Phytoplankton Community Response to Nutrients, Temperatures, and a Heat Wave in Shallow Lakes: An Experimental Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Sampling and Analyses of Physical and Chemical Variables
2.3. Statistical Analyses
2.3.1. Effects of Nutrients, Warming and Heat Wave
2.3.2. Stability Analyses
2.3.3. Phytoplankton Size Structure
3. Results
3.1. Initial Conditions
3.2. Phytoplankton Community Composition Throughout the Study
3.3. Nutrient Effects
3.4. Temperature Treatments: Before, during, and after the Heat Wave
3.5. Ecosystem Stability
3.6. Phytoplankton Size Structure
4. Discussion
4.1. Nutrient Effect
4.2. Effects of Warming, Nutrient and Temperature Interactions, and Heat Waves
4.3. Ecosystem Stability
4.4. Phytoplankton Size Structure
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ptacnik, R.; Lepistö, L.; Willén, E.; Brettum, P.; Andersen, T.; Rekolainen, S.; Lyche Solheim, A.; Carvalho, L. Quantitative Responses of Lake Phytoplankton to Eutrophication in Northern Europe. Aquat. Ecol. 2008, 42, 227–236. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, C.S. Phytoplankton Assemblages and Their Periodicity in Stratifying Lake Systems. Ecogr. Cop. 1980, 3, 141–159. [Google Scholar] [CrossRef]
- Reynolds, C.S. Phytoplankton Periodicity: The Interactions of Form, Function and Environmental Variability. Freshw. Biol. 1984, 14, 111–142. [Google Scholar] [CrossRef]
- Reynolds, C.S. Towards a Functional Classification of the Freshwater Phytoplankton. J. Plankton Res. 2002, 24, 417–428. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A.; Schofield, O.M.; Falkowski, P.G. The Role of Functional Traits and Trade-Offs in Structuring Phytoplankton Communities: Scaling from Cellular to Ecosystem Level. Ecol. Lett. 2007, 10, 1170–1181. [Google Scholar] [CrossRef]
- Padisák, J.; Crossetti, L.O.; Naselli-Flores, L. Use and Misuse in the Application of the Phytoplankton Functional Classification: A Critical Review with Updates. Hydrobiologia 2009, 621, 1–19. [Google Scholar] [CrossRef]
- Kruk, C.; Peeters, E.T.H.M.; Van Nes, E.H.; Huszar, V.L.M.; Costa, L.S.; Scheffer, M. Phytoplankton Community Composition Can Be Predicted Best in Terms of Morphological Groups. Limnol. Oceanogr. 2011, 56, 110–118. [Google Scholar] [CrossRef]
- Salmaso, N.; Naselli-Flores, L.; Padisák, J. Functional Classifications and Their Application in Phytoplankton Ecology. Freshw. Biol. 2015, 60, 603–619. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.G.; Hong, S.; Kim, D.K.; Joo, G.J. Drivers Shaping Episodic and Gradual Changes in Phytoplankton Community Succession: Taxonomic versus Functional Groups. Sci. Total Environ. 2020, 734, 138940. [Google Scholar] [CrossRef]
- Litchman, E.; Klausmeier, C.A.; Yoshiyama, K. Contrasting Size Evolution in Marine and Freshwater Diatoms. Proc. Natl. Acad. Sci. USA 2009, 106, 2665–2670. [Google Scholar] [CrossRef] [Green Version]
- Pomati, F.; Shurin, J.B.; Andersen, K.H.; Tellenbach, C.; Barton, A.D. Interacting Temperature, Nutrients and Zooplankton Grazing Control Phytoplankton Size-Abundance Relationships in Eight Swiss Lakes. Front. Microbiol. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Montoya, J.M.; Trimmer, M.; Woodward, G. Warming Alters the Size Spectrum and Shifts the Distribution of Biomass in Freshwater Ecosystems. Glob. Chang. Biol. 2011, 17, 1681–1694. [Google Scholar] [CrossRef] [Green Version]
- Sommer, U.; Peter, K.H.; Genitsaris, S.; Moustaka-Gouni, M. Do Marine Phytoplankton Follow Bergmann’s Rule Sensu Lato? Biol. Rev. 2017, 92, 1011–1026. [Google Scholar] [CrossRef] [Green Version]
- Zohary, T.; Flaim, G.; Sommer, U. Temperature and the Size of Freshwater Phytoplankton. Hydrobiologia 2020, 6. [Google Scholar] [CrossRef]
- Daufresne, M.; Lengfellner, K.; Sommer, U. Global Warming Benefits the Small in Aquatic Ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12788–12793. [Google Scholar] [CrossRef] [Green Version]
