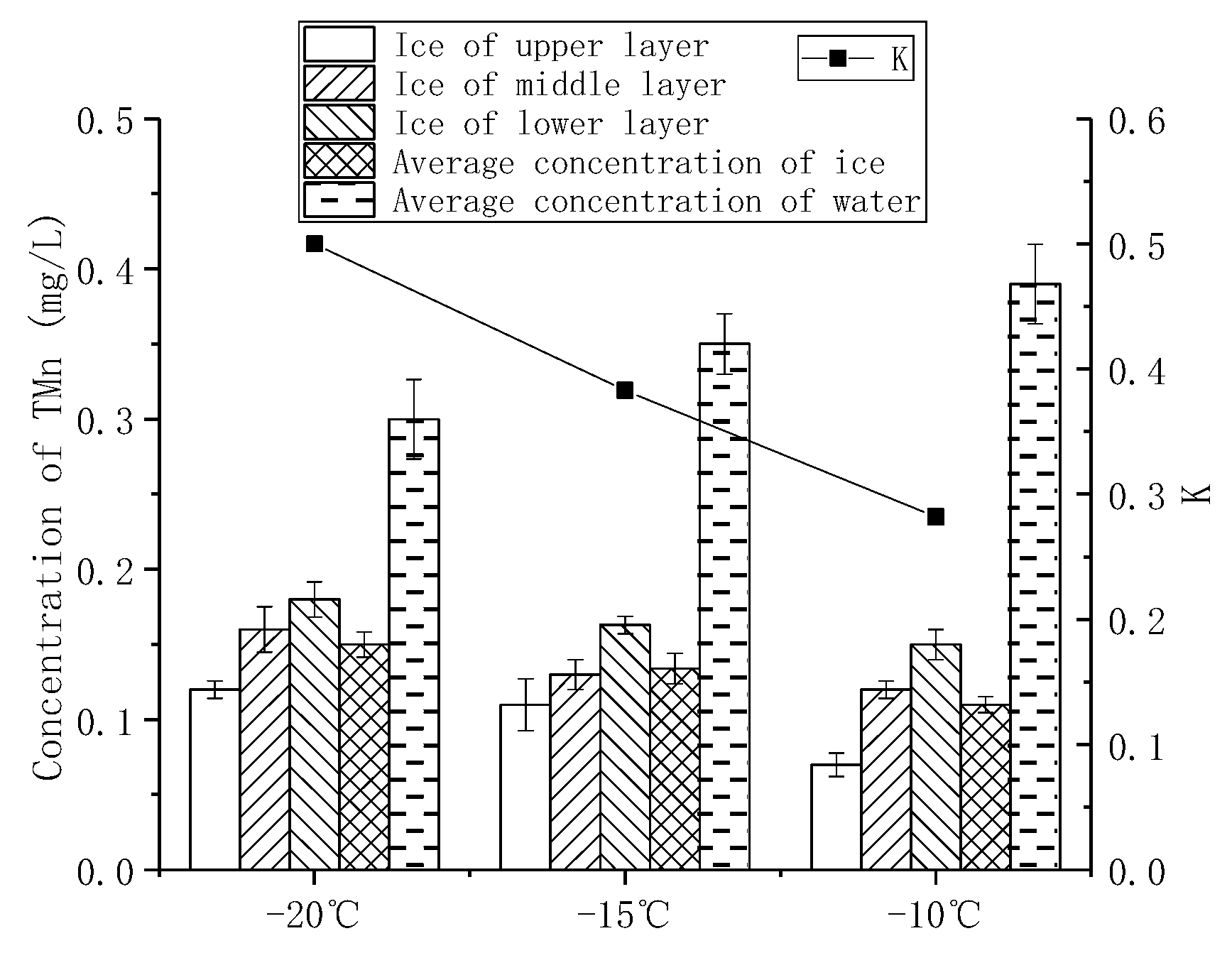
3.2.2. Effects of Freezing Temperatures on the Migration of TMn
3.2.3. Effects of Initial Concentrations on the Migration of TMn
4. Conclusions
5. Implication and Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Weyhenmeyer, G.A.; Livingstone, D.M.; Meili, M.; Jensen, O.; Benson, B.; Magnuson, J.J. Large geographical differences in the sensitivity of ice-covered lakes and rivers in the Northern Hemisphere to temperature changes. Glob. Chang. Biol. 2011, 17, 268–275. [Google Scholar] [CrossRef]
- Verpoorter, C.; Kutser, T.; Seekell, D.A.; Tranvik, L.J. A global inventory of lakes based on high-resolution satellite imagery. Geophys. Res. Lett. 2014, 41, GL060641. [Google Scholar] [CrossRef]
- Catalan, J. Evolution of dissolved and particulate matter during the ice-covered period in a deep, high-mountain lake. Can. J. Fish. Aquat. Sci. 1992, 49, 945–955. [Google Scholar] [CrossRef]
- Welch, H.E.; Legault, J.A.; Bergmann, M.A. Effects of snow and ice on the annual cycles of heat and light in Saqvaqjuac lakes. Can. J. Fish. Aquat. Sci. 1987, 44, 1451–1461. [Google Scholar] [CrossRef]
- Jewson, D.H.; Granin, N.G.; Zhdanov, A.A.; Gnatovsky, R.Y. Effect of snow depth on under-ice irradiance and growth of Aulacoseira baicalensis in Lake Baikal. Aquat. Ecol. 2009, 43, 673–679. [Google Scholar] [CrossRef]
- Pernica, P.; North, R.L.; Baulch, H.M. In the cold light of day: The potential importance of under-ice convective mixed layers to primary producers. Inland Waters 2017, 7, 138–150. [Google Scholar] [CrossRef]
- Fang, X.; Stefan, H.G. Simulations of climate effects on water temperature, dissolved oxygen, and ice and snow covers in lakes of the contiguous U.S. under past and future climate scenarios. Limnol. Oceanogr. 2009, 54, 2359–2370. [Google Scholar] [CrossRef]
- Golosov, S.; Maher, O.; Schipunova, E.; Terzhevik, A.; Zdorovennova, G.; Kirillin, G. Physical background of the development of oxygen depletion in ice-covered lakes. Oecologia 2007, 151, 331–340. [Google Scholar] [CrossRef]
- McBean, E.; Farquhar, G.; Kouwen, N.; Dubek, O. Predictions of ice-cover development in streams and its effect on dissolved oxygen modelling. Can. J. Civ. Eng. 1979, 6, 197–207. [Google Scholar] [CrossRef]
- Julie, T.; Amir, S.; Karl-Erich, L. Modelling Dissolved Oxygen/Sediment Oxygen Demand under Ice in a Shallow Eutrophic Prairie Reservoir. Water 2017, 9, 131–147. [Google Scholar]
- Hampton, S.E.; Galloway, A.W.E.; Powers, S.M.; Ozersky, T.; Woo, K.H.; Batt, R.D.; Labou, S.G.; O’Reilly, C.M.; Sharma, S.; Lottig, N.R. Ecology under lake ice. Ecol. Lett. 2017, 20, 98–111. [Google Scholar] [CrossRef]
- Salonen, K.; Leppäranta, M.; Viljanen, M.; Gulati, R. Perspectives in winter limnology: Closing the annual cycle of freezing lakes. Aquat. Ecol. 2009, 43, 609–616. [Google Scholar] [CrossRef]
- Lindenschmidt, K.E.; Baulch, H.; Cavaliere, E. River and Lake Ice Processes—Impacts of Freshwater Ice on Aquatic Ecosystems in a Changing Globe. Water 2018, 10, 1586. [Google Scholar] [CrossRef]
