Scaling up Microbial Fuel Cells for Treating Swine Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Swine Wastewater Used as Influent
2.2. Electricity Generation Using Two Different Anodes
2.3. Microbial Fuel Cells with 1.5 L Capacity
2.4. Microbial Fuel Cells with 12 L Capacity
2.5. Microbial Fuel Fells with 100 L Capacity
2.6. Chemical Analysis
2.7. Coulombic Efficiency
3. Results and Discussion
3.1. Electricity Generation Using Two Different Anodes
3.2. Performance of the 1.5 L-MFC
3.3. Performance of the 12 L-MFC
3.4. Performance of the 100 L-MFC
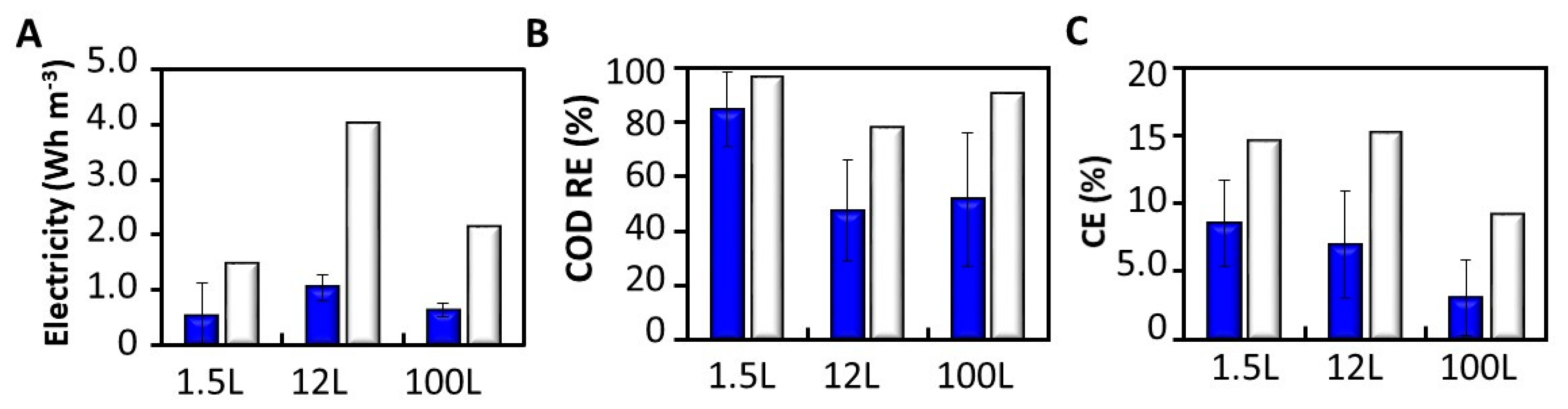
3.5. Effects of Scaling up Microbial Fuel Cells
3.6. Comparison of MFC Performance with Previous Reports
3.7. Net Energy Balance when Combining MFC and Post-Aeration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ministry of Agriculture, Forestry and Fisheries (MAFF). Production and Management of Livestock Manure; Ministry of Agriculture, Forestry and Fisheries: Tokyo, Japan, 2016. (In Japanese) [Google Scholar]
- Oshida, T.; Fukuyasu, T.; Kohzaki, K. Direct treatment of animal waste water by public sewerage in urban district. Waste Manag. Res. 1994, 5, 357–363. [Google Scholar] [CrossRef]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer—Can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Green, F.B.; Lundquist, T.J.; Oswald, W.J. Energetics of advanced integrated wastewater pond systems. Water Sci. Technol. 1995, 31, 9–20. [Google Scholar] [CrossRef]
- Kondusamy, D.; Kalamdhad, A.S. Pre-treatment and anaerobic digestion of food waste for high rate methane production—A review. J. Environ. Chem. Eng. 2014, 2, 1821–1830. [Google Scholar] [CrossRef]
- New Energy and Industrial Technology Development Organization (NEDO). White Paper on Renewable Energy Technologies, 2nd ed.; New Energy and Industrial Technology Development Organization: Kawasaki, Japan, 2014. (In Japanese) [Google Scholar]
- Gallert, C.; Bauer, S.; Winter, J. Effect of ammonia on the anaerobic degradation of protein by a mesophilic and thermophilic biowaste population. Appl. Microbiol. Biotechnol. 1998, 50, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Kato, K. Regulation of wastewater in the Water Pollution Control Law and waste water standards of nitrogen and phosphorus in livestock industry. Anim. Husb. Environ. Inf. 2004, 24, 19–25. (In Japanese) [Google Scholar]
- Watanabe, K. Recent developments in microbial fuel cell technologies for sustainable bioenergy. J. Biosci. Bioeng. 2008, 106, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [CrossRef]