- Irwin, A.J.; Finkel, Z.V.; Schofield, O.M.E.; Falkowski, P.G. Scaling-up from Nutrient Physiology to the Size-Structure of Phytoplankton Communities. J. Plankton Res. 2006, 28, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, D.A.; Molot, L.A.; Shuter, B.J. Specific Growth Rates of Freshwater Algae in Relation to Cell Size and Light Intensity. Can. J. Fish. Aquat. Sci. 1981, 38, 1052–1058. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Kitchell, J.F. Plankton Community Structure and Limnetic Primary Production. Am. Nat. 1984, 124, 159–172. [Google Scholar] [CrossRef]
- Brookes, J.D.; Carey, C.C. Resilience to Blooms. Science 2011, 334, 46–47. [Google Scholar] [CrossRef]
- Beklioǧlu, M.; Meerhoff, M.; Søndergaard, M.; Jeppesen, E. Eutrophication and Restoration of Shallow Lakes from a Cold Temperate to a Warm Mediterranean and a (Sub)Tropical Climate. In Eutrophication: Causes, Consequences and Control; Ansari, A.A., Gill, S.S., Lanza, G.R., Rast, W., Eds.; Springer: Cham, Switzerland, 2011; pp. 91–108. [Google Scholar] [CrossRef]
- Dodson, S.I.; Arnott, S.E.; Cottingham, K.L. The Relationship in Lake Communities between Primary Productivity and Species Richness. Ecology 2000, 81, 2662–2679. [Google Scholar] [CrossRef]
- Jeppesen, E.; Jensen, J.P.; Søndergaard, M.; Lauridsen, T.; Landkildehus, F. Trophic Structure, Species Richness and Diversity in Danish Lakes: Changes along a Phosphorus Gradient. Freshw. Biol. 2000, 45, 201–218. [Google Scholar] [CrossRef]
- Hodapp, D.; Hillebrand, H.; Striebel, M. Unifying the Concept of Resource Use Efficiency in Ecology. Front. Ecol. Evol. 2019, 6, 233. [Google Scholar] [CrossRef] [Green Version]
- Vitousek, P. Nutrient Cycling and Nutrient Use Efficiency. Am. Nat. 1982, 119, 553–572. [Google Scholar] [CrossRef]
- Ptacnik, R.; Solimini, A.G.; Andersen, T.; Tamminen, T.; Brettum, P.; Lepistö, L.; Willén, E.; Rekolainen, S. Diversity Predicts Stability and Resource Use Efficiency in Natural Phytoplankton Communities. Proc. Natl. Acad. Sci. USA 2008, 105, 5134–5138. [Google Scholar] [CrossRef] [Green Version]
- Olli, K.; Ptacnik, R.; Andersen, T.; Trikk, O.; Klais, R.; Lehtinen, S.; Tamminen, T. Against the Tide: Recent Diversity Increase Enhances Resource Use in a Coastal Ecosystem. Limnol. Oceanogr. 2014, 59, 267–274. [Google Scholar] [CrossRef]
- Duffy, J.E.; Cardinale, B.J.; France, K.E.; McIntyre, P.B.; Thébault, E.; Loreau, M. The Functional Role of Biodiversity in Ecosystems: Incorporating Trophic Complexity. Ecol. Lett. 2007, 10, 522–538. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Zhang, H.; Zhang, J.; Zhao, L.; Miao, M.; Huang, H. Biodiversity Effects on Resource Use Efficiency and Community Turnover of Plankton in Lake Nansihu, China. Environ. Sci. Pollut. Res. 2017, 24, 11279–11288. [Google Scholar] [CrossRef]
- Ersoy, Z.; Brucet, S.; Bartrons, M.; Mehner, T. Short-Term Fish Predation Destroys Resilience of Zooplankton Communities and Prevents Recovery of Phytoplankton Control by Zooplankton Grazing. PLoS ONE 2019, 14, e0212351. [Google Scholar] [CrossRef]
- Mccauley, E.; Briand, F. Zooplankton Grazing and Phytoplankton Species Richness: Field Tests of the Predation Hypothesis. Limnol. Oceanogr. 1979, 24, 243–252. [Google Scholar] [CrossRef]
- Jeppesen, E.; Nõges, P.; Davidson, T.A.; Haberman, J.; Nõges, T.; Blank, K.; Lauridsen, T.L.; Søndergaard, M.; Sayer, C.; Laugaste, R.; et al. Zooplankton as Indicators in Lakes: A Scientific-Based Plea for Including Zooplankton in the Ecological Quality Assessment of Lakes According to the European Water Framework Directive (WFD). Hydrobiologia 2011, 676, 279–297. [Google Scholar] [CrossRef]
- Carney, H.J.; Elser, J.J. Strength of Zooplankton-Phytoplankton Coupling in Relation to Lake Trophic State. In Large Lakes, Brock/Springer Series in Contemporary Bioscience; Tilzer, M.M., Serruya, C., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 615–631. [Google Scholar] [CrossRef]
- Winder, M.; Sommer, U. Phytoplankton Response to a Changing Climate. Hydrobiologia 2012, 5–16. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; González-Bergonzoni, I.; Teixeira-de Mello, F.; Declerck, S.A.J.; De Meester, L.; Søndergaard, M.; Lauridsen, T.L.; Bjerring, R.; et al. Impacts of Climate Warming on Lake Fish Community Structure and Potential Effects on Ecosystem Function. Hydrobiologia 2010, 646, 73–90. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Synthesis Report, Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzeland, 2014. [Google Scholar]