- Zhang, Y. Migration Mechanism of Pollutants and Its Application in Ulansuhai Lake in Freezing Process; Inner Mongolia Agriculture University: Inner Mongolia, China, 2012. (In Chinese) [Google Scholar]
- Kirillin, G. Physics of seasonally ice-covered lakes: A review. Aquat. Sci. 2012, 74, 659–682. [Google Scholar] [CrossRef]
- Powers, S.M.; Hampton, S.E. Winter Limnology as a New Frontier. Limnol. Oceanogr. Bull. 2016, 25, 103–108. [Google Scholar] [CrossRef]
- Hampton, S.E.; Moore, M.V.; Ozersky, T.; Stanley, E.H.; Polashenski, C.M.; Galloway, A.W.E. Heating up a cold subject: Prospectsfor under-ice plankton research in lakes. Plankton Res. 2015, 37, 277–284. [Google Scholar] [CrossRef]
- Belzile, C.; Gibson, J.A.E.; Vincent, W.F. Colored dissolved organic matter and dissolved organic carbon exclusion from lake ice: Implications for irradiance transmission and carbon cycling. Limnol. Oceanogr. 2002, 47, 1283–1293. [Google Scholar] [CrossRef] [Green Version]
- Powers, S.M.; Labou, S.G.; Baulch, H.M. Ice duration drives winter nitrate accumulation in north temperate lakes. Limnol. Oceanogr. Lett. 2017. [Google Scholar] [CrossRef]
- Roger, P.; Gregory, A.L. Effect of salt exclusion from lake ice on seasonal circulation. Limnol. Oceanogr. 2009, 54, 401–412. [Google Scholar]
- The Surface Water Environmental Quality Standard; China Environmental Science Press: Beijing, China, 2002. (In Chinese)
- Li, Q.Q. Research on Removal of Manganese from Groundwater by Chlorine Dioxide/Manganese Sand; Shenyang Jianzhu University: Liaoning, China, 2012. (In Chinese) [Google Scholar]
- Huang, B.; Li, Z.; Chen, Z. Study and health risk assessment of the occurrence of iron and manganese in groundwater at the terminal of the Xiang jiang River. Environ. Sci. Pollut. Res. 2015, 22, 19912–19921. [Google Scholar] [CrossRef]
- Kaiser, J. Manganese: A high-octane dispute. Science 2003, 300, 926–928. [Google Scholar] [CrossRef]
- Figá-Talamanca, I.; Petrelli, G. Reduction in male births among workers exposed to metal fumes. Int. J. Epidemiol. 2000, 29, 381. [Google Scholar] [CrossRef]
- Tandon, S.K.; Chandra, S.V.; Singh, J. Chelation in metal intoxication. I. In vivo effect of chelating a-gents on liver and testis of manganese administered rats. Environ. Res. 1975, 9, 18–25. [Google Scholar] [CrossRef]
- Wittczak, T.; Dudek, W.; Krakowiak, A. Occupational asthma due to manganese exposure: A case report. Int. J. Occup. Med. Environ. Health 2008, 21, 81–83. [Google Scholar] [CrossRef]
- Luo, S.S.; Wan, G.J.; Roland, S. Characters of Cycle and Transference of Iron and Manganese at Interface Between Sediment-Water in Wallersee, Austria. Agro-Environ. Prot. 2001, 3, 137–140. (In Chinese) [Google Scholar]
- The State Environmental Protection Administration the Water and Wastewater Monitoring Analysis Method Editorial Board. Water and Wastewater Monitoring Analysis Method, 4th ed.; China Environmental Science Press: Beijing, China, 2002. (In Chinese) [Google Scholar]