- Malaeb, L.; Katuri, K.P.; Logan, B.E.; Maab, H.; Nunes, S.P.; Saikaly, P.E. A hybrid microbial fuel cell membrane bioreactor with a conductive ultrafiltration membrane biocathode for wastewater treatment. Environ. Sci. Technol. 2013, 47, 11821–11828. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, X.; Logan, B.E.; Lee, H. Brewery wastewater treatment using air-cathode microbial fuel cells. Appl. Microbiol. Biotechnol. 2008, 78, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Feng, Y.J.; Lee, H. Electricity production from beer brewery wastewater using single chamber microbial fuel cell. Water Sci. Technol. 2008, 57, 1117–1121. [Google Scholar] [CrossRef]
- Clauwaert, P.; Rabaey, K.; Aelterman, P.; De Schamphelaire, L.; Pham, T.H.; Boeckx, P.; Boon, N.; Verstraete, W. Biological denitrification in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 3354–3360. [Google Scholar] [CrossRef]
- Zhuang, L.; Zheng, Y.; Zhou, S.; Yuan, Y.; Yuan, H.; Chen, Y. Scalable microbial fuel cell (MFC) stack for continuous real wastewater treatment. Bioresour. Technol. 2012, 106, 82–88. [Google Scholar] [CrossRef]
- Feng, Y.; He, W.; Liu, J.; Wang, X.; Qu, Y.; Ren, N. A horizontal plug flow and stackable pilot microbial fuel cell for municipal wastewater treatment. Bioresour. Technol. 2014, 156, 132–138. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wallack, M.J.; Kim, K.Y.; Zhang, X.; Yang, W.; Zhu, X.; Feng, Y.; Logan, B.E. The effect of flow modes and electrode combinations on the performance of a multiple module microbial fuel cell installed at wastewater treatment plant. Water Res. 2016, 105, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ge, Z.; Grimaud, J.; Hurst, J.; He, Z. Long-term performance of liter-scale microbial fuel cells treating primary effluent installed in a municipal wastewater treatment facility. Environ. Sci. Technol. 2013, 47, 4941–4948. [Google Scholar] [CrossRef]
- Dong, Y.; Qu, Y.; He, W.; Du, Y.; Liu, J.; Han, X.; Feng, Y.A. A 90-liter stackable baffled microbial fuel cell for brewery wastewater treatment based on energy self-sufficient mode. Bioresour. Technol. 2015, 195, 66–72. [Google Scholar] [CrossRef]
- Lu, M.; Chen, S.; Babanova, S.; Phadke, S.; Salvacion, M.; Mirhosseini, A.; Chan, S.; Carpenter, K.; Cortese, R.; Bretschger, O. Long-term performance of a 20-L continuous flow microbial fuel cell for treatment of brewery wastewater. J. Power Sources 2017, 356, 274–287. [Google Scholar] [CrossRef]
- Oh, S.T.; Kim, J.R.; Premier, G.C.; Lee, T.H.; Kim, C.; Sloan, W.T. Sustainable wastewater treatment: How might microbial fuel cells contribute. Biotechnol. Adv. 2010, 28, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Goto, Y.; Miyata, Y. Selective growth of and electricity production by marine exoelectrogenic bacteria in self-aggregated hydrogel of microbially reduced graphene oxide. C 2016, 2, 15. [Google Scholar] [CrossRef]
- Yoshida, N.; Miyata, Y.; Doi, K.; Goto, Y.; Nagao, Y.; Tero, R.; Hiraishi, A. Graphene oxide-dependent growth and self-aggregation into a hydrogel complex of exoelectrogenic bacteria. Sci. Rep. 2016, 6, 21867. [Google Scholar] [CrossRef]