- Bartosiewicz, M.; Przytulska, A.; Deshpande, B.N.; Antoniades, D.; Cortes, A.; MacIntyre, S.; Lehmann, M.F.; Laurion, I. Effects of Climate Change and Episodic Heat Events on Cyanobacteria in a Eutrophic Polymictic Lake. Sci. Total Environ. 2019, 693, 133414. [Google Scholar] [CrossRef]
- Gallina, N.; Anneville, O.; Beniston, M. Impacts of Extreme Air Temperatures on Cyanobacteria in Five Deep Peri-Alpine Lakes. J. Limnol. 2011, 70, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Huber, V.; Wagner, C.; Gerten, D.; Adrian, R. To Bloom or Not to Bloom: Contrasting Responses of Cyanobacteria to Recent Heat Waves Explained by Critical Thresholds of Abiotic Drivers. Oecologia 2012, 169, 245–256. [Google Scholar] [CrossRef]
- Jöhnk, K.D.; Huisman, J.; Sharples, J.; Sommeijer, B.; Visser, P.M.; Stroom, J.M. Summer Heatwaves Promote Blooms of Harmful Cyanobacteria. Glob. Chang. Biol. 2008, 14, 495–512. [Google Scholar] [CrossRef] [Green Version]
- Urrutia-Cordero, P.; Zhang, H.; Chaguaceda, F.; Geng, H.; Hansson, L.A. Climate Warming and Heat Waves Alter Harmful Cyanobacterial Blooms along the Benthic–Pelagic Interface. Ecology 2020, 101, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Huisman, J. Blooms like It Hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, E.; Kronvang, B.; Meerhoff, M.; Søndergaard, M.; Hansen, K.M.; Andersen, H.E.; Lauridsen, T.L.; Liboriussen, L.; Beklioglu, M.; Özen, A.; et al. Climate Change Effects on Runoff, Catchment Phosphorus Loading and Lake Ecological State, and Potential Adaptations. J. Environ. Qual. 2009, 38, 1930. [Google Scholar] [CrossRef]
- Bloch, I.; Weyhenmeyer, G.A. Long-Term Changes in Physical and Chemical Conditions of Nutrient-Poor Lakes along a Latitudinal Gradient: Is There a Coherent Phytoplankton Community Response? Aquat. Sci. 2012, 74, 77–85. [Google Scholar] [CrossRef]
- Thies, H.; Tolotti, M.; Nickus, U.; Lami, A.; Musazzi, S.; Guilizzoni, P.; Rose, N.L.; Yang, H. Interactions of Temperature and Nutrient Changes: Effects on Phytoplankton in the Piburger See (Tyrol, Austria). Freshw. Biol. 2012, 57, 2057–2075. [Google Scholar] [CrossRef]
- Battarbee, R.W.; Bennion, H. Using Palaeolimnological and Limnological Data to Reconstruct the Recent History of European Lake Ecosystems: Introduction. Freshw. Biol. 2012, 57, 1979–1985. [Google Scholar] [CrossRef] [Green Version]
- Elliott, J.A. The Seasonal Sensitivity of Cyanobacteria and Other Phytoplankton to Changes in Flushing Rate and Water Temperature. Glob. Chang. Biol. 2010, 16, 864–876. [Google Scholar] [CrossRef]
- Moss, B.; Mckee, D.; Atkinson, D.; Collings, S.E.; Eaton, J.W.; Gill, A.B.; Harvey, I.; Hatton, K.; Heyes, T.; Wilson, D. How Important Is Climate? Effects of Warming, Nutrient Addition and Fish on Phytoplankton in Shallow Lake Microcosms. J. Appl. Ecol. 2003, 40, 782–792. [Google Scholar] [CrossRef] [Green Version]
- Anneville, O.; Souissi, S.; Ibanez, F.; Ginot, V.; Druart, J.C.; Angeli, N. Temporal Mapping of Phytoplankton Assemblages in Lake Geneva: Annual and Interannual Changes in Their Patterns of Succession. Limnol. Oceanogr. 2002, 47, 1355–1366. [Google Scholar] [CrossRef]
- Elliott, J.A.; Jones, I.D.; Thackeray, S.J. Testing the Sensitivity of Phytoplankton Communities to Changes in Water Temperature and Nutrient Load, in a Temperate Lake. Hydrobiologia 2006, 559, 401–411. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.M.; Becares, E.; Costa, L.S.; van Donk, E.; Hansson, L.A.; Jeppesen, E.; Kruk, C.; Lacerot, G.; Mazzeo, N.; et al. Warmer Climates Boost Cyanobacterial Dominance in Shallow Lakes. Glob. Chang. Biol. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- Wagner, C.; Adrian, R. Cyanobacteria Dominance: Quantifying the Effects of Climate Change. Limnol. Oceanogr. 2009, 54, 2460–2468. [Google Scholar] [CrossRef]
- Weisse, T.; Gröschl, B.; Bergkemper, V. Phytoplankton Response to Short-Term Temperature and Nutrient Changes. Limnologica 2016, 59, 78–89. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [Green Version]