- Sun, C.; Li, C.Y.; Liu, J.J.; Shi, X.H.; Zhao, S.N.; Wu, Y.; Tian, W.D. First-Principles Study on the Migration of Heavy Metal Ions in Ice-Water Medium from Ulansuhai Lake. Water 2018, 10, 1149. [Google Scholar] [CrossRef]
- Standards for Drinking Water Quality; GB5749-2006; Ministry of Health of the People’s Republic of China: Beijing, China, 2006. (In Chinese)
- Wang, J. Accumulation of Heavy Metal in Different Tissues of Patinopecten Yessoensis and the Primary Study on the Environment Correlation; Ocean University of China: Shandong, China, 2009. (In Chinese) [Google Scholar]
- Zhang, Y.X.; Li, L.F.; Chai, T.Y.; Lin, D.; Zhang, H.M. Mechanisms of manganese toxicity and manganese tolerance in plants. Chin. Bull. Bot. 2010, 45, 506–520. [Google Scholar]
- Bigg, E.K. The supercooling of water. Proc. Phys. Soc. Lond. Sect. 1953, 66, 688–694. [Google Scholar] [CrossRef]
- Vali, G.; Stansbury, E.J. Time-dependent characteristics of the heterogeneous nucleation of ice. Can. J. Phys. 1966, 44, 477–502. [Google Scholar] [CrossRef]
- Song, X.L.; Huang, X.H. Fundamentals of Inorganic Materials Science; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Zhang, Y.; Li, C.Y.; Shi, X.H.; Chao, L.I. The migration of total dissolved solids during natural freezing process in Ulansuhai Lake. J. Arid. Land 2012, 4, 85–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.Y.; Zhang, S.; Shi, X.H.; Li, W.P. Analysis on polluting characteristics in Hulun Lake during ice-on period and its application in field of water treatment. Ecol. Environ. 2011, 20, 1289–1294. [Google Scholar]
- Fedotov, V.I. Studies of Antarctic fast ice. In Studies in Ice Physics and Ice Engineering; Iakovlev, G.N., Ed.; Proceedings Vol. 300; Hardin, R., Translator; Arctic and Antarctic Scientific Research Institute: Saint Petersburg, Russia, 1973; pp. 95–111. [Google Scholar]
- Zhang, Y.; Li, C.Y.; Shen, H.T.; Shi, X.H.; Qiao, L.M. Total nitrogen migration in Wuliangsuhai Lake during ice growth process. Adv. Water Sci. 2013, 24, 728–735. [Google Scholar]
- Maykut, G.A. The Surface Heat and Mass Balance. In The Geophysics of Sea Ice; Untersteiner, N., Ed.; Springer: Boston, MA, USA, 1986; Volume 146, pp. 418–463. [Google Scholar]
- Martin, S. A field study of brine drainage and oil entrainment in first-year sea ice. J. Glaciol. 1979, 22, 473–502. [Google Scholar] [CrossRef]
- Yu, T.; Ma, J. Application of Refrigeration in Wastewater Treatment and Recycle. J. Refrig. 2008, 29, 47–50. [Google Scholar]
- Schmidt, S.; Moskal, W.; De Mora, S.J.D. Limnological properties of Antarctic ponds during winter freezing. Antarct. Sci. 1991, 3, 379–388. [Google Scholar] [CrossRef]
- Wharton, R.A.; Mckay, C.P.; Clow, G.D. Perennial ice covers and their influence on Antarctic lake ecosystems. Antarct. Res. Ser. 1993, 59, 53–70. [Google Scholar]
- Waller, R. A Study of Aggregate Ice Formation as a Method of Concentrating Impurities in Aqueous Solution. Ph.D. Thesis, The Johns Hopkins University, Baltimore, MD, USA, 1967. [Google Scholar]