- Goto, Y.; Yoshida, N. Microbially reduced graphene oxide shows efficient electricity recovery from artificial dialysis wastewater. J. Gen. Appl. Microbiol. 2017, 63, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, N.; Miyata, Y.; Mugita, A.; Iida, K. Electricity recovery from municipal sewage wastewater using a hydrogel complex composed of microbially reduced graphene oxide and sludge. Materials 2016, 9, 742. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Goto, Y.; Yoshida, N. Preliminary evaluation of a microbial fuel cell treating artificial dialysis wastewater using graphene oxide. AIP Conf. Proc. 2016, 1709, 020007. [Google Scholar]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Logan, B.E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 2005, 39, 5488–5493. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Wallack, M.J.; Kim, K.-Y.; He, W.; Feng, Y.; Saikaly, P.E. Assessment of microbial fuel cell configurations and power densities. Environ. Sci. Technol. Lett. 2015, 2, 206–214. [Google Scholar] [CrossRef]
- Ghangrekar, M.M.; Shinde, V.B. Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production. Bioresour. Technol. 2007, 98, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.R.; Zuo, Y.; Regan, J.M.; Logan, B.E. Analysis of ammonia loss mechanisms in microbial fuel cells treating animal wastewater. Biotechnol. Bioeng. 2008, 99, 1120–1127. [Google Scholar] [CrossRef]
- Ge, Z.; He, Z. Long-term performance of a 200 liter modularized microbial fuel cell system treating municipal wastewater: Treatment, energy, and cost. Environ. Sci.: Water Res. Technol. 2016, 2, 274–281. [Google Scholar] [CrossRef]
- Kim, J.R.; Dec, J.; Bruns, M.A.; Logan, B.E. Removal of odors from swine wastewater by using microbial fuel cells. Appl. Environ. Microbiol. 2008, 74, 2540–2543. [Google Scholar] [CrossRef]
- Ichihashi, O.; Hirooka, K. Removal and recovery of phosphorus as struvite from swine wastewater using microbial fuel cell. Bioresour. Technol. 2012, 114, 303–307. [Google Scholar] [CrossRef]
- Ding, W.; Cheng, S.; Yu, L.; Huang, H. Effective swine wastewater treatment by combining microbial fuel cells with flocculation. Chemosphere 2017, 182, 567–573. [Google Scholar] [CrossRef]
- Min, B.; Kim, J.R.; Oh, S.E.; Regan, J.M.; Logan, B.E. Electricity generation from swine wastewater using microbial fuel cells. Water Res. 2005, 39, 4961–4968. [Google Scholar] [CrossRef] [PubMed]
- Nurmiyanto, A.; Kodera, H.; Kindaichi, T.; Ozaki, N.; Aoi, Y.; Ohashi, A. Dominant Candidatus Accumulibacter phosphatis enriched in response to phosphate concentrations in EBPR process. Microbes Environ. 2017, 32, 260–267. [Google Scholar] [CrossRef] [PubMed]
- He, Z. Microbial fuel cells: Now let us talk about energy. Environ. Sci. Technol. 2013, 47, 332–333. [Google Scholar] [CrossRef]