- Hillebrand, H.; Kunze, C. Meta-Analysis on Pulse Disturbances Reveals Differences in Functional and Compositional Recovery across Ecosystems. Ecol. Lett. 2020, 23, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Allen, A.P.; Cellamare, M.; Dossena, M.; Gaston, K.J.; Leitao, M.; Montoya, J.M.; Reuman, D.C.; Woodward, G.; Trimmer, M. Five Years of Experimental Warming Increases the Biodiversity and Productivity of Phytoplankton. PLoS Biol. 2015, 13, e1002324. [Google Scholar] [CrossRef] [Green Version]
- Verbeek, L.; Gall, A.; Hillebrand, H.; Striebel, M. Warming and Oligotrophication Cause Shifts in Freshwater Phytoplankton Communities. Glob. Chang. Biol. 2018, 24, 4532–4543. [Google Scholar] [CrossRef] [Green Version]
- Donohue, I.; Petchey, O.L.; Montoya, J.M.; Jackson, A.L.; Mcnally, L.; Viana, M.; Healy, K.; Lurgi, M.; O’Connor, N.E.; Emmerson, M.C. On the Dimensionality of Ecological Stability. Ecol. Lett. 2013, 16, 421–429. [Google Scholar] [CrossRef]
- Hillebrand, H.; Langenheder, S.; Lebret, K.; Lindström, E.; Östman, Ö.; Striebel, M. Decomposing Multiple Dimensions of Stability in Global Change Experiments. Ecol. Lett. 2018, 21, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Pimm, S.L. The Complexity and Stability of Ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Orians, G.H. Diversity, Stability and Maturity in Natural Ecosystems. In Unifying Concepts in Ecology; van Dobben, W.H., Lowe-McConnell, R.H., Eds.; Springer: The Hague, The Netherlands, 1975. [Google Scholar] [CrossRef]
- Liboriussen, L.; Landkildehus, F.; Meerhoff, M.; Bramm, M.E.; Søndergaard, M.; Christoffersen, K.; Richardson, K.; Søndergaard, M.; Lauridsen, T.L.; Jeppesen, E. Global Warming: Design of a Flow-through Shallow Lake Mesocosm Climate Experiment. Limnol. Oceanogr. Methods 2005, 3, 1–9. [Google Scholar] [CrossRef]
- Jeppesen, E.; Moss, B.; Bennion, H.; Carvalho, L.; Demeester, L.; Feuchtmayr, H.; Friberg, N.; Gessner, M.O.; Hefting, M.; Lauridsen, T.L.; et al. Interaction of Climate Change and Eutrophication; Wiley Online Library: Hoboken, NJ, USA, 2010. [Google Scholar] [CrossRef] [Green Version]
- IPCC. Climate Change 2007: Synthesis Report, Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Pachauri, R.K., Reisinger, A., Eds.; IPCC: Geneva, Switzerland, 2007; p. 104. [Google Scholar]
- IPCC. Climate change 2001: The Scientific Basis, Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Houghton, J.T., Ding, Y., Griggs, D.J., Noguer, M., van der Linden, P.J., Dai, X., Maskell, K., Johnson, C.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2001. [Google Scholar] [CrossRef]
- Landkildehus, F.; Søndergaard, M.; Beklioglu, M.; Adrian, R.; Angeler, D.G.; Hejzlar, J.; Papastergiadou, E.; Zingel, P.; Çakiroǧlu, A.I.; Scharfenberger, U.; et al. Climate Change Effects on Shallow Lakes: Design and Preliminary Results of a Cross-European Climate Gradient Mesocosm Experiment. Est. J. Ecol. 2014, 63, 71–89. [Google Scholar] [CrossRef] [Green Version]
- Işkın, U.; Filiz, N.; Cao, Y.; Neif, É.M.; Öğlü, B.; Lauridsen, T.L.; Davidson, T.A.; Søndergaard, M.; Tavşanoğlu, Ü.N.; Beklioǧlu, M.; et al. Impact of Nutrients, Temperature and a Heat Wave on Zooplankton Community Structure: An Experimental Approach. Water 2020, in press. [Google Scholar]
- Davidson, T.A.; Audet, J.; Svenning, J.C.; Lauridsen, T.L.; Sendergaard, M.; Landkildehus, F.; Larsen, S.E.; Jeppesen, E. Eutrophication Effects on Greenhouse Gas Fluxes from Shallow-Lake Mesocosms Override Those of Climate Warming. Glob. Chang. Biol. 2015, 21, 4449–4463. [Google Scholar] [CrossRef]
- Jespersen, A.; Christoffersen, K. Measurements of Chlorophyll―A from Phytoplankton Using Ethanol as Extraction Solvent. Arch. Hydrobiol. 1987, 109, 445–454. [Google Scholar]
- Søndergaard, M.; Jeppesen, E.; Jensen, J.P.; Amsinck, S.L. Water Framework Directive: Ecological Classification of Danish Lakes. J. Appl. Ecol. 2005, 42, 616–629. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. The Comprehensive R Archive Network. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 December 2020).