- Terwilliger, J.P.; Dizio, S.F. Salt rejection phenomena in the freezing of saline solutions. Chem. Eng. Sci. 1970, 25, 1331–1349. [Google Scholar] [CrossRef]
- Gao, W.; Habib, M.; Smith, D.W. Removal of organic contaminants and toxiciy from industrial effluents using freezing processes. Desalination 2009, 245, 108–119. [Google Scholar] [CrossRef]
- Yu, T.; Ma, J.Z.; Li, Q. Factors affecting ice crystal purity during freeze concentration process for urine treatment. J. Harbin Inst. Technol. 2007, 14, 593–597. [Google Scholar]
- Luo, C.S.; Chen, W.W.; Han, W.F. Experimental study on factors affecting the quality of ice crystal during the freezing concentration for the brackish water. Desalination 2010, 260, 231–238. [Google Scholar] [CrossRef]
- Weeks, W.F.; Ackley, S.F. The Growth, Structure and Properties of Sea Ice; U.S. Army Corps of Engineers, Cold Regions Research & Engineering Laboratory: Hanover, NH, USA, 1982. [Google Scholar]
- Ghosh, K.; Tyagi, N.; Kumar, P.; Singh, U.P.; Goel, N. Stabilization of TMn (II) and TMn (III) in mononuclear complexes derived from tridentate ligands with N2O donors: Synthesis, crystal structure, superoxide dismutase activity and DNA interaction studies. J. Inorg. Biochem. 2010, 104, 9–18. [Google Scholar] [CrossRef]
- Oldham, V.E.; Mucci, A.; Tebo, B.M.; Luther, G.W., III. Soluble TMn (III)–L complexes are abundant in oxygenated waters and stabilized by humic ligands. Geochim. Cosmochim. Acta 2017, 199, 238–246. [Google Scholar] [CrossRef]
- Chiswell, B.; Mokhtar, M.B. The speciation of manganese in freshwaters. Talanta 1986, 33, 669–677. [Google Scholar] [CrossRef]
- Jiang, J.; Pang, S.Y.; Ma, J. Oxidation of Triclosan by Permanganate (TMn (VII)): Importance of Ligands and In Situ Formed Manganese Oxides. Environ. Sci. Technol. 2009, 43, 8326–8331. [Google Scholar] [CrossRef]
- Lv, H.Z.; Li, C.Y.; Shi, X.H.; Zhao, S.N. Pollutant distribution under different conditions in Lake Ulansuhai ice-water system. J. Lake Sci. 2015, 27, 1151–1158. [Google Scholar]
- Liu, Y. Fate and Transport of Heavy Metals in Ice-Water-Sediment Multi-Medium—A Case Study in Ulansuhai during Its Ice Growthing and Non-Freezing Period; Inner Mongolia Agriculture University: Inner Mongolia, China, 2017. (In Chinese) [Google Scholar]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Tang, Y.; Yu, A.; Zhao, W.; Liu, Y. Research on the Migration of the Total Manganese during the Process of Water Icing. Water 2019, 11, 1626. https://doi.org/10.3390/w11081626
Zhang Y, Tang Y, Yu A, Zhao W, Liu Y. Research on the Migration of the Total Manganese during the Process of Water Icing. Water. 2019; 11(8):1626. https://doi.org/10.3390/w11081626
Chicago/Turabian StyleZhang, Yan, Yuanqing Tang, Aixin Yu, Wanli Zhao, and Yucan Liu. 2019. "Research on the Migration of the Total Manganese during the Process of Water Icing" Water 11, no. 8: 1626. https://doi.org/10.3390/w11081626
APA StyleZhang, Y., Tang, Y., Yu, A., Zhao, W., & Liu, Y. (2019). Research on the Migration of the Total Manganese during the Process of Water Icing. Water, 11(8), 1626. https://doi.org/10.3390/w11081626