- Maktabifard, M.; Zaborowska, E.; Makinia, J. Achieving energy neutrality in wastewater treatment plants through energy savings and enhancing renewable energy production. Rev. Environ. Sci. Bio/Technol. 2018, 17, 655–689. [Google Scholar] [CrossRef] [Green Version]
- Sugioka, M.; Yoshida, N.; Iida, K. On site evaluation of a tubular microbial fuel cell using an anion exchange membrane for sewage water treatment. Front. Energy Res. 2019, 7, 480714. [Google Scholar]




| Reference | Previous Research | This Study | ||||||
|---|---|---|---|---|---|---|---|---|
| 17 | 36 | 37 | 38 | 39 | 0.5 L | 4 L | 50 L | |
| Type | SAC a | SAC | SAC | SAC | SAC | SAC | SAC | AIC b |
| Unit scale (L) | 0.30 | 0.028 | 0.07 | 0.4 | 0.028 | 0.5 | 4 | 50 |
| CA c [cm2] | 187 | 7 | 47 | 400 | 7 | 39 | 400 | 1569 |
| CSSA [m2 m−3] | 64 | 25 | 67 | 100 | 25 | 7.8 | 10 | 3.1 |
| CC d | MnO2 e | Pt f | Pt g | AC h | Ptf | Pt g | Pt g | Pt g |
| Membrane | CEM i | Nafion | PTFE | PTFE | PTFE | PTFE | PTFE | PTFE |
| Temp. [°C] | 30 | 30 | RT j | 30 | 30 | RT | RT | 28.5–5.9 |
| IN-COD k [mg/L] | 5845 | 8270l | 60000 | 1313 | 8230 l | 2325 | 6500 | 7200 |
| EF-COD m [mg L−1] | 1110 | 1320l | 2900–9400 | 156 | 6090 l | 355 | 3400 | 3500 |
| Operation | C | FB n | FB | FB | FB | C | C | C |
| HRT | 5.7–23 h | 260 h | 3–17 d | 84 h | 44 h | 3 d | 3 d | 5 d |
| Electricity [Wh m−2] | 0.23 | Max: 0.23 | Max: 2.3 | Max: 0.27 | Max: 0.26 | Max: 0.35 Ave: 0.048 | Max: 0.15 Ave: 0.09 | Max: 1.2 Ave: 0.25 |
| [Wh m−3] | 15 | Max: 5.8 | Max: 154 | Max: 27 | Max: 6.6 | Max: 2.7 Ave: 1.2 | Max: 1.5 Ave: 0.9 | Max: 3.7 Ave: 0.8 |
| COD RE [%] | 81 | 84 | 91 | 88 | 27 | 85 | 49 | 52 |
| CE [%] | 0.3–0.5 | 47 | 14 | 8 | 5.6–15 | 1.0–15 | 0.7–9.2 | |
| EGE p [kwh kg-COD−1] | 0.044 | 0.035 | 0.026 | |||||
| Measurement | 1.5 L | 12 L | 100 L |
|---|---|---|---|
| COD-IN a (mg L−1) | 2300 | 6500 | 7200 |
| COD-EF b (MFC) (mg L−1) | 360 | 3400 | 3500 |
| COD-EF b (aeration) (mg L−1) | 10 | 10 | 10 |
| MFC-HRT c (d) | 3 | 3 | 5 |
| EGE d (kwh kg-COD−1) | 0.044 | 0.035 | 0.026 |
| EC e (kwh kg-COD−1) | ‒0.6 | ‒0.6 | ‒0.6 |
| MFC f (kwh m−3) | 0.086 | 0.11 | 0.096 |
| Aeration g (kwh m−3) | ‒1.4 | ‒3.9 | ‒4.3 |
| Post-aeration h (kwh m−3) | ‒0.21 | ‒2.0 | ‒2.1 |
| Net energy (kwh m−3) | ‒0.12 | ‒1.9 | ‒2.0 |
| Energy reduction (%) | 91 | 50 | 54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goto, Y.; Yoshida, N. Scaling up Microbial Fuel Cells for Treating Swine Wastewater. Water 2019, 11, 1803. https://doi.org/10.3390/w11091803
Goto Y, Yoshida N. Scaling up Microbial Fuel Cells for Treating Swine Wastewater. Water. 2019; 11(9):1803. https://doi.org/10.3390/w11091803
Chicago/Turabian StyleGoto, Yuko, and Naoko Yoshida. 2019. "Scaling up Microbial Fuel Cells for Treating Swine Wastewater" Water 11, no. 9: 1803. https://doi.org/10.3390/w11091803
APA StyleGoto, Y., & Yoshida, N. (2019). Scaling up Microbial Fuel Cells for Treating Swine Wastewater. Water, 11(9), 1803. https://doi.org/10.3390/w11091803