- Abonyi, A.; Horváth, Z.; Ptacnik, R. Functional Richness Outperforms Taxonomic Richness in Predicting Ecosystem Functioning in Natural Phytoplankton Communities. Freshw. Biol. 2017, 63, 178–186. [Google Scholar] [CrossRef] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015. [Google Scholar] [CrossRef]
- Feld, C.K.; Segurado, P.; Gutiérrez-Cánovas, C. Analysing the Impact of Multiple Stressors in Aquatic Biomonitoring Data: A ‘Cookbook’ with Applications in R. Sci. Total Environ. 2016. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. CAR—An R Companion to Applied Regression; Sage Publications: Texas, CA, USA, 2019. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analyzing Ecological Data; Springer: Cham, Switzeland, 2007. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where Is Positional Uncertainty a Problem for Species Distribution Modelling? Ecogr. Cop. 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Dolédec, S.; Chessel, D. Co-inertia Analysis: An Alternative Method for Studying Species–Environment Relationships. Freshw. Biol. 1994, 31, 277–294. [Google Scholar] [CrossRef]
- Öğlü, B.; Bhele, U.; Järvalt, A.; Tuvikene, L.; Timm, H.; Seller, S.; Haberman, J.; Agasild, H.; Nõges, P.; Silm, M.; et al. Is Fish Biomass Controlled by Abiotic or Biotic Factors? Results of Long-Term Monitoring in a Large Eutrophic Lake. J. Great Lakes Res. 2019, 46, 881–890. [Google Scholar] [CrossRef]
- Kruk, C.; Huszar, V.L.M.; Peeters, E.T.H.M.; Bonilla, S.; Costa, L.; LüRling, M.; Reynolds, C.S.; Scheffer, M. A Morphological Classification Capturing Functional Variation in Phytoplankton. Freshw. Biol. 2010, 55, 614–627. [Google Scholar] [CrossRef]
- Brucet, S.; Boix, D.; López-Flores, R.; Badosa, A.; Quintana, X.D. Size and Species Diversity of Zooplankton Communities in Fluctuating Mediterranean Salt Marshes. Estuar. Coast. Shelf Sci. 2006, 67, 424–432. [Google Scholar] [CrossRef]
- Quintana, X.D.; Brucet, S.; Boix, D.; López-Flores, R.; Gascón, S.; Badosa, A.; Sala, J.; Moreno-Amich, R.; Egozcue, J.J. A Nonparametric Method for the Measurement of Size Diversity with Emphasis on Data Standardization. Limnol. Oceanogr. Methods 2008, 6, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Ersoy, Z.; Jeppesen, E.; Sgarzi, S.; Arranz, I.; Cañedo-Argüelles, M.; Quintana, X.D.; Landkildehus, F.; Lauridsen, T.L.; Bartrons, M.; Brucet, S. Size-Based Interactions and Trophic Transfer Efficiency Are Modified by Fish Predation and Cyanobacteria Blooms in Lake Mývatn, Iceland. Freshw. Biol. 2017, 62, 1942–1952. [Google Scholar] [CrossRef]
- Pielou, E.C. An Introduction to Mathematical Ecology; Wiley-Inter-science: New York, NY, USA, 1969. [Google Scholar]
- Brucet, S.; Tavşanoğlu, Ü.N.; Özen, A.; Levi, E.E.; Bezirci, G.; Çakıroğlu, A.İ.; Jeppesen, E.; Svenning, J.C.; Ersoy, Z.; Beklioğlu, M. Size-Based Interactions across Trophic Levels in Food Webs of Shallow Mediterranean Lakes. Freshw. Biol. 2017, 62, 1819–1830. [Google Scholar] [CrossRef]
- Birk, S.; Chapman, D.; Carvalho, L.; Spears, B.M.; Andersen, H.E.; Argillier, C.; Auer, S.; Baattrup-Pedersen, A.; Banin, L.; Beklioğlu, M.; et al. Impacts of Multiple Stressors on Freshwater Biota across Spatial Scales and Ecosystems. Nat. Ecol. Evol. 2020, 4, 1060–1068. [Google Scholar] [CrossRef]
- Jeppesen, E.; Søndergaard, M.; Lauridsen, T.L.; Davidson, T.A.; Liu, Z.; Mazzeo, N.; Trochine, C.; özkan, K.; Jensen, H.S.; Trolle, D.; et al. Biomanipulation as a Restoration Tool to Combat Eutrophication. Recent Advances and Future Challenges; Academic Press: Cambridge, MA, USA, 2012; Volume 47. [Google Scholar] [CrossRef]
- Elser, J.J.; Goldman, C.R. Experimental Separation of the Direct and Indirect Effects of Herbivorous Zooplankton on Phytoplankton in a Subalpine Lake. Int. Ver. Theor. Angew. Limnol. Verh. 1990, 24, 493–498. [Google Scholar] [CrossRef]
- Filstrup, C.T.; Hillebrand, H.; Heathcote, A.J.; Harpole, W.S.; Downing, J.A. Cyanobacteria Dominance Influences Resource Use Efficiency and Community Turnover in Phytoplankton and Zooplankton Communities. Ecol. Lett. 2014, 17, 464–474. [Google Scholar] [CrossRef]
- Heathcote, A.; Filstrup, C.; Kendall, D.; Downing, J. Biomass Pyramids in Lake Plankton: Influence of Cyanobacteria Size and Abundance. Inl. Waters 2016, 6, 250–257. [Google Scholar] [CrossRef]
- Caputo, L.; Naselli-Flores, L.; Ordoñez, J.; Armengol, J. Phytoplankton Distribution along Trophic Gradients within and among Reservoirs in Catalonia (Spain). Freshw. Biol. 2008, 53, 2543–2556. [Google Scholar] [CrossRef]
- Striebel, M.; Behl, S.; Stibor, H. The Coupling of Biodiversity and Productivity in Phytoplankton Communities: Consequences for Biomass Stoichiometry. Ecology 2009, 90, 2025–2031. [Google Scholar] [CrossRef] [Green Version]
- Chai, Z.Y.; Wang, H.; Deng, Y.; Hu, Z.; Zhong Tang, Y. Harmful Algal Blooms Significantly Reduce the Resource Use Efficiency in a Coastal Plankton Community. Sci. Total Environ. 2020, 704, 135381. [Google Scholar] [CrossRef]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The Influence of Functional Diversity and Composition on Ecosystem Processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef] [Green Version]
- O’Neil, J.M.; Davis, T.W.; Burford, M.A.; Gobler, C.J. The Rise of Harmful Cyanobacteria Blooms: The Potential Roles of Eutrophication and Climate Change. Harmful Algae 2012, 14, 313–334. [Google Scholar] [CrossRef]
- Sukenik, A.; Quesada, A.; Salmaso, N. Global Expansion of Toxic and Non-Toxic Cyanobacteria: Effect on Ecosystem Functioning. Biodivers. Conserv. 2015, 24, 889–908. [Google Scholar] [CrossRef]
- Seip, K.L.; Reynolds, C.S. Phytoplankton Functional Attributes along Trophic Gradient and Season. Limnol. Oceanogr. 1995, 40, 589–597. [Google Scholar] [CrossRef]
- Litchman, E.; de Tezanos Pinto, P.; Klausmeier, C.A.; Thomas, M.K.; Yoshiyama, K. Linking Traits to Species Diversity and Community Structure in Phytoplankton. Hydrobiologia 2010, 653, 15–28. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of Temperature and Light on the Growth of Algae Species: A Review. Renew. Sustain Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Reynolds, C.S.; Padisák, J.; Sommer, U. Intermediate Disturbance in the Ecology of Phytoplankton and the Maintenance of Species Diversity: A Synthesis. Hydrobiologia 1993, 249, 183–188. [Google Scholar] [CrossRef]


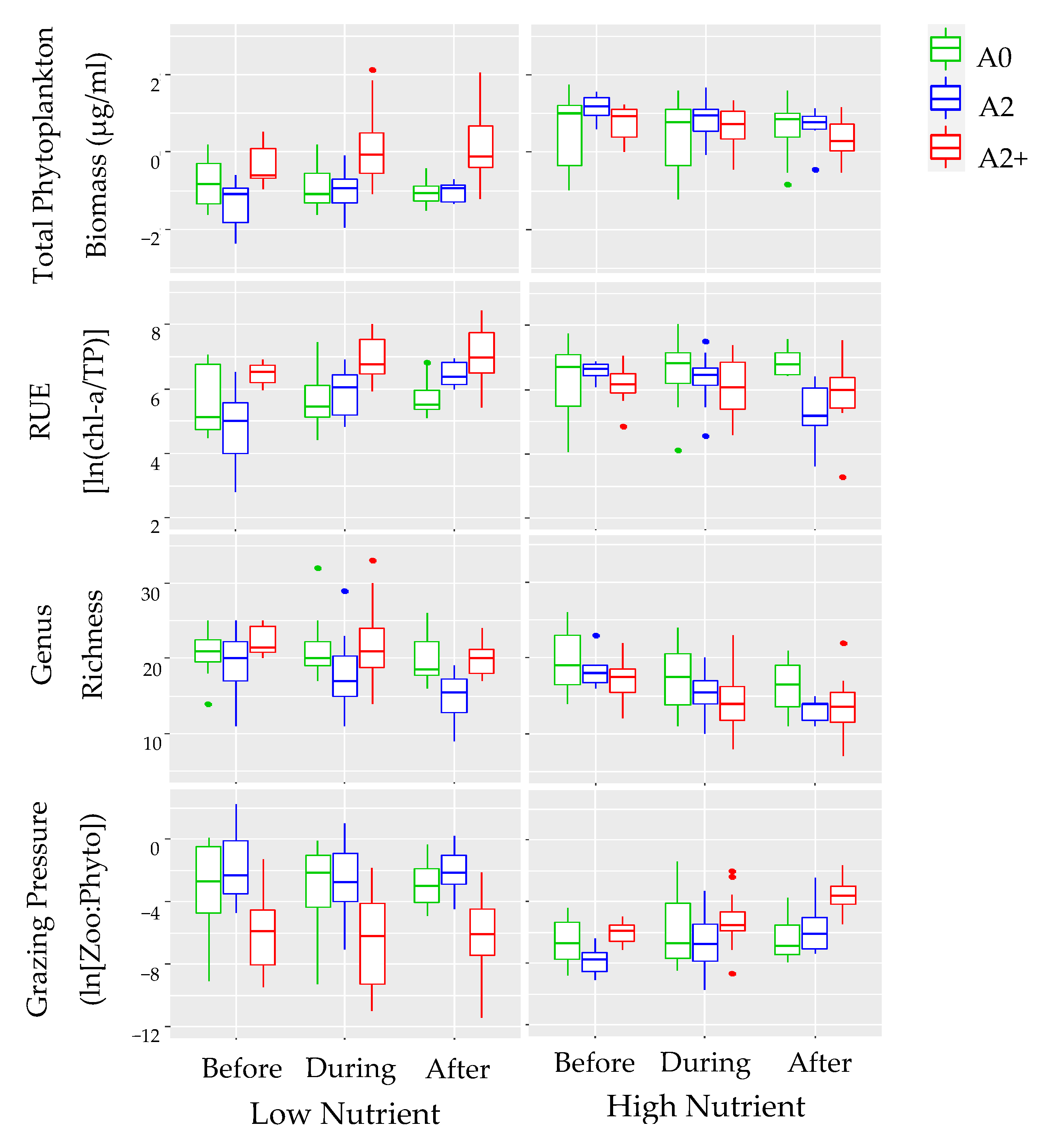




| LOW NUTRIENT (LN) | HIGH NUTRIENT (HN) | ||||||
|---|---|---|---|---|---|---|---|
| A0 | A2 | A2+ | A0 | A2 | A2+ | ||
| Temp. (°C) | Before | 17.34 ± 0.16 | 19.79 ± 0.15 | 20.96 ± 0.15 | 17.26 ± 0.12 | 19.70 ± 0.12 | 20.86 ± 0.12 |
| During | 20.05 ± 0.49 | 27.41 ± 0.49 | 28.68 ± 0.49 | 20.07 ± 0.49 | 27.05 ± 0.62 | 28.68 ± 0.49 | |
| After | 18.13 ± 1.29 | 21.89 ± 1.30 | 23.70 ± 1.28 | 18.22 ± 1.27 | 21.99 ± 1.27 | 23.78 ± 1.27 | |
| TP (mg/L) | 0.013 ± 0.002 | 0.009 ± 0.0003 | 0.014 ± 0.002 | 0.306 ± 0.06 | 0.570 ± 0.06 | 0.338 ± 0.06 | |
| TN (mg/L) | 0.31 ± 0.05 | 0.19 ± 0.02 | 0.35 ± 0.06 | 2.83 ± 0.48 | 3.43 ± 0.37 | 2.13 ± 0.25 | |
| Total Phyto. | Chloro-Phyta | Bacillario-Phyta | Cyano-Bacteria | Crypto-Phyta | Chryso-Phyta | Dino-Phyta | TZoo./TPhyto. | RUE | Genus Richness | Genus Evenness | Size Diversity | Size Evenness | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BEFORE | HNA0vsLNA0 (Nutrient effect) | 0.003 (+) | <0.001(+) | <0.001 (+) | 0.001 (+) | ns | ns | ns | 0.02 (−) | ns | 0.08 (−) | ns | ns | ns |
| LNA0vsLNA2 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| LNA0vsLNA2+ | ns | ns | ns | ns | ns | ns | 0.08 (+) | ns | ns | ns | ns | ns | ns | |
| HNA0vsHNA2 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | |
| HNA0vsHNA2+ | ns | ns | ns | ns | 0.02 (+) | ns | ns | ns | ns | ns | ns | ns | ns | |
| Nutrient*A2 | ns | NA | NA | NA | ns | NA | NA | ns | ns | NA | NA | NA | NA | |
| Nutrient*A2+ | ns | NA | NA | NA | 0.005 (+) | NA | NA | ns | ns | NA | NA | NA | NA | |
| DURING | HNA0vsLNA0 (Nutrient effect) | 0.002 (+) | 0.01 (+) | <0.001 (+) | <0.001(+) | <0.001 (−) | 0.004 (+) | ns | 0.03 (−) | 0.03 (+) | <0.001 (−) | ns | ns | ns |
| LNA0vsLNA2 | ns | ns | ns | 0.07 (+) | ns | 0.007 (+) | ns | ns | ns | 0.045 (−) | ns | ns | ns | |
| LNA0vsLNA2+ | 0.02 (+) | 0.02 (+) | ns | 0.08 (+) | ns | 0.04 (+) | 0.06 (+) | 0.044 (−) | 0.02 (+) | ns | ns | ns | ns | |
| HNA0vsHNA2 | ns | ns | ns | 0.07 (+) | ns | ns | ns | ns | ns | ns | ns | ns | 0.08 (−) | |
| HNA0vsHNA2+ | ns | ns | ns | ns | ns | ns | ns | s | ns | ns | ns | ns | 0.049 (−) | |
| Nutrient*A2 | ns | ns | NA | NA | NA | NA | 0.06 (−) | ns | ns | NA | NA | NA | 0.03 (−) | |
| Nutrient*A2+ | ns | 0.02 (-) | NA | NA | NA | NA | ns | 0.03 (+) | 0.004 (−) | NA | NA | NA | 0.04 (−) | |
| AFTER | HNA0vsLNA0 (Nutrient effect) | <0.001 (+) | 0.02 (+) | <0.001 (+) | <0.001(+) | ns | <0.001(+) | ns | 0.005 (−) | 0.02 (+) | 0.001 (−) | 0.06 (−) | ns | ns |
| LNA0vsLNA2 | ns | ns | ns | 0.05 (+) | 0.04 (+) | ns | ns | ns | ns | 0.002 (−) | ns | ns | ns | |
| LNA0vsLNA2+ | 0.005 (+) | 0.03 (+) | ns | ns | ns | ns | ns | 0.02 (−) | 0.01 (+) | ns | ns | ns | ns | |
| HNA0vsHNA2 | ns | ns | 0.02 (−) | ns | ns | ns | 0.08 (−) | ns | 0.004(−) | ns | ns | ns | 0.03 (−) | |
| HNA0vsHNA2+ | ns | ns | 0.007 (−) | ns | ns | ns | ns | 0.003(+) | 0.04 (−) | ns | ns | ns | 0.01 (−) | |
| Nutrient*A2 | ns | ns | ns | NA | 0.01 (−) | 0.04 (+) | 0.03 (−) | ns | 0.001(−) | NA | NA | NA | 0.07 (−) | |
| Nutrient*A2+ | 0.02 (−) | <0.001 (−) | 0.004 (−) | NA | ns | Ns | ns | 0.001 (+) | <0.001(−) | NA | NA | NA | 0.02 (−) |
| SC1 (<5 µm) | SC2 (5–20 µm) | SC3 (20–100 µm) | SC4 (>100 µm) | ||
|---|---|---|---|---|---|
| Before | HN-A0vsHN-A2 | 0.01 (+) | ns | 0.06 (+) | 0.004 (+) |
| HN-A0vsHN-A2+ | 0.03 (+) | ns | ns | ns | |
| During | HN-A0vsHN-A2 | 0.008 (+) | ns | 0.04 (+) | 0.01 (+) |
| HN-A0vsHN-A2+ | 0.007 (+) | ns | ns | 0.03 (+) | |
| After | HN-A0vsHN-A2 | ns | 0.04 (+) | ns | ns |
| HN-A0vsHN-A2+ | ns | ns | ns | 0.04 (+) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filiz, N.; Işkın, U.; Beklioğlu, M.; Öğlü, B.; Cao, Y.; Davidson, T.A.; Søndergaard, M.; Lauridsen, T.L.; Jeppesen, E. Phytoplankton Community Response to Nutrients, Temperatures, and a Heat Wave in Shallow Lakes: An Experimental Approach. Water 2020, 12, 3394. https://doi.org/10.3390/w12123394
Filiz N, Işkın U, Beklioğlu M, Öğlü B, Cao Y, Davidson TA, Søndergaard M, Lauridsen TL, Jeppesen E. Phytoplankton Community Response to Nutrients, Temperatures, and a Heat Wave in Shallow Lakes: An Experimental Approach. Water. 2020; 12(12):3394. https://doi.org/10.3390/w12123394
Chicago/Turabian StyleFiliz, Nur, Uğur Işkın, Meryem Beklioğlu, Burak Öğlü, Yu Cao, Thomas A. Davidson, Martin Søndergaard, Torben L. Lauridsen, and Erik Jeppesen. 2020. "Phytoplankton Community Response to Nutrients, Temperatures, and a Heat Wave in Shallow Lakes: An Experimental Approach" Water 12, no. 12: 3394. https://doi.org/10.3390/w12123394






